Abstract
Recovery after acute kidney injury is impaired in the elderly, but mechanistic information regarding why this occurs is limited. In this study, aged mouse kidneys displayed a reduced epithelial proliferative reserve in vivo and in vitro. Microarray analysis identified increased expression of zinc-α (2)-glycoprotein (Zag) in aged proximal tubular cells. The addition of recombinant Zag to primary renal epithelial cell cultures decreased proliferation, whereas knockdown of Zag increased proliferation. In vivo, systemic small interference RNA suppressed expression of Zag in the mouse proximal tubule; this increased the rate of epithelial cell proliferation after renal ischemia/reperfusion in aged mice but also increased parenchymal fibrosis. These results demonstrate that increased Zag expression in the aged kidney acts to suppress the proliferative response to injury and introduce Zag as a modifier of the aging phenotype.
The incidence of acute kidney injury (AKI) has steadily increased in recent years, and this increase is strongly associated with the advancing age of our population.1 Aging is thought to increase the susceptibility to AKI2–5 as a result of functional changes in the vasoregulatory reserve4,6 and a loss of cellular stress resistance.7 The age-dependent increase in the susceptibility to AKI coincides with a decline in functional renal recovery. According to our recent meta-analysis,8 it is estimated that patients who are older than 65 yr carry a 28% higher risk for failing to recover renal function completely after surviving an episode of AKI. Despite significant progress in our understanding of the cellular and molecular aspects of AKI in recent years,9,10 the role of aging in this process has not been considered in most studies; therefore, the identification of age-specific factors that mediate the altered response to AKI in the elderly are lacking.
In this study, we demonstrated that aged mouse kidneys displayed a reduced epithelial proliferative reserve, and we investigated the hypothesis that this loss of proliferative capacity contributes to the age-dependent decline in renal repair. Using a microarray approach, we found that old kidneys displayed increased proximal tubular expression of zinc-α (2)-glycoprotein (Zag), which was previously implicated in epithelial proliferative inhibition.11,12 The addition of recombinant Zag to primary renal epithelial cell cultures decreased proliferation rates, whereas knockdown of Zag increased proliferation in vitro. In vivo, we found that suppression of tubular Zag expression in aged mice resulted in higher rates of epithelial cell proliferation during the repair phase after ischemia/reperfusion (I/R); however, this desired effect was accompanied by increased parenchymal fibrosis, with no net functional benefit.
RESULTS
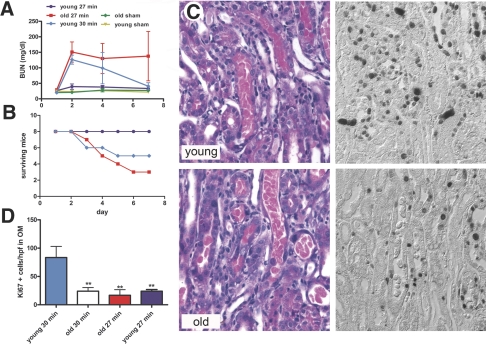
Experimental studies of rats indicated that the aging kidney is more likely to develop AKI after I/R.2,3,5 We found that this is also the case in mice in which renal function after unilateral nephrectomy and 27 min of contralateral I/R was dramatically reduced in old mice and only moderately affected in young (Figure 1A). For equalization of the severity of the initial injury, an additional group of young mice underwent unilateral nephrectomy and 30 min of contralateral I/R, which resulted in an initial rise in blood urea nitrogen (BUN) that was comparable to that of old mice with 27 min of I/R. In young mice, this was followed by a steady decrease of BUN, whereas a lack of functional recovery and an increased death rate were observed in old mice (Figure 1, A and B). Because the regulated burst of epithelial cell proliferation is an integral part of the renal repair process after I/R injury,9,13 we tested whether the proliferative response is changed in the old kidney by quantifying Ki-67 and proliferating cell nuclear antigen (PCNA)-positive nuclei. Kidneys from young mice had significantly higher rates of proliferation in the outer medulla (OM) at 72 h after 30 min of I/R than did old kidneys after 27 min and after 30 min (Figure 1, C and D; Supplemental Figure 1, A through C). To rule out the possibility that initial damage in the aged mice even at 27 min resulted in more cell loss and thus fewer surviving cells available to proliferate, we also performed 24 min of clamping time in old mice to allow greater cell survival. Reduction of clamping time to 24 min resulted in minimal histologic damage and only sporadic cell proliferation (data not shown). Although this approach cannot formally exclude the possibility that a subtle difference in the level of injury or cell survival accounts at least in part for the observed failure of epithelial proliferation, these data are most consistent with the hypothesis that the proliferative potential of surviving tubular epithelial cells is reduced in aged kidneys.
Figure 1.
Survival and renal function of young and old mice after I/R. (A and B) BUN values (A) and survival curves (B) for mice that underwent left-sided nephrectomy and contralateral renal clamping for 27 or 30 min (n = 8). (C) Renal histology (hematoxylin and eosin) and Ki-67 immunostaining at 72 h after 30 min of ischemia (representative images from the OM). (D) Quantification of Ki-67–positive cells/high-power field in outer medulla (OM) of kidneys at 72 h after 30 or 27 min of ischemia (n = 6). **P < 0.01; versus young 30 min. Magnification, ×400.
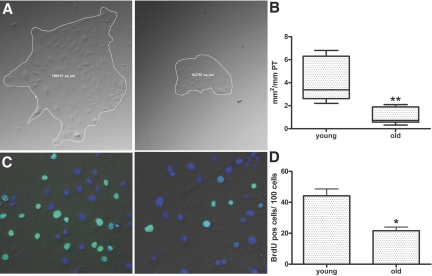
To test whether old tubular cells have an intrinsically diminished proliferative capacity that is independent of the amount of initial injury by I/R, we isolated microdissected proximal tubules (PT) from healthy young and old mice for in vitro growth assays of primary PT epithelial cells (PTEC). When freshly isolated tubules were cultured in epithelial growth medium, cell colonies that expanded more rapidly in size and cell number in the young age group were observed within 2 to 3 d. Cell proliferation measured at days 6 through 8 by bromodeoxyuridine (BrdU) uptake was two-fold greater in young colonies as compared with old (Figure 2, C and D). When PTEC colony growth reached a plateau phase at 10 d, colony surface sizes were found to be significantly smaller in colonies grown from old tubules than those from young tubules (Figure 2, A and B), even though the surface area of individual young and old cells was not significantly different (average cell surface area 816.2 ± 452 μm2 in young and 938.4 ± 350.9 in old). Although additional mechanisms such as reduced cellular migration may contribute to reduced colony expansion of old PTEC, these results demonstrate that tubular epithelial cells from old mice have a reduced proliferative rate.
Figure 2.
Age-dependent differences in tubular epithelial cell proliferation in vitro. (A and B) Epithelial colonies grown from microdissected PT of young and old mice (A). After 10 d of culture, colony surface sizes were significantly larger when derived from young tubules (quantified in B). (C and D) BrdU incorporation studies showed a higher rate of proliferation in young colonies (C; quantified in D). (n = 7, each n represents approximately 10 mm of microdissected PT cultured from a separate mouse.) **P < 0.01; *P < 0.05 old versus young. Magnifications: ×200 in A; ×400 in C.
These results suggest that there is a cell-autonomous, age-dependent decline of renal epithelial proliferative capacity. To identify genes that might be causative of this decline, we performed microarray analysis on microdissected PT from young and aged mice. Isolated tubules were chosen rather than whole kidney or PTEC cultures to circumvent the confounding influence of age-induced alterations in interstitial and peritubular capillary cell composition14,15 while maintaining the cell within the context of the normal tubular architecture. Manually microdissected PT were pooled from eight young and eight old mice to obtain sufficient RNA for a single Affymetrix Mouse Genome 430 2.0 array per age group. The validity of the tubule identification was confirmed by the expression profile of tubule- and cell-specific markers (Table 1). A total of 310 mRNA were differentially expressed by two-fold or more between young and old tubules. Onto-Express software16 was used to cluster these genes into functionally relevant categories, resulting in identification of 15 mRNA grouped into cell cycle–related groups (Table 2). Among these differentially regulated mRNA, the secreted protein Zag demonstrated the highest difference in expression with a 6.4-fold increase in transcript levels in aged tubules. Zag has been described as a negative regulator of epithelial proliferation11,12,17 but has not previously been examined in the context of normal aging, tissue repair, or renal disease.
Table 1.
Expression of marker genes by microdissected proximal tubules as shown by Affymetrix Mouse Genome 430 2.0 arrays
| Kidney Compartment | Gene | Probe Set ID | Young (Absolute Signal) | Old (Absolute Signal) |
|---|---|---|---|---|
| Glomerulus | Nephrin | 1422142_at | Absent | Absent |
| Podocin | 1460297_at | Absent | Absent | |
| PT | Alk. phos. | 1423611_at | 5091 | 5335 |
| Nhe3 | 1438115_at | 12648 | 15064 | |
| NaPi2a | 1449330_at | 3304 | 3329 | |
| Collecting duct | Aqp2 | 1418903_at | Absent | Absent |
| ENaC α | 1425088_at | Absent | Absent | |
| Vessels | Pecam 1 | 1421287_at | Absent | Absent |
| α-sm-actin | 1456658_at | Absent | Absent | |
| Interstitium | Vimentin | 1450641_at | Absent | Absent |
| S100A4 | 1424542_at | Absent | Absent |
Table 2.
Cell cycle genes differentially regulated between young and old PT
| Gene Symbol | Probe Set ID | Young (Absolute Signal) | Old (Absolute Signal) | Signal Log Ratio (Old versus Young) |
|---|---|---|---|---|
| AZGP1 (Zag) | 1417776_at | 1698 | 11320 | 2.7 |
| Hrasls3 | 1451611_at | 170 | 757 | 1.8 |
| Trim7 | 1421398_at | 228 | 709 | 1.5 |
| Gas6 | 1459585_at | 190 | 439 | 1.4 |
| Myc | 1424942_a_at | 150 | 447 | 1.3 |
| Nfib | 1438072_at | 369 | 1040 | 1.2 |
| Tlk2 | 1457357_at | 82 | 245 | 1.2 |
| Fgf1 | 1423136_at | 2559 | 5223 | 1.1 |
| Cxcl12 | 1417574_at | 324 | 811 | 1.0 |
| Egf | 1418093_a_at | 1969 | 921 | −1.0 |
| Odc1 | 1427364_a_at | 8446 | 4010 | −1.2 |
| Ppard | 1439797_at | 500 | 174 | −1.2 |
| Col8a1 | 1455627_at | 398 | 201 | −1.3 |
| Camk2b | 1455869_at | 1082 | 326 | −1.4 |
| Trim35 | 1454650_at | 1982 | 605 | −1.8 |
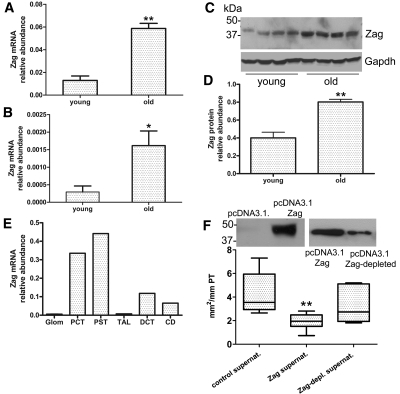
Real-time quantitative PCR (qPCR) for Zag mRNA from whole-kidney homogenates revealed a 5.8-fold increase in Zag message from old kidneys as compared with young kidneys (Figure 3A), and in vitro studies of PTEC confirmed that Zag mRNA levels were 5.5-times higher in epithelial cells obtained from old tubules as compared with those from young tubules (Figure 3B). Examination by Western analysis of whole kidneys confirmed that Zag was upregulated in the aged kidney, although the difference between young and old was less pronounced at the protein level (Figure 3C, quantified in 3D). Because available reagents were of insufficient quality to localize Zag reliably by immunohistochemistry in murine kidney tissue, qPCR was performed on microdissected nephron segments. The greatest expression of Zag was found in proximal convoluted and straight tubules, with lower levels in distal convoluted tubules and collecting ducts. Zag expression was nearly undetectable in glomeruli and thick ascending limbs of the loop of Henle (Figure 3E). This pattern was consistent with the published immunolocalization of Zag in human kidney and with our own immunostaining of human renal tissue, where the strongest signal was found in epithelial cells of the PT and weaker signal in the distal convoluted tubule. In these localizations, signal intensity was homogeneous between the investigated specimen (n = 5; age range 71 to 76 yr). No signal was found in any sample in glomeruli, interstitial cells, or vessels (Supplemental Figure 1, D and E).
Figure 3.
Expression of Zag in the aging kidney is increased and suppresses growth of young PTEC in vitro. qPCR of Zag mRNA in whole-kidney homogenates (A; n = 5) and PTEC of old mice cultured for 10 d (B; Zag mRNA expressed as 2dCT relative to Gapdh; n = 4; **P < 0.01; *P < 0.05). (C) Western blot for Zag protein expression in whole-kidney homogenates from young and old mice (each lane represents a separate mouse). (D) Quantification of Zag protein as in C normalized to Gapdh (n = 4; **P < 0.01). (E) qPCR demonstrates the relative abundance of Zag mRNA in microdissected nephron segments from old mice. Glom, glomerulus; PCT, proximal convoluted tubule; PST, proximal straight tubule; TAL, thick ascending limb of the loop of Henle; DCT, distal convoluted tubule; CD, collecting duct (n = 2). (F) Representative Western blots of supernatants of pcDNA3.1.Zag-transfected or pcDNA3.1 control transfected HEK293 cells before and after nickel-chelator resin depletion of polyhistidine-tagged Zag. Graph shows effect of control, Zag-containing, and Zag-depleted supernatant on colony size of young PTEC. **P < 0.01.
Higher levels of Zag expression have been demonstrated to correlate with reduced epithelial proliferation in tumor-derived cells11,12; therefore, for examination of whether Zag might contribute to the age-dependent decline in renal epithelial proliferative repair, the mRNA for murine Zag was cloned and transfected into HEK293 cells, yielding supernatants containing high levels of Zag (Figure 3F, insert). Addition of Zag-enriched supernatant to the culture medium of freshly isolated PT from young kidneys resulted in a significant inhibition of PTEC colony growth (Figure 3F). This effect was Zag specific because reduction of the polyhistidine-tagged Zag concentration in supernatants by precipitation with nickel-chelator agarose partially reversed the growth inhibitory effect (Figure 3F). BrdU incorporation studies confirmed that Zag inhibited the rate of proliferation of young PTEC (31 ± 3.1 BrdU-positive cells/100 cells in vector controls and 20 ± 2.4 BrdU-positive cells/100 cells in Zag-conditioned medium; P < 0.05; data not shown). Of note, direct transfection of PTEC with pcDNA3.1. Zag resulted in a transfection efficiency of only approximately 5%, undetectable levels of Zag in the culture supernatant, and no change in overall colony size; however, BrdU uptake studies revealed that proliferation of those cells that were successfully transfected with Zag was inhibited by 69%, suggesting an autocrine role of Zag to suppress proliferation in these cells (Supplemental Figure 1, F through H).
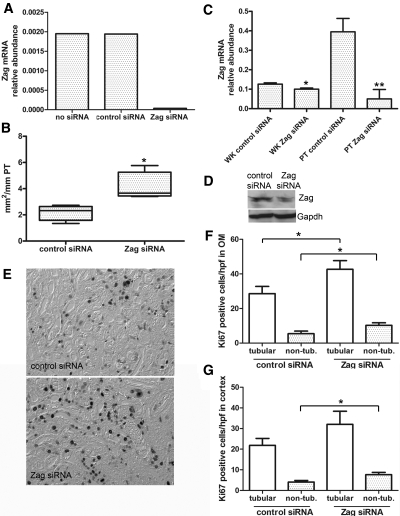
For clarification of the importance of endogenous Zag expression in proliferation of old PTEC, a Zag targeting small interference RNA (siRNA) that yielded >95% suppression of Zag mRNA levels was used (Figure 4A) in old PTEC. Transfection of this siRNA at days 3 and 6 of old PTEC culture resulted in a significant increase in colony growth as compared with control siRNA-treated cells (Figure 4B). BrdU incorporation studies confirmed an increase in cell proliferation after Zag suppression (25 ± 2.8 BrdU-positive cells/100 Zag siRNA-treated cells versus 14 ± 2.3 in control siRNA-treated cells; P < 0.05; data not shown). For investigation of the in vivo role of Zag in renal proliferative responses, intravenous injection of the Zag siRNA was performed in aged mice to induce gene specific knockdown in cells of the PT as has been previously demonstrated.18 Twenty-four hours after three daily injections of Zag or control siRNA (50 μg), Zag mRNA levels were decreased by 21.5 ± 5.2% in the whole kidney and by 77.3 ± 17.4% specifically in the PT (Figure 4C). Western analysis revealed that Zag protein expression decreased by 65% in cortex homogenate (Figure 4D). On the basis of these findings, mice were treated with injections of control or Zag siRNA for 3 d before and 3 d after unilateral I/R. This resulted in a significantly enhanced tubular cell proliferation in OM after I/R in mice treated with Zag siRNA as compared with controls (Figure 4E, quantified in 4F). Epithelial proliferation was also increased in cortex, but the difference did not reach statistical significance (Figure 4G). Analysis of the nontubular cell compartment revealed a less pronounced but also significant increase in interstitial cell proliferation in cortex and OM after Zag siRNA treatment (Figure 4, F and G).
Figure 4.
Zag knockdown increases epithelial proliferation. (A) qPCR of cultured PTEC transfected with Zag or control siRNA. Results expressed as 2dCT relative to Gapdh (n = 2). (B) Colony size (in mm2/mm of cultured tubule) of PTEC from old kidneys transfected with Zag siRNA or control siRNA (n = 5; each n represents approximately 10 mm of microdissected PT cultured from a separate mouse; *P < 0.05). (C) qPCR from whole kidney (WK) or isolated PT from old mice after three daily injections of 50 μg of Zag or control siRNA (n = 4, **P < 0.01; *P < 0.05). (D) Representative Western blot of kidney cortex after control or Zag siRNA injection daily for 3 d. (E) Ki-67 staining of kidney OM 72 h after 27 min of I/R in aged mice treated with control or Zag siRNA. (F and G) Quantification of tubular and nontubular Ki-67–positive cells/high-power field in OM (F) and cortex (G; n = 7; *P < 0.05). Magnification, ×400.
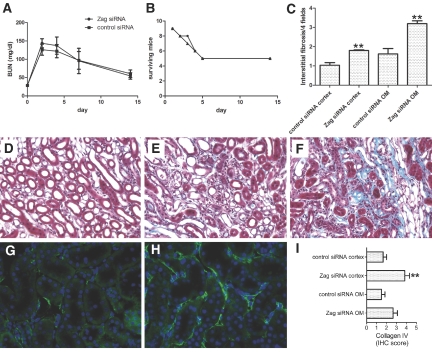
The functional importance of the Zag-dependent inhibition of proliferation was assessed using systemic siRNA injection to knock down Zag in 19-mo-old mice in the setting of unilateral I/R (27 min of ischemia time) with contralateral nephrectomy. Nineteen-month-old mice were chosen for these studies because they exhibited increased Zag expression as compared with young mice but had better survival than 24-mo-old mice after I/R with a higher potential for functional recovery. After I/R, a marked increase in BUN was observed in both Zag siRNA–and control siRNA–injected mice (Figure 5A), accompanied by identical death rates of 44.4% during the first 4 d after I/R (Figure 5B). Thus the reduction of Zag expression did not protect aged mice from developing AKI; however, because it is widely accepted that epithelial proliferation is required for successful renal repair and these studies have identified an increased proliferative capacity after Zag siRNA treatment, surviving mice were followed to 14 d after I/R to examine the reparative response. Mice in both the Zag siRNA and control siRNA groups demonstrated an indistinguishable improvement in BUN at 2 wk after injury (Figure 5A) and seemed to be normally active and healthy. Although there was no functional difference between the groups, histologic analysis of the kidneys revealed areas of interstitial fibrosis (Figure 5, C through F) and peritubular collagen IV deposition (data not shown) that were significantly more pronounced in both the cortex and the OM of Zag siRNA–treated mice. To elucidate whether this unexpected difference was a direct consequence of Zag suppression, we analyzed noninjured control kidneys after 6 d of Zag siRNA or control siRNA treatment. Although kidneys did not display any morphologic differences or changes in the baseline numbers of proliferating cells (data not shown), immunostaining revealed significantly more peritubular deposition of collagen IV in Zag siRNA–treated mice (Figure 5, G through I). These results suggest that Zag plays a role in the regulation of tubulointerstitial homeostasis and extracellular matrix production that is independent of its antiproliferative effects. Taken together, we propose that suppression of Zag has the potential to restore the tubular proliferative capacity in the aging kidney but that this beneficial effect might be counteracted by the accompanying acceleration of interstitial fibrosis.
Figure 5.
Zag suppression causes increased fibrosis in aged mice. (A and B) BUN values (A) and survival curves (B) for Zag- and control siRNA-treated old mice undergoing left-sided nephrectomy and contralateral renal clamping for 27 min reveal no significant difference in renal function or survival (n = 9). (C) Interstitial fibrosis in kidneys of surviving mice at day 14 after I/R is more pronounced after Zag siRNA treatment (n = 4; **P < 0.01 compared with control siRNA). (D through F) Representative images from Masson Trichrome–stained OM of an old uninjured kidney (D) or kidneys 2 wk after I/R with control siRNA (E) or Zag siRNA (F) injection. Tubular atrophy, interstitial cell infiltrates, and collagen deposition are increased in both groups after injury but are more pronounced in Zag siRNA–treated animals (F). (G and H) Representative pictures of immunostaining for collagen IV on noninjured control kidneys of old mice after 6 d of treatment with control siRNA (G) or Zag siRNA (H). (I) semiquantitative analysis of collagen IV in cortex and OM (n = 6; **P < 0.01). Magnification, ×400.
DISCUSSION
These data suggest that aged tubular epithelial cells have a decrease of proliferative reserve that contributes to the diminished repair response in the aged kidney. Using mRNA and protein expression studies, we identified increased Zag expression by the aged epithelial cell as an important mediator of the diminished proliferation. Unexpected, Zag also seems to influence the turnover of extracellular matrix components; therefore, this study not only introduces Zag as a novel regulator of cell proliferation in the aging kidney but also suggests that Zag might be implicated in the regulation of renal interstitial homeostasis.
Zag is a secreted protein that was first detected by Burgi and Schmid19 in human plasma. Its name derives from the tendency to precipitate with zinc combined with its electrophoretic migration in the region of the α2-globulins. Zag expression was recently shown to be much wider than previously thought, including expression in the kidney, liver, and heart.20 Structurally, Zag is a single polypeptide chain that exhibits an open apical groove with similarity to the class I MHC.20 There is biochemical evidence that Zag, in analogy with other MHC class I–related proteins, can bind a ligand in this groove.21 The ligand seems to be a low molecular mass glycolipid rather than a peptide, although it has yet to be definitively identified.22 It has been shown that the amino acids that are positioned directly adjacent to the putative ligand in the Zag groove are identical across multiple mammalian species,22 suggesting that the Zag–ligand interaction is specific and functionally important and that the physiologic effects of Zag may depend on ligand delivery and/or sequestration. It is unclear whether Zag and/or its putative ligand bind to a cell surface receptor or whether uptake of the complex into the cell is required. It has been suggested that Zag can signal via a β3-adrenoreceptor–dependent pathway,23 although a β3-adrenoreceptor agonist was unable to rescue Zag-mediated effects in adipocytes that were isolated from Zag-deficient mice.20 Studies using these mice focused on the role of Zag in lipid metabolism because the animals displayed altered fat repartition, increased body weight, and reduced lipolytic activity.
Some studies have demonstrated that low levels of Zag expression seem to correlate with metastatic potential and a higher risk for recurrence of prostate cancer,24,25 compatible with the suggestion that Zag can act as an inhibitor of proliferation.12 Consistent with this possibility, several pathologic states associated with an increase in epithelial cell proliferation, including psoriasis7 and polycystic kidney disease,26 are accompanied by decreased Zag abundance. This putative antiproliferative effect of Zag has also been demonstrated in vitro in several carcinoma cell lines,11,12 as well as hepatic stellate cells, bovine endothelial cells, and 3T6 fibroblasts.17
Although our in vivo studies of I/R revealed that Zag knockdown indeed resulted in improved tubular cell proliferation after I/R injury, these mice did not exhibit accelerated renal recovery. The lack of functional benefit might have been caused by the accompanying fibrotic response. Interestingly, we also observed signs of accelerated fibrosis (peritubular deposition of collagen IV) after Zag suppression in noninjured kidneys despite the lack of differences in cell proliferation. Although the mechanistic basis of the fibrotic response is unknown, our results suggest that Zag has an unrecognized effect on extracellular matrix production that is independent of its role in cell proliferation. On the basis of these findings, it is conceivable that the age-dependent increase in tubular Zag expression might act to counterbalance the increase in profibrotic factors observed with renal aging.14,15
An alternative explanation for the lack of functional benefit despite an increased rate of proliferation after Zag knockdown might be that the importance of proliferation in recovery of GFR in rodents after AKI is generally overstated. In p21 knockout mice, for example, it has been demonstrated that uncoordinated enhancement of the proliferative potential can be disadvantageous for renal injury and regeneration in the setting of I/R.27 These results illustrate the complex downstream effects of manipulating biologic responses to injury and underscore the potential problems of nonselective pro-proliferative strategies as treatment for AKI.
In summary, we found that renal epithelial cells exhibit an age-dependent, cell-autonomous loss of proliferative capacity, correlating with a significant increase in the expression of Zag. Furthermore, Zag expression levels were shown to regulate directly epithelial proliferation in vitro, and suppression of Zag in aged kidneys increased the in vivo proliferative response during the reparative phase after ischemic injury. Future studies will elucidate whether the lack of functional benefit after this desired response can be linked to the contrasting outcome of increased interstitial fibrosis.
CONCISE METHODS
Mice
All experiments were performed using male C57Bl/6 mice at the ages of 2 to 3 mo (young group) or 19 to 24 mo (old group). Old mice were purchased from the National Institute on Aging and young mice from the National Institutes of Health. All experiments were in accordance with a protocol approved by the Yale Institutional Animal Care and Use Committee.
I/R Experiments
I/R was performed as described previously,28 with the modification that functional studies included nephrectomy of the contralateral kidney. Sham-operated mice were subjected to the identical surgery except for uninephrectomy and renal pedicle clamping. BUN measurements were performed using an automated analyzer (COBAS FARA; Roche, Indianapolis, IN) in the laboratory of Gerald Shulman (Yale University School of Medicine).
Histology and Immunohistochemistry
Mice underwent perfusion fixation with 4% paraformaldehyde through the left ventricle. For detection of Ki-67, PCNA, and collagen IV, deparaffinized kidney sections were boiled in Retrievagen A buffer (BD Pharmingen, San Jose, CA) and incubated overnight with rabbit anti–Ki-67 (1:200; Lab Vision, Fremont, CA), rabbit anti-PCNA antibody (Santa Cruz Biotechnology, Santa Cruz, CA), or goat anti–collagen IV (Southern Biotech, Birmingham, AL). This was followed by antibody visualization using the ABC Vectastain kit (Vector Laboratories, Burlingame, CA) for Ki-67 and PCNA or Alexa 488 secondary antibody (Molecular Probes, Eugene, OR) for collagen IV. For histologic evaluation of renal injury, sections were stained with hematoxylin and eosin or with Masson Trichrome. For quantification of Ki-67–and PCNA-positive cells, a minimum of five separate ×400 high-power fields were analyzed per section in cortex and OM. Quantification of interstitial fibrosis was performed as described previously29 using Masson Trichrome–stained sections with slight modifications. A minimum of 12 nonoverlapping ×400 fields were superimposed with a four-section grid, and the number of fibrotic sections was recorded for each field in a blinded manner. For the semiquantitative analysis of interstitial collagen IV, immunofluorescence intensity for each sample was scored on a scale from 1 to 5 in five separate high-power fields per section in cortex and OM. Immunohistochemistry for Zag was performed on tumor-free tissue sections from nephrectomies of patients with renal carcinoma (provided by W. Weichert, Department of Pathology, Charitè, Berlin, Germany) using a goat polyclonal anti-Zag antibody (H-21; Santa Cruz Biotechnology) and Alexa 488 secondary antibody (Molecular Probes).
Isolation of Renal PT and Cell Culture Experiments
Isolation of mouse PT was done according to a modified protocol by Schafer et al.30 This procedure caused no differences in numbers of dead cells between young and old in freshly isolated PT as shown by staining with propidium iodide (PI; 12 ± 4 PI+ cells/mm in young and 10 ± 6 PI+ cells /mm in old PT). PT were cultured in 24-well plates containing renal epithelial growth medium (Lonza, Walkersville, MD). Medium was changed after 5 d, and the number and total surface area of outgrowing colonies were quantified after 10 d using the Spot Image microscope software (Diagnostic Instruments, Sterling Heights, MI). No differences in cell numbers/mm PT were found between old and young tubules (310 ± 21/mm in young and 322 ± 27/mm in old). For cell proliferation experiments, cells were incubated with 20 μm of BrdU for 30 to 90 min, fixed with 4% paraformaldehyde, permeabilized with 2 M HCl and 0.5% Triton X, and stained with anti-BrdU antibody (1:100; Sigma, St. Louis, MO). Positive nuclei were quantified as a percentage of total nuclei. For immunocytochemical analysis of Zag in transfected cells, cells were fixed in methanol and stained with goat anti-Zag antibody (E-20, 1:200; Santa Cruz Biotechnology) or with anti-Myc antibody (1:500; Upstate Scientific, Lake Placid, NY).
Gene Expression Microarray
PT from young and old mice were microdissected under RNAse-free conditions as described already and snap-frozen for RNA isolation. Preparation times for PT were restricted to 30 min after termination of the digestion to minimize ex vivo synthesis of mRNA. Total RNA was extracted using the TRIzol protocol (Invitrogen, Carlsbad, CA). For obtaining sufficient RNA for microarray hybridization without amplification steps, total RNA from PT of eight mice per age group was pooled for one chip (n = 1 per group). cRNA preparation and array hybridization were carried out by the Yale W.M. Keck Foundation Biotechnology Resource Laboratory. Gene expression data were analyzed according to standard guidelines provided by Affymetrix and using Onto-Express software to identify candidate genes.16
qPCR Analysis
Two-step qPCR was performed to determine Zag expression in tissue homogenate from whole kidney, isolated PT, and in PTEC. Total RNA was extracted using the RNeasy Kit (Qiagen), and 1 μg of RNA was reverse-transcribed using random hexamer primers according to the manufacturer's instructions (SuperScript II; Invitrogen). qPCR was conducted using power SYBR green mix (Applied Biosystems, Foster City, CA) with a 7300 AB Real-time PCR machine (Applied Biosystems). Primer pairs were selected for their specificity and efficiency: Zag primers (5′-ATG GTG CCT GTC CTG CTG TC, 5′-TCG CAA CCA AAC ATT CCC TG) and as housekeeping control genes Hprt1 (5′-CAG TAC AGC CCC AAA ATG GT, 5′-CAA GGG CAT ATC CAA CAA CA) and GAPDH (5′-GAC CCC TTC ATT GAC CTC AAC and 5′-CTT CTC CAT GGT GGT GAA GA). Target gene expression levels were determined by the comparative threshold cycle (dCt) method,31 and mRNA ratios are given by 2dCT. PCR controls run in absence of template were constantly negative.
Western Analysis
Protein electrophoresis was performed as described previously.32 Proteins were transferred to polyvinylidene difluoride membranes, blocked with 5% milk in TBST, and probed over night at 4°C with goat anti-Zag antibody (1:400, E-20; Santa Cruz Biotechnology) at a dilution of 1:400 and visualized by enhanced chemiluminescence (Pharmacia, Piscataway, NJ). GAPDH (1:5000, mAb; Novus Biologicals, Littleton, CO) was used as an internal loading control and for normalization of Zag quantification using NIH image.
Cloning and Overexpression of Murine Zag
Zag was cloned from murine kidney cDNA using primers that cover the full length of the mRNA sequence and 15 nucleotides of the 5′ untranslated region (5′-GGC ACT ACC GTA GCA ATG GT-3′ and 5′-CTG AGG CTG AGC TAC AAC AT-3′). The PCR yielded a single product of the correct size that was cloned into pGEM-T Easy Vector (Promega, Madison, WI), then digested with NotI and ligated into pcDNA3.1 in frame with the pcDNA3.1 c-myc and the polyhistidine tag. The integrity of the sequence was verified before subcloning by DNA sequencing. For cell transfection, 5 μg of pcDNA3.1. Zag or 5 μg of empty vector was used with 7.5 μl of Lipofectamine 2000 (Invitrogen) in OptiMEM for 10-cm dishes. For generation of supernatants containing secreted recombinant Zag, medium was removed 12 h after transfection and cells were subsequently cultured in DMEM/F12 without FBS. Supernatants were harvested 48 h later, concentrated five-fold by using centrifugal filters with a cutoff of 5 kD (Amicon Ultra, Millipore, Bedford, MA) and directly used for primary culture experiments without further storage. Zag content was confirmed by Western blot analysis. Cultures of PT from young mice that were isolated as described already were supplemented with concentrated supernatants at a final dilution of 1:4 in renal epithelial growth medium. Additional concentrated supernatant was added on day 6 at a final concentration of 1:5. For Zag depletion, concentrated supernatants were incubated with sterile ProCatch His Resin nickel-chelator agarose (Miltenyi Biotech, Bergisch Gladbach, Germany) and spun at 8000 rpm for 10 min to precipitate the histidine-tagged Zag-ProCatch His Resin complexes. This procedure was repeated twice before using supernatants for culture experiments as described already.
Knockdown of Zag Expression In Vitro
siRNA oligonucleotides were designed to target Zag and synthesized by the Yale Pathology Laboratory. siRNA were desalted using NAP10 columns (GE Healthcare, Piscataway, NJ) and eluted in 0.9% NaCl. The highest knockdown efficiency of Zag was achieved with siRNA targeting the sequence between nucleotides 406 and 423 of Zag transcript (5′-GGAGAGGAUUUCAUCGAAUtt-3′, antisense 5′-AUUCGAUGAAAUCCUCUCCtt-3′). A previously reported siRNA33 directed against green fluorescence protein served as a control (sense 5′-GGCUACGUCCAGGAGCGCACCtt-3′, antisense 5′-UGCGCUCCUGGACGUAGCCUUtt-3′). In cell culture experiments, transfections were performed using Lipofectamine 2000 together with 0.8 μg of siRNA per well in six-well plates. Cells were lysed 48 h after transfection for qPCR and immunoblot analysis of Zag expression. For proliferation studies, siRNA transfection was performed on days 3 and 6 after starting the culture. Colony growth evaluation and BrdU uptake were performed as described already.
Knockdown of Zag Expression In Vivo
For in vivo experiments, 50 μg of siRNA in 200 μl of 0.9% NaCl was injected via the retro-orbital sinus. For the initial evaluation of Zag knockdown, either Zag or control siRNA was injected daily for 3 d. Mice were killed 24 h after the third injection, and kidneys were homogenized or used for PT isolation and qPCR or immunoblot analysis. For I/R experiments, mice received three administrations of siRNA at 24-h intervals before the surgery, a fourth injection of siRNA immediately after the surgery, and three daily injections after surgery. Renal function was monitored by BUN measurements, and mice were killed on day 14 after I/R for morphologic analysis.
Statistical Analysis
All results are expressed as means ± SEM. Statistical significance was assessed by t test using GraphPad Prism (GraphPad Software, San Diego, CA). P < 0.05 was considered to be statistically significant.
DISCLOSURES
None.
Acknowledgments
This work was supported by an award from the National Institute on Aging to L.G.C. (AG24544) and an Emmy Noether Stipend from the Deutsche Forschungsgemeinschaft to R.S.
Some of the data were presented in abstract form at the annual meeting of the American Society of Nephrology, November 14 through 19, 2006, San Diego, CA; and November 2 through 5, 2007, San Francisco, CA.
We thank Heino Velazquez, Rachel Gallagher, Anil Karihaloo, and Jiankan Guo for assistance.
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, Molitoris BA, Himmelfarb J, Collins AJ: Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol 17: 1135–1142, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Miura K, Goldstein RS, Morgan DG, Pasino DA, Hewitt WR, Hook JB: Age-related differences in susceptibility to renal ischemia in rats. Toxicol Appl Pharmacol 87: 284–296, 1987 [DOI] [PubMed] [Google Scholar]
- 3.Qiao X, Chen X, Wu D, Ding R, Wang J, Hong Q, Shi S, Li J, Xie Y, Lu Y, Wang Z: Mitochondrial pathway is responsible for aging-related increase of tubular cell apoptosis in renal ischemia/reperfusion injury. J Gerontol A Biol Sci Med Sci 60: 830–839, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Sabbatini M, Pisani A, Uccello F, Serio V, Seru R, Paterno R, Cianciaruso B, Fuiano G, Andreucci M: Atorvastatin improves the course of ischemic acute renal failure in aging rats. J Am Soc Nephrol 15: 901–909, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Zager RA, Alpers CE: Effects of aging on expression of ischemic acute renal failure in rats. Lab Invest 61: 290–294, 1989 [PubMed] [Google Scholar]
- 6.Esposito C, Plati A, Mazzullo T, Fasoli G, De Mauri A, Grosjean F, Mangione F, Castoldi F, Serpieri N, Cornacchia F, Dal Canton A: Renal function and functional reserve in healthy elderly individuals. J Nephrol 20: 617–625, 2007 [PubMed] [Google Scholar]
- 7.Chen G, Bridenbaugh EA, Akintola AD, Catania JM, Vaidya VS, Bonventre JV, Dearman AC, Sampson HW, Zawieja DC, Burghardt RC, Parrish AR: Increased susceptibility of aging kidney to ischemic injury: Identification of candidate genes changed during aging, but corrected by caloric restriction. Am J Physiol Renal Physiol 293: F1272–F1281, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmitt R, Coca S, Kanbay M, Tinetti ME, Cantley LG, Parikh CR: Recovery of kidney function after acute kidney injury in the elderly: A systematic review and meta-analysis. Am J Kidney Dis 52: 262–271, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Devarajan P: Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol 17: 1503–1520, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Schrier RW, Wang W, Poole B, Mitra A: Acute renal failure: Definitions, diagnosis, pathogenesis, and therapy. J Clin Invest 114: 5–14, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lei G, Brysk H, Arany I, Tyring SK, Srinivasan G, Brysk MM: Characterization of zinc-alpha(2)-glycoprotein as a cell adhesion molecule that inhibits the proliferation of an oral tumor cell line. J Cell Biochem 75: 160–169, 1999 [DOI] [PubMed] [Google Scholar]
- 12.He N, Brysk H, Tyring SK, Ohkubo I, Brysk MM: Zinc-alpha(2)-glycoprotein hinders cell proliferation and reduces cdc2 expression. J Cell Biochem Suppl [Suppl 36]: 162–169, 2001 [PubMed]
- 13.Witzgall R, Brown D, Schwarz C, Bonventre JV: Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney: Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest 93: 2175–2188, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang DH, Anderson S, Kim YG, Mazzalli M, Suga S, Jefferson JA, Gordon KL, Oyama TT, Hughes J, Hugo C, Kerjaschki D, Schreiner GF, Johnson RJ: Impaired angiogenesis in the aging kidney: Vascular endothelial growth factor and thrombospondin-1 in renal disease. Am J Kidney Dis 37: 601–611, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Thomas SE, Anderson S, Gordon KL, Oyama TT, Shankland SJ, Johnson RJ: Tubulointerstitial disease in aging: Evidence for underlying peritubular capillary damage, a potential role for renal ischemia. J Am Soc Nephrol 9: 231–242, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Draghici S, Khatri P, Bhavsar P, Shah A, Krawetz SA, Tainsky MA: Onto-Tools, the toolkit of the modern biologist: Onto-Express, Onto-Compare, Onto-Design and Onto-Translate. Nucleic Acids Res 31: 3775–3781, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim KY, Choi I, Kim SS: Purification and characterization of a novel inhibitor of the proliferation of hepatic stellate cells. J Biochem 127: 23–27, 2000 [DOI] [PubMed] [Google Scholar]
- 18.van de Water FM, Boerman OC, Wouterse AC, Peters JG, Russel FG, Masereeuw R: Intravenously administered short interfering RNA accumulates in the kidney and selectively suppresses gene function in renal proximal tubules. Drug Metab Dispos 34: 1393–1397, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Burgi W, Schmid K: Preparation and properties of Zn-alpha 2-glycoprotein of normal human plasma. J Biol Chem 236: 1066–1074, 1961 [PubMed] [Google Scholar]
- 20.Rolli V, Radosavljevic M, Astier V, Macquin C, Castan-Laurell I, Visentin V, Guigne C, Carpene C, Valet P, Gilfillan S, Bahram S: Lipolysis is altered in MHC class I zinc-alpha(2)-glycoprotein deficient mice. FEBS Lett 581: 394–400, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Sanchez LM, Chirino AJ, Bjorkman P: Crystal structure of human ZAG, a fat-depleting factor related to MHC molecules. Science 283: 1914–1919, 1999 [DOI] [PubMed] [Google Scholar]
- 22.McDermott LC, Freel JA, West AP, Bjorkman PJ, Kennedy MW: Zn-alpha2-glycoprotein, an MHC class I-related glycoprotein regulator of adipose tissues: Modification or abrogation of ligand binding by site-directed mutagenesis. Biochemistry 45: 2035–2041, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Russell ST, Zimmerman TP, Domin BA, Tisdale MJ: Induction of lipolysis in vitro and loss of body fat in vivo by zinc-alpha2-glycoprotein. Biochim Biophys Acta 1636: 59–68, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Lapointe J, Li C, Higgins JP, van de Rijn M, Bair E, Montgomery K, Ferrari M, Egevad L, Rayford W, Bergerheim U, Ekman P, DeMarzo AM, Tibshirani R, Botstein D, Brown PO, Brooks JD, Pollack JR: Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc Natl Acad Sci U S A 101: 811–816, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Descazeaud A, de la Taille A, Allory Y, Faucon H, Salomon L, Bismar T, Kim R, Hofer MD, Chopin D, Abbou CC, Rubin MA: Characterization of ZAG protein expression in prostate cancer using a semi-automated microscope system. Prostate 66: 1037–1043, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Donauer J, Rumberger B, Klein M, Faller D, Wilpert J, Sparna T, Schieren G, Rohrbach R, Dern P, Timmer J, Pisarski P, Kirste G, Walz G: Expression profiling on chronically rejected transplant kidneys. Transplantation 76: 539–547, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Megyesi J, Andrade L, Vieira JM Jr, Safirstein RL, Price PM: Positive effect of the induction of p21WAF1/CIP1 on the course of ischemic acute renal failure. Kidney Int 60: 2164–2172, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Kale S, Karihaloo A, Clark PR, Kashgarian M, Krause DS, Cantley LG: Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest 112: 42–49, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lange-Sperandio B, Schimpgen K, Rodenbeck B, Chavakis T, Bierhaus A, Nawroth P, Thornhill B, Schaefer F, Chevalier RL: Distinct roles of Mac-1 and its counter-receptors in neonatal obstructive nephropathy. Kidney Int 69: 81–88, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Schafer JA, Watkins ML, Li L, Herter P, Haxelmans S, Schlatter E: A simplified method for isolation of large numbers of defined nephron segments. Am J Physiol 273: F650–F657, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Livak KJ, Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Ishibe S, Haydu JE, Togawa A, Marlier A, Cantley LG: Cell confluence regulates hepatocyte growth factor-stimulated cell morphogenesis in a beta-catenin-dependent manner. Mol Cell Biol 26: 9232–9243, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamar P, Song E, Kokeny G, Chen A, Ouyang N, Lieberman J: Small interfering RNA targeting Fas protects mice against renal ischemia-reperfusion injury. Proc Natl Acad Sci U S A 101: 14883–14888, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]