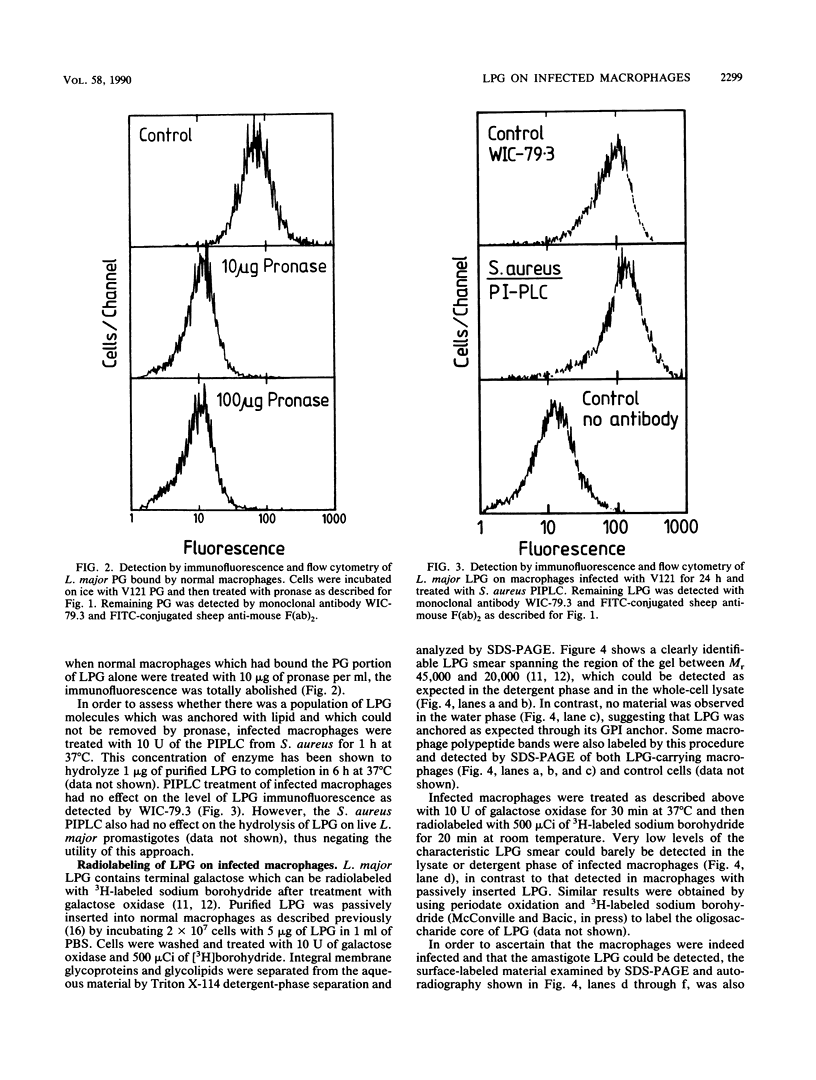
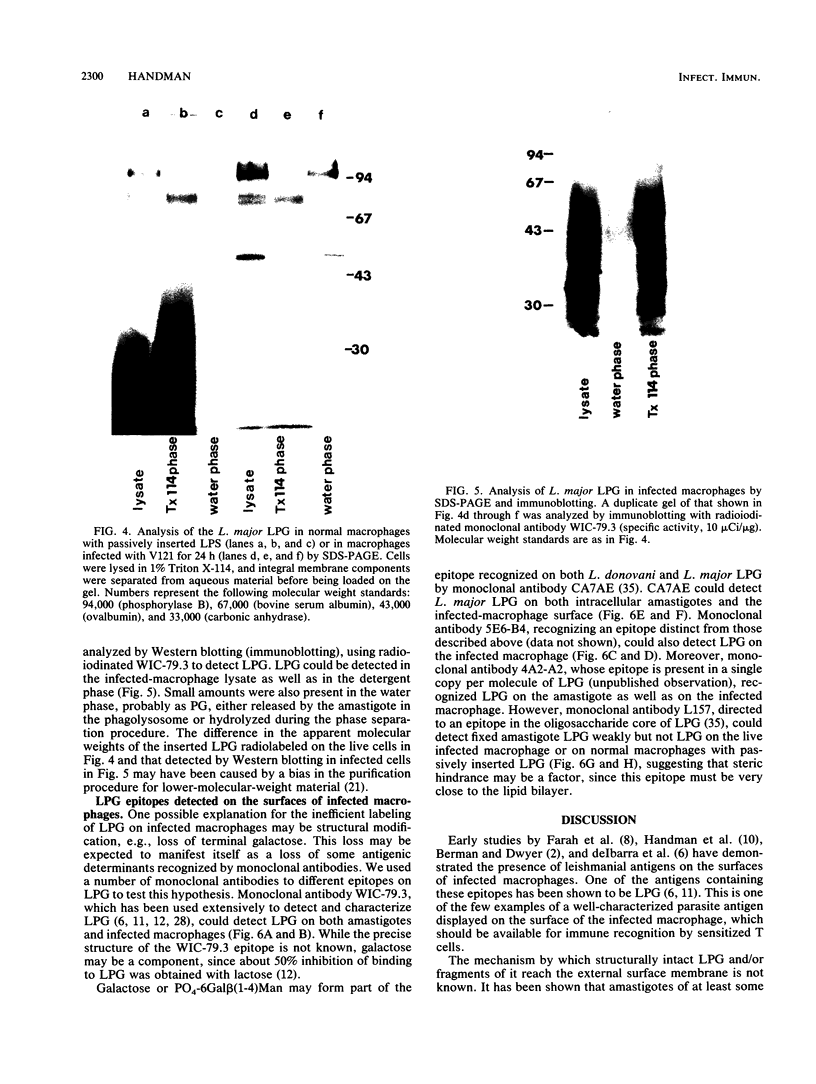
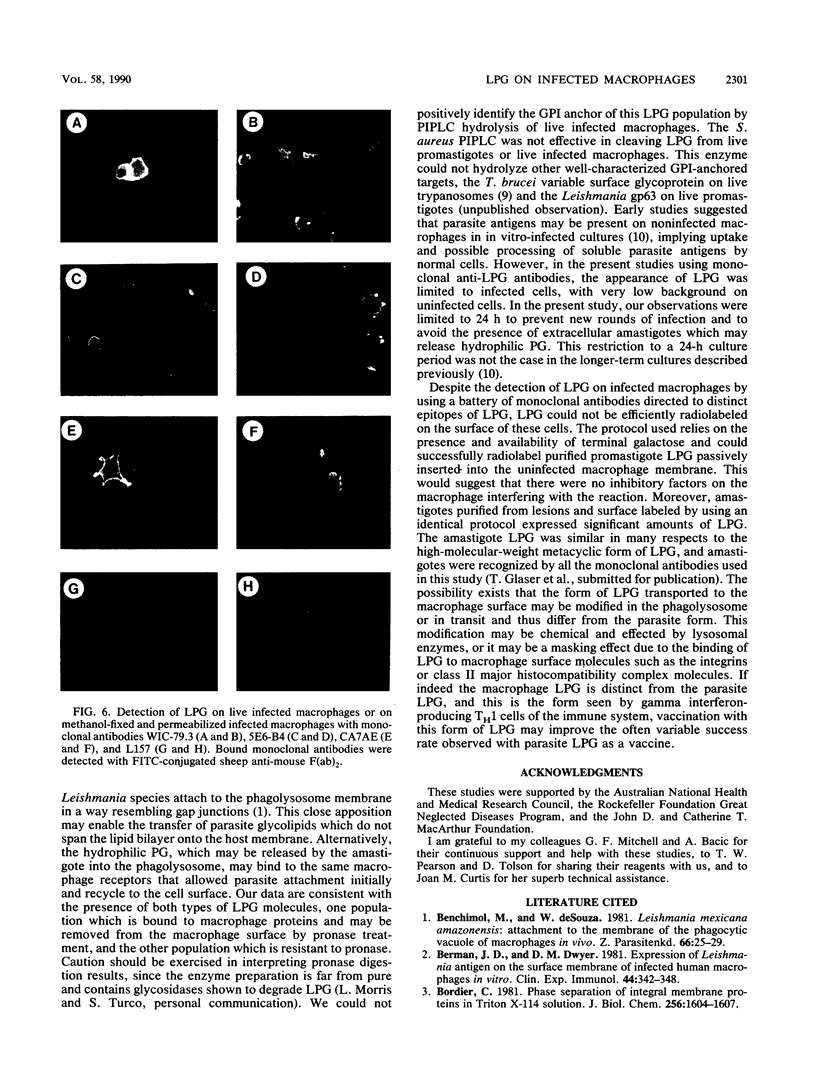
Abstract
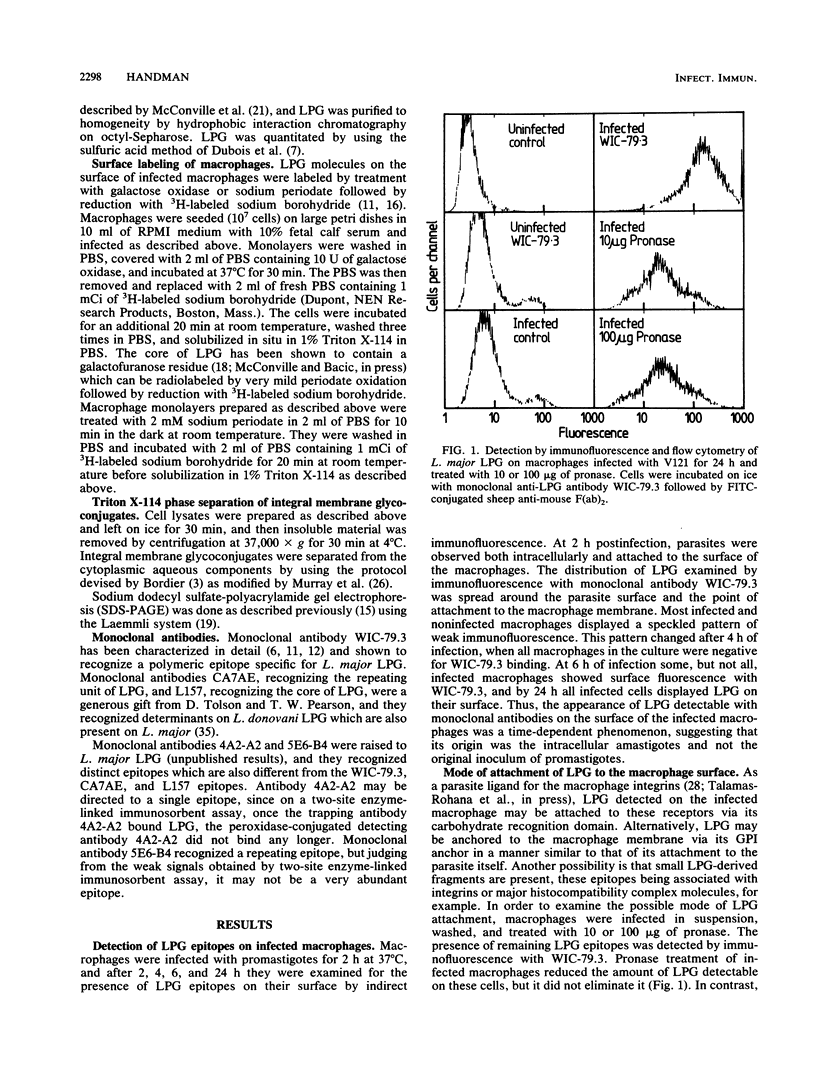
Leishmania major infection of macrophages is followed by a time-dependent appearance of lipophosphoglycan (LPG) that can be detected on the surface of infected cells by monoclonal antibodies. The origin of these LPG epitopes is probably the intracellular amastigote. LPG epitopes could be detected on the amastigote and the infected macrophage by a number of monoclonal antibodies directed to several distinct determinants on the phosphoglycan moiety. The macrophage-expressed LPG may be modified because, unlike the parasite LPG as expressed on promastigotes or amastigotes, it could not be radiolabeled by galactose oxidase or periodate treatment of infected cells followed by reduction with 3H-labeled sodium borohydride. Some LPG epitopes displayed on the macrophage may be anchored with glycosylphosphatidylinositol, and some may be in the water-soluble phosphoglycan form bound to macrophage integrins involved in its specific recognition. The water-soluble population could be released from the infected macrophage by gentle protease treatment.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benchimol M., de Souza W. Leishmania mexicana amazonensis: attachment to the membrane of the phagocytic vacuole of macrophages in vivo. Z Parasitenkd. 1981;66(1):25–29. doi: 10.1007/BF00941942. [DOI] [PubMed] [Google Scholar]
- Berman J. D., Dwyer D. M. Expression of Leishmania antigen on the surface membrane of infected human macrophages in vitro. Clin Exp Immunol. 1981 May;44(2):342–348. [PMC free article] [PubMed] [Google Scholar]
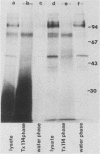
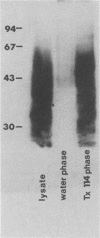
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Chang K. P. Human cutaneous lieshmania in a mouse macrophage line: propagation and isolation of intracellular parasites. Science. 1980 Sep 12;209(4462):1240–1242. doi: 10.1126/science.7403880. [DOI] [PubMed] [Google Scholar]
- Cross G. A. Eukaryotic protein modification and membrane attachment via phosphatidylinositol. Cell. 1987 Jan 30;48(2):179–181. doi: 10.1016/0092-8674(87)90419-3. [DOI] [PubMed] [Google Scholar]
- Farah F. S., Samra S. A., Nuwayri-Salti N. The role of the macrophage in cutaneous leishmaniasis. Immunology. 1975 Oct;29(4):755–764. [PMC free article] [PubMed] [Google Scholar]
- Ferguson M. A., Williams A. F. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu Rev Biochem. 1988;57:285–320. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]
- Handman E., Ceredig R., Mitchell G. F. Murine cutaneous leishmaniasis: disease patterns in intact and nude mice of various genotypes and examination of some differences between normal and infected macrophages. Aust J Exp Biol Med Sci. 1979 Feb;57(1):9–29. doi: 10.1038/icb.1979.2. [DOI] [PubMed] [Google Scholar]
- Handman E., Goding J. W. The Leishmania receptor for macrophages is a lipid-containing glycoconjugate. EMBO J. 1985 Feb;4(2):329–336. doi: 10.1002/j.1460-2075.1985.tb03633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Greenblatt C. L., Goding J. W. An amphipathic sulphated glycoconjugate of Leishmania: characterization with monoclonal antibodies. EMBO J. 1984 Oct;3(10):2301–2306. doi: 10.1002/j.1460-2075.1984.tb02130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Hocking R. E., Mitchell G. F., Spithill T. W. Isolation and characterization of infective and non-infective clones of Leishmania tropica. Mol Biochem Parasitol. 1983 Feb;7(2):111–126. doi: 10.1016/0166-6851(83)90039-7. [DOI] [PubMed] [Google Scholar]
- Handman E., Mitchell G. F., Goding J. W. Identification and characterization of protein antigens of Leishmania tropica isolates. J Immunol. 1981 Feb;126(2):508–512. [PubMed] [Google Scholar]
- Handman E., Mitchell G. F. Immunization with Leishmania receptor for macrophages protects mice against cutaneous leishmaniasis. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5910–5914. doi: 10.1073/pnas.82.17.5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Schnur L. F., Spithill T. W., Mitchell G. F. Passive transfer of Leishmania lipopolysaccharide confers parasite survival in macrophages. J Immunol. 1986 Dec 1;137(11):3608–3613. [PubMed] [Google Scholar]
- Howard J. G., Liew F. Y. Mechanisms of acquired immunity in leishmaniasis. Philos Trans R Soc Lond B Biol Sci. 1984 Nov 13;307(1131):87–98. doi: 10.1098/rstb.1984.0111. [DOI] [PubMed] [Google Scholar]
- King D. L., Chang Y. D., Turco S. J. Cell surface lipophosphoglycan of Leishmania donovani. Mol Biochem Parasitol. 1987 May;24(1):47–53. doi: 10.1016/0166-6851(87)90114-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liew F. Y. Cell-mediated immunity in experimental cutaneous Leishmaniasis. Parasitol Today. 1986 Oct;2(10):264–270. doi: 10.1016/0169-4758(86)90135-3. [DOI] [PubMed] [Google Scholar]
- McConville M. J., Bacic A., Mitchell G. F., Handman E. Lipophosphoglycan of Leishmania major that vaccinates against cutaneous leishmaniasis contains an alkylglycerophosphoinositol lipid anchor. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8941–8945. doi: 10.1073/pnas.84.24.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G. F., Handman E. Leishmania tropica major in mice: vaccination against cutaneous leishmaniasis in mice of high genetic susceptibility. Aust J Exp Biol Med Sci. 1983 Feb;61(Pt 1):11–25. doi: 10.1038/icb.1983.2. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F., Handman E., Moll H., McConville M. J., Spithill T. W., Kidane G. Z., Samaras N., Elhay M. J. Resistance and susceptibility of mice to Leishmania major: a view from Melbourne. Ann Inst Pasteur Immunol. 1987 Sep-Oct;138(5):738–743. doi: 10.1016/s0769-2625(87)80029-6. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F., Handman E., Spithill T. W. Examination of variables in the vaccination of mice against cutaneous leishmaniasis using living avirulent cloned lines and killed promastigotes of Leishmania major. Int J Parasitol. 1985 Dec;15(6):677–684. doi: 10.1016/0020-7519(85)90015-3. [DOI] [PubMed] [Google Scholar]
- Modabber F. Experiences with vaccines against cutaneous leishmaniasis: of men and mice. Parasitology. 1989;98 (Suppl):S49–S60. doi: 10.1017/s0031182000072243. [DOI] [PubMed] [Google Scholar]
- Murray P. J., Spithill T. W., Handman E. Characterization of integral membrane proteins of Leishmania major by Triton X-114 fractionation and analysis of vaccination effects in mice. Infect Immun. 1989 Jul;57(7):2203–2209. doi: 10.1128/iai.57.7.2203-2209.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi P. A., Jr, Turco S. J. Structure of the lipid moiety of the Leishmania donovani lipophosphoglycan. J Biol Chem. 1987 Jul 25;262(21):10384–10391. [PubMed] [Google Scholar]
- Puentes S. M., Sacks D. L., da Silva R. P., Joiner K. A. Complement binding by two developmental stages of Leishmania major promastigotes varying in expression of a surface lipophosphoglycan. J Exp Med. 1988 Mar 1;167(3):887–902. doi: 10.1084/jem.167.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. G., Alexander J. Effective immunization against cutaneous leishmaniasis with defined membrane antigens reconstituted into liposomes. J Immunol. 1988 Feb 15;140(4):1274–1279. [PubMed] [Google Scholar]
- Russell D. G., Talamas-Rohana P. Leishmania and the macrophage: a marriage of inconvenience. Immunol Today. 1989 Oct;10(10):328–333. doi: 10.1016/0167-5699(89)90188-6. [DOI] [PubMed] [Google Scholar]
- Russell D. G. The macrophage-attachment glycoprotein gp63 is the predominant C3-acceptor site on Leishmania mexicana promastigotes. Eur J Biochem. 1987 Apr 1;164(1):213–221. doi: 10.1111/j.1432-1033.1987.tb11013.x. [DOI] [PubMed] [Google Scholar]
- Russell D. G., Wilhelm H. The involvement of the major surface glycoprotein (gp63) of Leishmania promastigotes in attachment to macrophages. J Immunol. 1986 Apr 1;136(7):2613–2620. [PubMed] [Google Scholar]
- Russell D. G., Wright S. D. Complement receptor type 3 (CR3) binds to an Arg-Gly-Asp-containing region of the major surface glycoprotein, gp63, of Leishmania promastigotes. J Exp Med. 1988 Jul 1;168(1):279–292. doi: 10.1084/jem.168.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P. The role of TH1 and TH2 cells in experimental cutaneous leishmaniasis. Exp Parasitol. 1989 Apr;68(3):369–372. doi: 10.1016/0014-4894(89)90120-3. [DOI] [PubMed] [Google Scholar]
- Tolson D. L., Turco S. J., Beecroft R. P., Pearson T. W. The immunochemical structure and surface arrangement of Leishmania donovani lipophosphoglycan determined using monoclonal antibodies. Mol Biochem Parasitol. 1989 Jun 15;35(2):109–118. doi: 10.1016/0166-6851(89)90113-8. [DOI] [PubMed] [Google Scholar]
- de Ibarra A. A., Howard J. G., Snary D. Monoclonal antibodies to Leishmania tropica major: specificities and antigen location. Parasitology. 1982 Dec;85(Pt 3):523–531. doi: 10.1017/s0031182000056304. [DOI] [PubMed] [Google Scholar]