Abstract
Reducing medical errors and improving patient safety have become a national priority. Patients with chronic kidney disease (CKD) may be at higher risk for adverse consequences of medical care, but few studies have evaluated this question. Here, data for patients hospitalized in the Veteran's Health Administration during 2004 to 2005 was analyzed to conduct a cross-sectional study of CKD and adverse safety events. Outcomes included 13 patient safety indicators (PSI) defined by the Agency for Healthcare Research and Quality and six experimental PSI relevant to CKD. The 71,666 (29%) hospitalized veterans with CKD had a higher risk for several PSI, even after case-mix adjustment. Among surgical hospitalizations, CKD was associated with increased risk for hip fracture, physiologic/metabolic derangements, and complications of anesthesia. Among all acute hospitalizations, the PSI with the highest risk in patients with CKD were infection as a result of medical care and death among those in diagnosis-related groups normally associated with low mortality. Furthermore, as preadmission estimated GFR decreased, a significant trend of increasing risk for all PSI was observed (P = 0.001). In conclusion, hospitalized patients with CKD are at increased risk for adverse safety events, measured by established PSI. Further investigation is needed to develop and test interventions to reduce this risk.
Patient safety is a high priority area for improvement in health care. The 1999 Institute of Medicine (IOM) report entitled “To Err Is Human: Building a Safer Health System” concluded that 44,000 to 98,000 in-hospital deaths each year were due to medical errors.1 With increasing attention to this problem, the Agency for Healthcare Research and Quality (AHRQ) developed a set of “patient safety indicators” (PSI) to monitor rates of adverse events among hospitalized patients across multiple domains of medical care.2,3 These PSI are intended for use with administrative hospital discharge data to ascertain potentially preventable complications of medical care. Previous studies have used these PSI definitions to examine trends in patient safety events and have identified significant associations of PSI rates with hospital-specific4–7 and patient-level factors.8,9
Chronic kidney disease (CKD) is a common condition with several attributes that have the potential to increase the risk for medical errors and lapses in patient safety. People with CKD often have greater rates of hospitalizations and encounters with the medical system, which leave them susceptible to interventions with the potential for medical errors.10 Moreover, CKD is associated with pathophysiologic processes—anemia, osteopenia, susceptibility to hypervolemia, electrolyte abnormalities, and infection—that can increase the risk for adverse complications of medical care. CKD is also characterized by impaired renal clearance of numerous medications, raising the risk for incorrect dosing and toxicity of therapeutic agents.11 Perhaps most important, CKD is frequently underrecognized in most health care settings,12 leaving providers unprepared to address the preventable consequences of this disease and its complexities. These factors, along with the high prevalence of CKD in the United States,13 make it a good candidate to explain a significant portion of potentially avoidable adverse safety events, with opportunities for measurable improvements in the identified problem of patient safety. The purpose of this study was to estimate the association of CKD with the risk for adverse safety events, using the PSI developed by the AHRQ and applied to a national sample of hospitalized patients.
RESULTS
Participants
We identified 315,213 Veterans Health Administration (VHA) patients with at least one acute hospitalization within the study period; of these, demographic information including race was available on 298,556 (95%) people. An outpatient serum creatinine was measured among 84% of these individuals within 1 wk to 1 yr preceding their index hospitalization (median 61 d), resulting in a study population of 247,176. This represents the sample of patients with a hospitalization included in at least one PSI analysis. The patient set included in the analysis of each specific PSI varied according to the various exclusion criteria established for each indicator.
CKD was present among 29% (n = 71,666) of the study population, and these patients were older; slightly less likely to be black; and more likely to have diabetes, cardiovascular disease (CVD), cancer, and length of stay (LOS) >3 d than those without CKD (Table 1). Similar differences between those with and without CKD were observed when examined among the veterans included in each specific PSI analysis. Mean estimated GFR (eGFR) among those with CKD was 42.5 ml/min per 1.73 m2; 50% had an eGFR between 45 and 60 ml/min per 1.73 m2.14
Table 1.
Characteristics of study population among those with and without CKDa
| Characteristic | CKD (29%) | No CKD (71%) |
|---|---|---|
| Age (yr; n [%]) | ||
| <45 | 636 (0.9) | 10,737 (6.1) |
| 45 to 59 | 12,432 (17.4) | 74,827 (42.6) |
| 60 to 74 | 28,395 (39.6) | 57,607 (32.8) |
| ≥75 | 30,203 (42.1) | 32,339 (18.4) |
| Male (n [%]) | 69,338 (96.8) | 167,692 (95.6) |
| Black (n [%]) | 11,450 (16.0) | 34,098 (19.4) |
| GFR (ml/min per 1.73 m2; mean [SD]) | 42.5 (14.0) | 87.9 (22.5) |
| Diabetes (n [%]) | 37,130 (51.8) | 60,360 (34.4) |
| CVD (n [%])b | 43,589 (60.8) | 61,871 (35.3) |
| Cancer (n [%])c | 21,742 (30.3) | 45,814 (26.1) |
| Charlson Comorbidity Index (n [%]) | ||
| 0 to 1 | 12,652 (17.7) | 64,926 (37.0) |
| 2 to 3 | 22,434 (31.3) | 57,846 (33.0) |
| ≥4 | 36,580 (51.0) | 52,738 (30.1) |
| LOS (d; n [%]) | ||
| <3 days | 24,337 (34.0) | 67,941 (38.7) |
| ≥3 | 47,329 (66.0) | 107,569 (61.3) |
| eGFR (n [%]) | ||
| ≥60 | 176,020 (100) | |
| 45 to <60 | 37,768 (53.1) | |
| 30 to <45 | 20,268 (28.5) | |
| <30 | 13,120 (18.4) |
All comparisons were significant at P < 0.001.
One or more of the following: Congestive heart failure, coronary artery disease, or cerebrovascular disease.
Excluding nonmelanomatous skin cancer.
Specific PSI
Rates of PSI related to surgical care among those with and without CKD are shown in the first section of Table 2 along with the adjusted incidence rate ratios (IRR) for each PSI. Compared with those without CKD, hospitalized patients with CKD were more likely to experience complications of anesthesia (IRR 1.60; 95% confidence interval [CI] 1.07 to 2.37), postoperative hip fracture (IRR 4.89; 95% CI 2.79 to 8.57), and postoperative physiologic or metabolic derangement (IRR 4.00; 95% CI 3.18 to 5.02) and to have postoperative respiratory failure (IRR 1.37; 95% CI 1.19 to 1.57).
Table 2.
Rates of PSI among hospitalized patients with and without CKDa
| PSI | PSI Description | No. of Events | Rate in CKDb | Rate in Non-CKDb | aIRR (95% CI)c | P |
|---|---|---|---|---|---|---|
| Complications of surgical care | ||||||
| 1 | complications of anesthesia | 58 | 0.12 | 0.08 | 1.60 (1.07 to 2.37) | 0.020 |
| 8 | postoperative hip fracture | 17 | 0.11 | 0.02 | 4.89 (2.79 to 8.57) | <0.001 |
| 9 | postoperative hemorrhage or hematoma | 170 | 0.30 | 0.26 | 0.96 (0.72 to 1.29) | 0.800 |
| 10 | postoperative physiological/metabolic derangement | 239 | 0.96 | 0.20 | 4.00 (3.18 to 5.02) | <0.001 |
| 11 | postoperative respiratory failure | 1086 | 4.01 | 1.97 | 1.37 (1.19 to 1.57) | <0.001 |
| 12 | postoperative PE or DVT | 645 | 1.23 | 0.96 | 1.04 (0.88 to 1.22) | 0.700 |
| 13 | postoperative sepsis | 407 | 2.47 | 1.33 | 1.39 (1.14 to 1.71) | 0.001 |
| 14 | postoperative wound dehiscence | 80 | 0.90 | 0.59 | 1.12 (0.74 to 1.70) | 0.600 |
| Complications of any acute hospitalization | ||||||
| 2 | death in low-mortality DRG | 107 | 0.47 | 0.18 | 1.53 (1.14 to 2.05) | 0.005 |
| 3 | decubitus ulcer | 1176 | 1.64 | 1.20 | 0.95 (0.84 to 1.08) | 0.500 |
| 4 | failure to rescue | 1491 | 11.0 | 11.2 | 0.95 (0.85 to 1.05) | 0.300 |
| 7 | infection as a result of medical care | 359 | 0.34 | 0.16 | 2.33 (1.92 to 2.82) | <0.001 |
DRG, diagnosis-related group; DVT, deep vein thrombosis; PE, pulmonary embolism.
Events per 100 hospitalizations.
Adjusted for age, gender, race, diabetes, CVD, and cancer.
Table 2 also shows rates of PSI related to any acute hospitalization (surgical or nonsurgical) among those with and without CKD, along with the adjusted IRR for each PSI. Patients with CKD were significantly more likely to die during a hospitalization for a condition considered to be at low mortality risk (IRR 1.53; 95% CI 1.14 to 2.05) and to develop an infection as a result of medical care (IRR 2.33; 95% CI 1.92 to 2.82). Additional adjustment for the LOS of each hospitalization (as a categorical variable) did not materially change the magnitude of the adjusted IRR for both the surgery-specific PSI and the PSI associated with general medical care (data not shown).
Table 3 compares the risk between those with and without CKD of the six additional PSI considered as secondary outcomes. Adjusted incidence rate ratios (aIRR) for patients with CKD were significantly higher for postoperative in-hospital myocardial infarction (aIRR 1.18; 95% CI 1.05 to 1.34) and for metabolic/physiologic derangements during any acute hospitalization (aIRR 1.36; 95% CI 1.30, 1.41). After additional adjustment for length of hospital stay, the association of CKD with these two PSI did not change.
Table 3.
Rates of additional PSI among hospitalized patients with and without CKDa
| PSI | No. of Events | Rate in CKDb | Rate in Non-CKDb | aIRR (95% CI)c | P |
|---|---|---|---|---|---|
| Technical difficulty with procedure | 1535 | 0.51 | 0.66 | 0.91 (0.80 to 1.03) | 0.100 |
| Physiological derangement, medical and surgical admissions | 29,628 | 15.70 | 10.80 | 1.36 (1.30 to 1.41) | <0.001 |
| Aspiration pneumonia | 376 | 0.77 | 0.57 | 0.91 (0.74 to 1.11) | 0.400 |
| CABG after PTCA | 32 | 0.69 | 0.64 | 0.83 (0.45 to 1.50) | 0.500 |
| Postoperative in-hospital MI | 665 | 2.14 | 0.83 | 1.18 (1.05 to 1.34) | 0.007 |
| Postoperative cardiogenic iatrogenic complications | 1505 | 3.31 | 2.10 | 1.11 (0.99 to 1.25) | 0.060 |
CABG, coronary artery bypass grafting; PTCA, percutaneous coronary angioplasty; MI, myocardial infarction.
Events per 100 hospitalizations.
Adjusted for age, gender, race, diabetes, CVD, and cancer.
Composite PSI
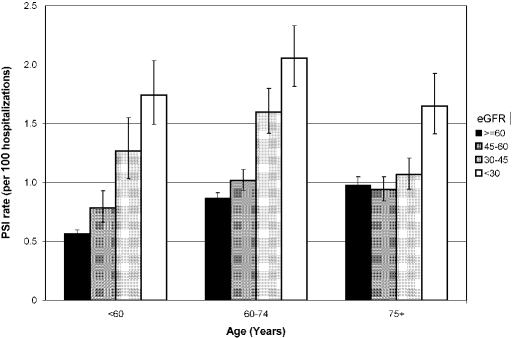
When we examined the association of CKD with the combined outcome of all of the PSI listed in Table 2, a 19% greater risk for this combined outcome was observed (aIRR 1.19; 95% CI 1.13 to 1.25). Additional adjustment for length of hospital stay did not effect the results (aIRR 1.19). A significant linear trend in increased PSI rate across eGFR categories was observed (Table 4), which was not changed by additional adjustment for LOS (data not shown). Likewise, relative risks were unchanged when all hospitalizations (first and recurrent) were included. However, the differences in risk associated with CKD severity varied across age categories (Figure 1); a stepwise increment in risk with decrements in eGFR was observed among those <60 yr of age, but increased risk was observed only at an eGFR <30 ml/min per 1.73 m2 among those ≥75 yr of age. In fully adjusted regression models, the interaction between eGFR and age was significant (P = 0.001). In contrast, the association between eGFR and PSI rates did not differ between those with and without diabetes or CVD (tests of interaction, P > 0.05).
Table 4.
IRR of all PSI events combined, by CKD severity
| Parameter | eGFR (ml/min per 1.73 m2)
|
|||
|---|---|---|---|---|
| <30 | 30 to <45 | 45 to <60 | ≥60 | |
| Unadjusted IRR | 1.93 (1.77 to 2.10) | 1.59 (1.46 to 1.73) | 1.25 (1.17 to 1.34) | Reference |
| aIRRa | 1.59 (1.49 to 1.69) | 1.20 (1.13 to 1.28) | 1.03 (0.97 to 1.08) | Reference |
Adjusted for age, gender race, diabetes, cardiovascular disease, and cancer.
Figure 1.
Rates of all PSI combined, by age and severity of CKD. Bars indicate 95% CI assuming a Poisson distribution.
DISCUSSION
Since the landmark 1999 IOM report “To Err Is Human,” there have been major efforts to identify causes and consequences of medical errors and lapses in patient safety and to track the frequency of these events over time and across health care systems. The set of PSI developed by the AHRQ serves as a tool to quantify rates of adverse safety events in the acute-care inpatient setting, using readily available administrative data. Previous reports have used the PSI tools to identify discrepancies in patient safety events across hospital-level factors,4,7 payer status,5 and demographic factors including race and ethnicity.8
In this analysis, we set out to explain variations in adverse safety event rates in the VHA and the degree to which they relate to a specific disease process. The results demonstrated that CKD was a significant risk factor for many of the predefined PSI included in the analysis after adjustment for many known demographic and comorbid differences between veterans with and without CKD. Recognizing that some of the PSI were relatively uncommon events, the findings also revealed that CKD was a significant predictor of a composite of all PSI.
This association between CKD and the composite outcome of all PSI was modified by age, however, with a stepwise increase in risk with decrements in eGFR among those <60 yr of age but with increased risk observed only at an eGFR <30 ml/min per 1.73 m2 for those ≥75 yr. A similar age-dependent effect of lower eGFR was described with regard to mortality in the VHA by O'Hare et al.15 This age modification may represent inaccuracies in the estimation of GFR in the elderly; alternatively, a cut point of 60 ml/min per 1.73 m2 as recommended by the National Kidney Foundation may fail to distinguish true renal disease from the decline in eGFR associated with advanced aging.14
Since the 1999 IOM report, improvements in the frequency of medical errors have been documented, but they are sporadic and the health care system at large remains a high-risk setting for patient safety lapses.16–18 For example, a survey of physicians and members of the public found that 35 and 42%, respectively, had experienced a medical error in their own or a family member's medical care, with approximately 20% of these medical errors considered serious.19 The persistence of this problem may relate to the failure to identify important root causes that may account for the diverse set of adverse outcomes considered to represent lapses in patient safety. Previous studies have predominately focused on system-level or hospital-level processes related to patient safety4–7; however, it is plausible that specific patient comorbidities such as CKD represent an important precondition to adverse safety outcomes.
The work examining this question to date is limited; however, investigators have identified a high frequency of medication errors among patients who have kidney failure and are on dialysis20 and with earlier stages of CKD.11,21 Acute kidney injury—often medication induced—is also common among patients with CKD, although how frequently these events relate to lapses in patient safety are unclear.22 Patients with CKD have much higher rates of hospitalization in general than patients with preserved renal function.10 This analysis demonstrates for the first time that, after being admitted to a hospital, these patients are also more likely to have adverse events related to potential lapses in patient safety.
Several of the PSI at greater risk in CKD after case-mix adjustment relate to excess cardiovascular events or all-cause mortality. It is uncertain whether this excess risk relates specifically to adverse consequences of health care or more generally to the underlying pathophysiology of CKD, as demonstrated by the well-established association between CKD and CVD in multiple previous studies; however, the AHRQ software is designed to use secondary diagnostic codes to ascertain events and to exclude hospitalizations for which vascular conditions are the primary diagnoses. These PSI events are meant to reflect in-hospital events and in this way identify CVD events more likely to be a byproduct of health care process than underlying disease. This distinction is more than semantic, because modification of health care processes may have more effect on reducing CVD events in this high-risk population than any currently available medical therapies.
Strengths of the study include the examination of patient safety events across an integrated national health system including a large number of hospitals. The use of outpatient creatinine measures to define CKD rather than inpatient creatinine or diagnosis codes improves the accuracy of the assigned diagnosis of CKD and minimizes the likelihood of mischaracterizing acute renal failure as CKD; nevertheless, residual misclassification of acute kidney disease versus CKD may have occurred.
Limitations include the administrative nature of the data used to identify potential adverse safety events. As with most studies using administrative data, there is a lack of certainty about the precision of coding, especially in the case of diagnoses that might be construed as measures of poor care. We cannot be certain whether those adverse events identified using the PSI algorithm represent truly preventable events related to medical care or unavoidable events related to comorbidity or underlying pathophysiology. Nevertheless, we propose that these results provide a “signal” of a greater risk for potentially preventable adverse events among inpatients with CKD that warrants further investigation.
An additional limitation is that these PSI were not designed to evaluate safety measures specific to a chronic disease such CKD but rather were designed for a general inpatient population. The development and validation of disease-specific safety indicators may provide a more complete estimation of preventable adverse safety events in the CKD population.
In conclusion, the presence of CKD was associated with a greater risk for adverse patient safety events in hospitalized patients in the VHA. Further investigation is needed to examine this association in other health care systems and to define more specific safety measures, with the goal of improving patient safety for patients with CKD.
CONCISE METHODS
Setting and Data Sources
This was a cross-sectional observational study using national data on patients who received inpatient and outpatient care in VHA facilities. The study data set was derived from the fiscal year 2005 acute inpatient data file and linked to outpatient laboratory data, outpatient encounters, and vital status data for those veterans. The source data files included the Patient Treatment File, the Outpatient Care File, and the Decision Support System Laboratory Result files.23,24 This study was approved by the institutional review board of the University of Maryland, Baltimore, and the research and development committee of the Maryland VA Healthcare System.
Study Population
The study population consisted of patients who had an acute care hospitalization from October 1, 2004, through September 30, 2005, and had complete demographic data (age, gender, and race) and an outpatient serum creatinine measured before the admission date of their hospitalization and during the interval defined below (see “Variables of Interest”). The analyses excluded hospitalizations (and sections of hospitalizations) occurring in non–acute care facilities, according to previously described methods.6 Because patients with CKD were more likely to have multiple hospitalizations than those without CKD, we considered only the first hospitalization for each individual during this period. We explored in sensitivity analyses whether including repeated hospitalizations influenced the results.
Variables of Interest
The primary exposure variable was the presence of CKD, defined by an eGFR <60 ml/min per 1.73 m2 using the abbreviated Modification of Diet in Renal Disease (MDRD) equation25 and the most recent outpatient serum creatinine performed within 1 wk to 1 yr preceding the admission date of the index hospitalization. In additional analyses, we categorized CKD severity according to eGFR level (<30, 30 to <45, or 45 to <60 ml/min per 1.73 m2). Demographic characteristics used in the analysis include gender, age at date of hospital admission, and race, which was characterized as black or other.
Comorbidities of interest included cancer (excluding nonmelanomatous skin cancer), diabetes, and CVD (a composite of cerebrovascular disease, myocardial infarction, and/or congestive heart failure). These comorbidity variables were defined using International Classification of Diseases, Ninth Revision diagnosis codes from the acute care inpatient and outpatient data sets from October 1, 1999, through the date of index hospitalization, as shown in Supplemental Appendix 1. LOS was computed using only the acute care portions of each hospitalization. Charlson comorbidity score was also computed for each patient for descriptive purposes, using both inpatient and outpatient International Classification of Diseases, Ninth Revision diagnosis codes.26
Patient Safety Indicators
To estimate rates of specific PSI within subgroups of patients, we used publicly available software provided by the AHRQ. The process by which the software was developed was previously described.27 The AHRQ PSI 3.0a was used for this analysis.2 The software identifies which hospitalizations are considered in the “at-risk” set for each PSI and counts the total number of hospitalizations with each type of PSI. The at-risk set differs for each PSI according to the exclusion/inclusion criteria established by the AHRQ. For example, some PSI include only hospitalizations with surgical procedures; others exclude patients with acute conditions that confer a markedly increased risk for a specific adverse event (e.g., septic shock in the PSI for “physiologic derangements”). The output from the PSI software was generated for multiple subgroups defined by unique combinations of categorical patient characteristics (demographic factors and comorbidities categorically classified) that were used in multivariate regression models (see the Statistical Analysis section).
From the list of 20 standard PSI not relating to complications of obstetric care, we a priori selected 13 PSI that were plausibly related to the presence of CKD or otherwise of clinical significance to renal patients (Table 2, PSI numbers 1 through 4, 7 through 14, and 16).2 For PSI 16 (“transfusion reaction”), there were too few events (n = 8) for meaningful analysis, and it was excluded. The remaining 12 PSI were considered the primary study outcomes.
In addition, we selected as secondary outcomes five PSI thought to be relevant to CKD from a set of “experimental” indicators. These indicators were originally considered by the AHRQ review committee as potentially useful markers of patient safety but requiring additional research on their construct validity or operational definition. Finally, we created a sixth secondary outcome modified from approved PSI 10 (“postoperative physiologic and metabolic derangements”). The at-risk hospitalizations for this modified PSI included hospitalizations for both surgical and medical conditions.
The standard input file structure used for the AHRQ PSI software was modified to account for unique features of the VHA administrative data, as described previously by Rosen et al.28 In addition, we made slight modifications to inclusion/exclusion criteria for six of the approved PSI (2 through 4, 7, and 13) to account for the unique comorbidity characteristics of the older and largely male patient population and for the primary predictor of interest (CKD), as shown in Supplemental Appendix 2.
Statistical Analysis
Rates of each PSI were computed for patients with and without CKD by dividing the number of hospitalizations with a specific PSI by the total number of hospitalizations at risk for that PSI. For each PSI, we used Poisson regression models to determine the relationship between CKD and the PSI event rate, controlling for age, race, gender, and comorbidity (cancer, diabetes, and CVD). The dependent variable was the number of events, and the logarithm transformation of the number of hospitalizations was used as an offset variable.
The Poisson model relaxed the assumption that the variance of expected number of events is the same as the observed by introducing a scale parameter σ, such that Var(Y) = μσ2, as described previously.29 Because the causal relationship between days in hospital and a PSI was likely bidirectional, LOS was not included as a primary adjustment covariate, but the effect of additional adjustment for this factor was examined in sensitivity analyses.
To summarize the association between CKD and a composite of all PSI of interest (as listed in Table 1), we developed a single model in which the number of patient safety events for each PSI and within each subgroup was entered as the dependent variable. The number of hospitalizations considered “at risk” for each PSI within each of these subgroups was entered as the offset (logarithmically transformed). The model included the presence of CKD as the primary independent variable, with adjustment covariates added as described already, and indicator variables for each PSI were entered as fixed effects. In this model, the coefficient for CKD can be considered as the association between CKD and rate of all PSI events combined. Effect modification by age and diabetes were examined through the use of multiplicative interaction terms. Analysis was carried out using the GENMOD procedure in SAS 9 (SAS Institute, Cary, NC).
DISCLOSURES
None.
Acknowledgments
This study was funded by grants R21 DK075675-01 from the National Institutes of Health and P60 MD000532-01 from the National Center on Minority Health and Health Disparities.
The authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Published online ahead of print. Publication date available at www.jasn.org.
The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Kohn KT, Corrigan JM, Donaldson MS: To Err is Human: Building a Safer Health System, Washington, DC, National Academies Press, 1999 [PubMed]
- 2.Agency for Healthcare Research and Quality: AHRQ Quality Indicators: Guide to Patient Safety Indicators, Rockville, MD, Agency for Healthcare Research and Quality, 2003
- 3.Committee on Quality of Health Care in America: Crossing the Quality Chasm: A New Health System for the 21st Century, Washington, DC, National Academies Press, 2001 [PubMed]
- 4.Weiner BJ, Alexander JA, Baker LC, Shortell SM, Becker M: Quality improvement implementation and hospital performance on patient safety indicators. Med Care Res Rev 63: 29–57, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Clement JP, Lindrooth RC, Chukmaitov AS, Chen HF: Does the patient's payer matter in hospital safety? A study of urban hospitals. Med Care 45: 131–138, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Rosen AK, Zhao S, Rivard P, Loveland S, Montez-Rath ME, Elixhauser A, Romano PS: Tracking rates of patient safety indicators over time: Lessons from the Veterans Administration. Med Care 44: 850–861, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Encinosa WE, Bernard DM: Hospital finances and patients safety outcomes. Inquiry 42: 60–72, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Coffey RM, Andrews RM, Moy E: Racial, ethnic, and socioeconomic disparities in estimates of AHRQ patient safety indicators. Med Care 43: I48–I57, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Daumit GL, Pronovost PJ, Anthony CB, Guallar E, Steinwachs DM, Ford DE: Adverse events during medical and surgical hospitalizations for persons with schizophrenia. Arch Gen Psychiatry 63: 267–272, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH: Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 164: 659–663, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Chertow GM, Lee J, Kuperman GJ, Burdick E, Horsky J, Seger DL, Lee R, Mekala A, Song J, Komaroff AL, Bates DW: Guided medication dosing for inpatients with renal insufficiency. JAMA 286: 2839–2844, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Stevens LA, Fares G, Fleming J, Martin D, Murthy K, Qiu J, Stark PC, Uhlig K, Van Lente F, Levey AS: Low rates of testing and diagnostic codes usage in a commercial clinical laboratory: Evidence for lack of physician awareness of chronic kidney disease. J Am Soc Nephrol 16: 2439–2448, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Coresh J, Selvin E, Stevens LA: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Kidney Disease Outcome Quality Initiative (K/DOQI): Clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39: S1–S242, 2002 [PubMed] [Google Scholar]
- 15.O'Hare AM, Choi AI, Bertenthal D, Bacchetti P, Garg AX, Kaufman JS, Walter LC, Mehta KM, Steinman MA, Allon M, McClellan WM, Landefeld CS: Age affects outcome in chronic kidney disease. J Am Soc Nephrol 18: 2758–2765, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Leape LL, Berwick DM: Five years after To Err is Human: What have we learned? JAMA 293: 2384–2390, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Zhan C, Kelley E, Yang HP, Keyes M, Battles J, Borotkanics RJ, Stryer D: Assessing patient safety in the United States: Challenges and opportunities. Med Care 43: I42–I47, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Zhan C, Miller M: Excess length of stay, charges, and mortality attributable to medical injuries during hospitalization. JAMA 290: 1868–1874, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Blendon RJ, DesRosches CM, Brodie M, Benson JM, Rosen AB, Schneider E, Altman DE, Zapert K, Herrmann MJ, Steffenson AE: Views of practicing physicians and the public on medical errors. N Engl J Med 347: 1933–1939, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Manley HJ, Cannella CA, Bailie GR, St Peter WL: Medication-related problems in ambulatory hemodialysis patients: A pooled analysis. Am J Kidney Dis 46: 669–680, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Blix HS, Viktil KK, Moger TA, Reikvam A: Use of renal risk drugs in hospitalized patients with impaired renal function: An underestimated problem? Nephrol Dial Transplant 21: 3164–3171, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Liangos O, Wald R, O'Bell JW, Price L, Pereira BJ, Jaber BL: Epidemiology and outcomes of acute renal failure in hospitalized patients: A national survey. Clin J Am Soc Nephrol 1: 43–51, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Cowper DC, Hynes DM, Kubal JD, Murphy PA: Using administrative databases for outcomes research: Select examples from VA Health Services Research and Development. J Med Syst 23: 249–259, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Maynard C, Chapko MK: Data resources in the Department of Veterans Affairs. Diabetes Care 27: B22–B26, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Greene T, Kusek JW, Beck GJ: A simplified equation to predict glomerular filtration rate from serum creatinine [Abstract]. J Am Soc Nephrol 111, 155A, 2000 [Google Scholar]
- 26.Deyo RA, Cherkin DC, Ciol MA: Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45: 613–619, 1992 [DOI] [PubMed] [Google Scholar]
- 27.McDonald K, Romano P, Geppert J: Measures of Patient Safety Based on Hospital Administrative Data. The Patient Safety Indicators Technical Review 5 [AHRQ Publication No. 02-0038], Rockville, MD, Agency for Healthcare Research and Quality, 2002 [PubMed]
- 28.Rosen AK, Rivard P, Zhao S, Loveland S, Tsilimingras D, Christiansen CL, Elixhauser A, Romano PS: Evaluating the patient safety indicators: How well do they perform on Veterans Health Administration data? Med Care 43: 873–884, 2005 [DOI] [PubMed] [Google Scholar]
- 29.SAS Institute Inc.: PROC GENMOD: SAS/STAT 9.1 User's Guide, Cary NC, SAS Institute Inc., 2004