Abstract
Vitamin D receptor (VDR)-null mice develop polyuria, but the underlying mechanism remains unknown. In this study, we investigated the relationship between vitamin D and homeostasis of water and electrolytes. VDR-null mice had polyuria, but the urine osmolarity was normal as a result of high salt excretion. The urinary responses to water restriction and to vasopressin were similar between wild-type and VDR-null mice, suggesting intact fluid-handling capacity in VDR-null mice. Compared with wild-type mice, however, renin and angiotensin II were dramatically upregulated in the kidney and brain of VDR-null mice, leading to a marked increase in water intake and salt appetite. Angiotensin II–mediated upregulation of intestinal NHE3 expression partially explained the increased salt absorption and excretion in VDR-null mice. In the brain of VDR-null mice, expression of c-Fos, which is known to associate with increased water intake, was increased in the hypothalamic paraventricular nucleus and the subfornical organ. Treatment with an angiotensin II type 1 receptor antagonist normalized water intake, urinary volume, and c-Fos expression in VDR-null mice. Furthermore, despite a salt-deficient diet to reduce intestinal salt absorption, VDR-null mice still maintained the increased water intake and urinary output. Together, these data indicate that the polyuria observed in VDR-null mice is not caused by impaired renal fluid handling or increased intestinal salt absorption but rather is the result of increased water intake induced by the increase in systemic and brain angiotensin II.
The homeostasis of the extracellular volume and osmotic conditions in animals is maintained by neural and endocrine hormones such as arginine vasopressin (AVP), angiotensin II (AngII), aldosterone, and atrial natriuretic peptide (ANP). These hormones control the homeostasis by regulation of water drinking, blood flow to osmoregulatory organs, and salt and water transport across epithelia in the kidney and intestine. Usually, alterations in intravascular volume and osmolality lead to changes in production and secretion of hormones, which act to maintain the homeostasis. For example, AVP is secreted from the hypothalamus/posterior pituitary in response to intravascular osmotic pressure and acts on the collecting duct in the kidney to increase water reabsorption. AVP activates the basolateral V2 receptor in renal collecting duct epithelial cells, leading to increased association of water channel aquaporin 2 (AQP-2) to the apical membrane, which increases water transport across the tubular epithelium.1 AngII, the main effector of the renin-angiotensin system (RAS), stimulates central regulation of water intake and aldosterone production from the adrenal glands.2 Several regions in the brain, including the subfornical organ (SFO), median preoptic nucleus (MnPO), and hypothalamic paraventricular nucleus (PVN), are known to be involved in the dipsogenic action of AngII.3 AngII is also known to stimulate salt and water absorption from the intestine.4 Aldosterone, in addition to being stimulated by AngII, responds to hyperkalemia and acts on the distal nephron to increase sodium reabsorption and potassium excretion.5 ANP, however, is released from the heart in response to volume expansion and acts on the kidney to increase diuresis and natriuresis.6 The interaction of these hormones plays key roles in the regulation of the water and salt balance in the body.
Polyuria is characterized by unusually high urinary output. We previously reported that mice lacking the vitamin D receptor (VDR) develop polyuria, with urinary volume increased several-fold compared with wild-type mice,7 but the mechanism underlying the development of polyuria remains unclear. In this study we demonstrated that the polyuria seen in VDR(−/−) mice is not due to impaired kidney function to handle fluid or increased intestinal salt absorption but is caused by increased water intake as a result of AngII overproduction in the plasma and the brain. This study unveils novel aspects of the vitamin D endocrine system in the regulation of hydromineral homeostasis.
RESULTS
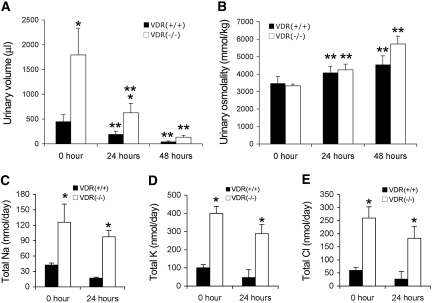
Under the basal condition, the 24-h urinary volume of VDR(−/−) mice was three to four times higher than that of VDR(+/+) mice (Figure 1A, at 0 h). Surprisingly, despite the higher urinary output, the urinary osmotic strength (Figure 1B) was indistinguishable between VDR(−/−) and VDR(+/+) mice at the baseline. The plasma osmolality and plasma salt concentration of these two genotypes were also the same (data not shown). As a result, the total urinary excretion of Na+, K+, and Cl− per day was higher in VDR(−/−) mice than in VDR(+/+) mice at the baseline (Figure 1, C through E). To explore whether the polyuria seen in VDR(−/−) mice was caused by impaired renal ability to concentrate urine, we carried out a water restriction experiment. As shown in Figure 1, water deprivation for 24 and 48 h led to a dramatic decrease in urinary volume in both VDR(+/+) and VDR(−/−) mice (Figure 1A). The reduction was 57 and 65% at 24 h and 90 and 93% at 48 h, for VDR(+/+) and VDR(−/−) mice, respectively. After the water restriction, the urinary volume of VDR(−/−) mice remained higher than that of VDR(+/+) mice; however, the osmolality of the urine increased with time in both VDR(+/+) and VDR(−/−) mice: 18 and 27% at 24 h and 31 and 71% at 48 h, respectively, in VDR(+/+) and VDR(−/−) mice (Figure 1B). Consequently, the urinary osmolality remained indistinguishable between VDR(+/+) and VDR(−/−) mice after the water restriction. Total urinary excretion of Na+, K+, and Cl− decreased in VDR(+/+) and VDR(−/−) mice 24 h after water restriction; however, as expected, salt excretion of VDR(−/−) mice remained higher than that of VDR(+/+) mice (Figure 1, C through E). These data suggest that the urine-concentrating capacity of VDR(−/−) mice is intact.
Figure 1.
VDR(−/−) mice have high urinary volume and normal urine-concentrating ability. (A) Twenty-four-hour urinary volume of VDR(+/+) and VDR(−/−) mice at baseline (0 h) and at 24 and 48 h after water restriction. (B) Urinary osmolality of VDR(+/+) and VDR(−/−) mice at baseline and at 24 and 48 h after water restriction. (C through E) Total urinary excretion of Na+ (C), K+ (D), and Cl− (E) per day at baseline and at 24 h after water restriction, calculated by salt concentration × 24-h volume. n ≥ 7 in each genotype. *P < 0.05 versus VDR(+/+) mice; **P < 0.01 versus 0 h.
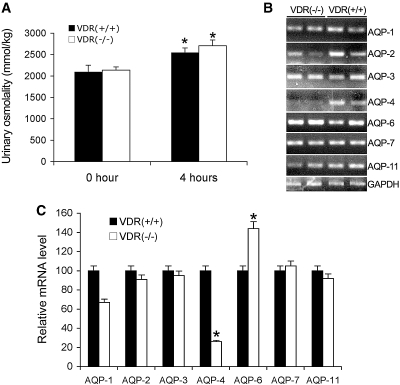
To examine further the fluid-handling ability of VDR(−/−) mice, we compared the response of VDR(+/+) and VDR(−/−) mice to AVP, a hormone known to stimulate water retention in the kidney. As shown in Figure 2, 4 h after desamino d-arginine vasopressin administration, urinary osmolality was significantly increased to the same extent in VDR(+/+) and VDR(−/−) mice (Figure 2A). These data confirm that the kidney's urine-concentrating capacity in response to AVP is normal in VDR(−/−) mice.
Figure 2.
VDR(−/−) mice display the same response to arginine vasopressin as VDR(+/+) mice to concentrate urine. (A) Response to vasopressin challenge. Shown are urinary osmolality of VDR(+/+) and VDR(−/−) mice at baseline and at 4 h after receiving one dose of 1 μg/kg d-arginine vasopressin. *P < 0.05 versus 0 h. (B and C) Expression of water channels in the kidney of VDR(+/+) and VDR(−/−) mice. Water channels AQP-1 to AQP-12 mRNA levels were determined by RT-PCR (B) and real time RT-PCR (C). AQP-5, -8, -9, -10, and -12 were undetectable. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
To test this conclusion further at the molecular level, we compared the expression of water channels ranging from AQP-1 to AQP-12 in the kidneys of VDR(+/+) and VDR(−/−) mice. Reverse transcription–PCR (RT-PCR) showed that, except for AQP-4 (relatively lower in VDR(−/− mice at baseline but was induced to the normal level during water restriction) and AQP-6 (relatively higher in VDR(−/−) mice), the mRNA levels of AQP-1, -2, -3, -6, -7, and -11 were similar in VDR(+/+) and VDR(−/−) kidneys (Figure 2B). Further quantification of these AQP with real-time RT-PCR confirmed this result (Figure 2C). AQP-5, -8, -9, -10, and -12 mRNA were undetectable in both genotypes. Because AQP (particularly AQP2) play key roles in water transport/reabsorption in the kidney, these data support the conclusion that VDR(−/−) mice still maintain an intact urine-concentrating process; therefore, polyuria is unlikely caused by impaired renal functions in VDR(−/−) mice.
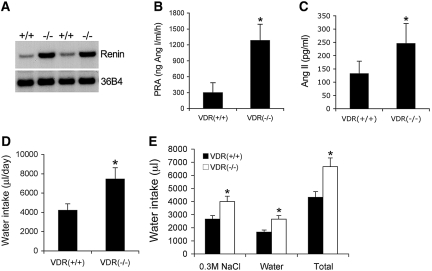
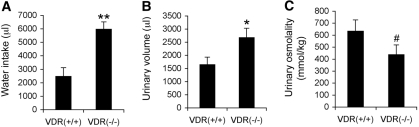
To continue the investigation into the mechanism of polyuria, we then examined the RAS. We reported previously that VDR inactivation leads to hyperreninemia,7 because 1,25-hydroxyvitamin D suppresses renin gene transcription.8 As shown in Figure 3, renin mRNA expression in the kidney (Figure 3A) was dramatically upregulated in VDR(−/−) mice, leading to an approximately 300% increase in plasma renin activity relative to the VDR(+/+) counterparts (Figure 3B). Consequently, plasma AngII levels in VDR(−/−) mice were markedly increased by approximately 200% (Figure 3C). AngII is widely known to stimulate central regulation of water drinking.9 Accompanied with the higher urinary output, water intake in VDR(−/−) mice was also markedly increased by approximately 90 to 100% compared with VDR(+/+) mice (Figure 3D). When given a choice of tap water and hypertonic saline, VDR(−/−) mice showed an increased preference for sodium consumption (Figure 3E), consistent with the previous finding that AngII plays dual central roles in water and sodium intake.10–12
Figure 3.
Activation of the RAS is associated with increased water drinking in VDR(−/−) mice. (A) Northern blot showing renin mRNA levels in the kidney of VDR(+/+) and VDR(−/−) mice. (B) Plasma renin activity (PRA) in VDR(+/+) and VDR(−/−) mice. (C) Plasma AngII levels in VDR(+/+) and VDR(−/−) mice. (D) Daily water intake volume of VDR(+/+) and VDR(−/−) mice. (E) Increased salt appetite in VDR(−/−) mice. VDR(+/+) and VDR(−/−) mice were provided two separate burettes simultaneously: One contains 0.3 M NaCl saline, and the other contains tap water. The volumes of 24-h intake were determined from these two burettes. Total is the two volumes combined. n ≥ 5 in each genotype. *P < 0.05 versus VDR(+/+) mice.
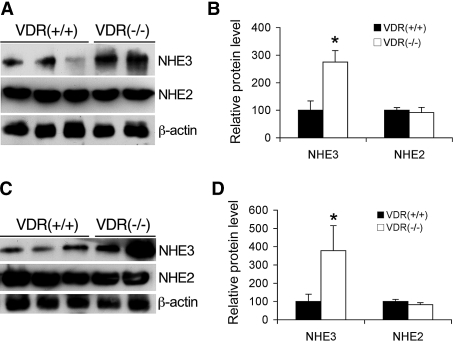
AngII is known to stimulate intestinal salt transport. On the basis of the observations that VDR(−/−) mice had higher salt excretion than VDR(+/+) mice while maintaining normal plasma salt concentrations7 and the food intake of VDR(−/−) mice was basically the same as that of VDR(+/+) mice [150.2 ± 5.3 g/kg body wt per d in VDR(−/−) mice versus 140.8 ± 4.2 in VDR(+/+) mice; n = 6], we speculated that VDR(−/−) mice most likely have increased salt absorption in the intestine to maintain the salt balance. As an initial step to address this issue, we compared the protein levels of apical intestinal Na+/H+ exchangers (NHE) between VDR(+/+) and VDR(−/−) mice, using antibodies specific to NHE2 and NHE3.13 As shown in Figure 4, NHE3 expression in the jejunum (Figure 4, A and B) and ileum (Figure 4, C and D) was increased in VDR(−/−) mice, whereas the levels of NHE2 were the same in VDR(+/+) and VDR(−/−) mice (Figure 4, A through C). These data are consistent with the speculation of higher intestinal sodium absorption in VDR(−/−) mice.
Figure 4.
Expression of intestinal NHE in VDR(+/+) and VDR(−/−) mice. (A and C) Intestinal mucosa were isolated from the jejunum (A) and ileum (C) of VDR(+/+) and VDR(−/−) mice, and protein levels of NHE3 and NHE2 in the mucosa were determined by Western blotting with antibody against NHE3 or NHE2 as indicated. (B and D) Densitometric quantification of jejunum (B) and ileum (D) NHE3 and NHE2 protein levels. *P < 0.05 versus VDR(+/+).
We further investigated the involvement of the brain in AngII-stimulated water intake. Previous studies demonstrated an association of increased c-Fos expression in the SFO and hypothalamic PVN neurons with stimulation of water drinking.14 Moreover, the SFO is long known to be an important region in the brain to control water drinking in response to AngII.15,16 To link AngII overproduction to overdrinking in VDR(−/−) mice, we examined c-Fos expression in the brain. Immunostaining showed that c-Fos expression in the SFO (Figure 5, A and B) and PVN (Figure 5, C and D) was clearly increased in VDR(−/−) mice. These data strongly suggest that AngII overproduced in VDR(−/−) mice stimulates water drinking by acting on the central nervous system.
Figure 5.
Increased c-Fos expression in the brain of VDR(−/−) mice. (A through D) Immunostaining shows increased c-Fos expression in the SFO (A and B) and in the hypothalamic PVN (C and D) of VDR(−/−) mice (B and D) compared with VDR(+/+) mice (A and C).
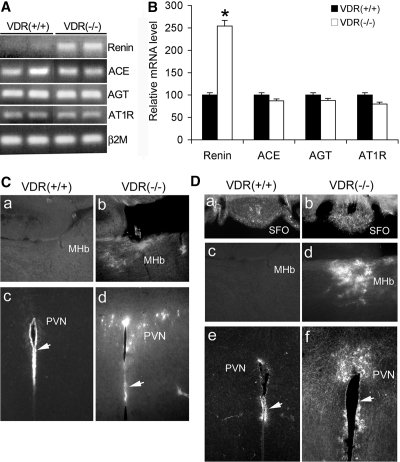
The brain is known to have a local RAS. All components of the RAS have been found within the brain.17 Renin has previously been located in multiple regions in the brain.18–20 Because vitamin D suppresses renin expression8 and VDR inactivation leads to renin upregulation in the kidney,7 we examined renin and other components of the RAS in the brain of VDR(+/+) and VDR(−/−) mice. RT-PCR data showed that, similar to the kidney, renin mRNA in the brain was markedly elevated in VDR(−/−) mice; however, the levels of angiotensin-converting enzyme, angiotensinogen, and AngII type 1 receptor (AT1R) mRNA were the same in VDR(+/+) and VDR(−/−) mice (Figure 6A). Further quantification of these proteins with real-time RT-PCR confirmed this finding (Figure 6B).
Figure 6.
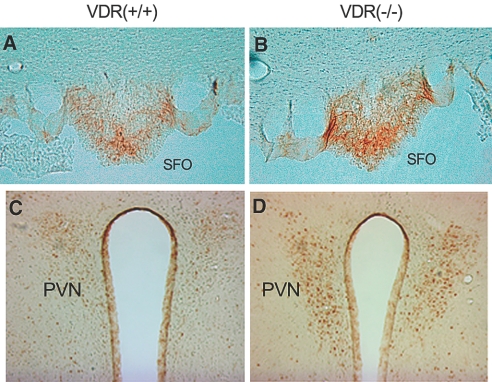
Increased renin expression and AngII production in the brain of VDR(−/−) mice. (A and B) RT-PCR determination (A) and real-time RT-PCR quantification (B) of the mRNA levels of renin, angiotensin-converting enzyme (ACE), angiotensinogen (AGT), and AT1R in the brain in VDR(+/+) and VDR(−/−) mice. Total RNA were extracted from the −1 to −4 region relative to the bregma as described in the Concise Methods section. Note the higher renin mRNA level in VDR(−/−) mice. *P < 0.05. (C) Immunostaining of renin in the brain. Note increased renin staining in the MHb (a and b) and hypothalamic PVN (c and d) of VDR(−/−) mice (b and d) compared with VDR(+/+) mice (a and c). (D) Immunostaining of AngII in the brain. Note increased AngII staining in the SFO (a and b), the MHb (c and d), and the hypothalamic PVN (e and f) of VDR(−/−) mice (b, d, and f) compared with VDR(+/+) mice (a, c, and e). Arrows indicate the renin-positive endothelial cells lining the third ventricle.
We further used immunostaining to determine renin expression in the brain. Although high renin expression was detected in the endothelial layer of the third ventricle in both VDR(+/+) and VDR(−/−) mice (Figure 6C, c and d), renin upregulation was clearly seen in the medial habenular nucleus (MHb; Figure 6Cb) and hypothalamic PVN (Figure 6Cd) of VDR(−/−) mice.
Renin upregulation in the brain is expected to lead to an increase in the local production of AngII. Indeed, immunostaining with AngII-specific antibody revealed markedly increased AngII staining in the MHb (Figure 6Dd) and hypothalamic PVN (Figure 6Df) regions, as well as in the SFO (Figure 6Db) of VDR(−/−) mice. Both PVN and SFO are known to be involved in regulation of water drinking14; therefore, in addition to the systemic AngII, the brain-derived AngII most likely plays a critical role in the induction of water intake in VDR(−/−) mice.
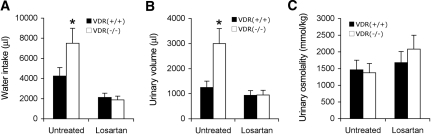
To confirm that AngII was indeed responsible for the increase in water intake, we treated the mice with losartan, an AT1R blocker. As shown in Figure 7, 7-d losartan treatment reduced water intake (Figure 7A) as well as urinary output (Figure 7B) in both VDR(+/+) and VDR(−/−) mice, and both water intake and urinary volume were indistinguishable between VDR(+/+) and VDR(−/−) mice after the treatment. As expected, urinary osmolality was increased in VDR(+/+) and VDR(−/−) mice after losartan treatment (Figure 7C), because of the reduction in water excretion.
Figure 7.
Losartan treatment normalizes water drinking and urinary output in VDR(−/−) mice. (A through C) VDR(+/+) and VDR(−/−) mice were untreated or treated with losartan for 7 d, and water drinking (A), urinary volume (B), and urinary osmolality (C) were determined. n ≥ 4 in each genotype. *P < 0.05 versus VDR(+/+) mice.
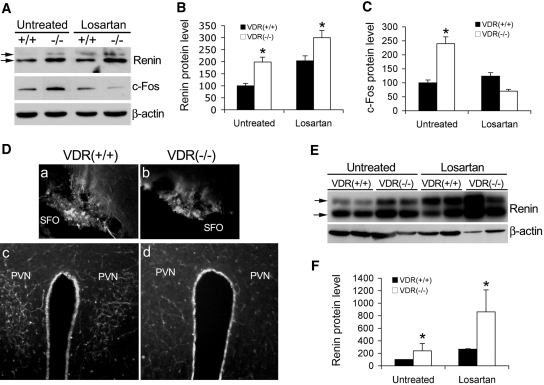
At the molecular level, brain c-Fos levels were higher in VDR(−/−) mice at the baseline, and losartan markedly reduced c-Fos expression in both VDR(+/+) and VDR(−/−) mice (Figure 8, A and C), and immunostaining showed that c-Fos expression in the SFO and PVN regions of VDR(−/−) mice was similar to or lower than that seen in VDR(+/+) mice after the treatment (Figure 8D, a versus b, and c versus d). These data confirm that AngII is the main factor responsible for increased water drinking in VDR(−/−) mice, which leads to polyuria.
Figure 8.
Effect of losartan treatment on c-Fos and renin expression in the brain and the kidney. (A) Western blots showing renin and c-Fos levels in the brain of VDR(+/+) and VDR(−/−) mice before and after losartan treatment. The two arrows indicate the prorenin and renin polypeptide bands. Note that losartan markedly suppresses c-Fos expression in the brain of both VDR(+/+) and VDR(−/−) mice. (B and C) Densitometric quantification of renin (B) and c-Fos (C) protein levels from the Western blots of untreated and losartan-treated VDR(+/+) and VDR(−/−) brains. (D) Brain immunostaining of c-Fos in the SFO (a and b) and PVN (c and d) regions of losartan-treated VDR(+/+) (a and c) and VDR(−/−) (b and d) mice. Note the marked decreased staining in the PVN of VDR(−/−) mice. (E) Western blot showing that losartan upregulates renin expression in the kidney of VDR(+/+) and VDR(−/−) mice. The two arrows indicate the prorenin and renin polypeptide bands. (F) Densitometric quantification of renin protein levels in untreated and losartan-treated kidneys of VDR(+/+) and VDR(−/−) mice. *P < 0.05 versus VDR(+/+).
Because inhibition of AT1R disrupts the feedback inhibition loop of renin regulation, as expected, losartan treatment caused a dramatic upregulation of renin expression in the kidney of both VDR(+/+) and VDR(−/−) mice (Figure 8, E and F). Much smaller renin upregulation was seen in the brain in both mice (Figure 8, A and B). Thus, renin regulation seems to be subject to the feedback inhibition in both the kidney and the brain.
Because the high urinary output was accompanied by a high salt excretion in VDR(−/−) mice, it is possible that polyuria was driven by solute diuresis caused by the increased intestinal salt absorption, rather than by increased water intake. To address this question, we placed VDR(+/+) and VDR(−/−) mice on a special diet deficient in Na+, K+, and Cl− to avoid the interference of intestinal salt absorption on the development of polyuria. As shown in Figure 9, 2 wk after the mice were on this diet, the urinary osmolality was reduced by >80% in VDR(+/+) and VDR(−/−) mice compared with mice on the regular diet (Figure 9C versus Figure 1B). Despite the low salt excretion, water intake (Figure 9A) and urinary output (Figure 9B) in VDR(−/−) mice remained significantly higher than in VDR(+/+) mice. Importantly, the urine of VDR(−/−) mice became osmotically dilute under this dietary condition compared with VDR(+/+) mice (Figure 9C). These data demonstrate that the development of polyuria seen in VDR(−/−) mice is largely driven by water intake and independent of intestinal salt absorption.
Figure 9.
Effect of a salt-deficient diet on water drinking, urinary output, and osmolality. (A through C) VDR(+/+) and VDR(−/−) mice were placed on a Na+-, K+-, and Cl−-deficient diet for 2 wk, and 24-h water drinking (A), urinary volume (B), and urinary osmolality (C) were then determined in these mice. n = 6 in each genotype. *P < 0.05 versus VDR(+/+); **P < 0.01 versus VDR(+/+); #P = 0.05 versus VDR(+/+).
DISCUSSION
Polyuria can originate from kidney or brain causes. Impaired renal fluid handling, abnormal central regulation of water drinking, or both can lead to polyuria. Although in our early studies we reported polyuria in VDR(−/−) mice,7,21 the exact mechanism remains unclear. Given the broad spectrum of functionalities of the vitamin D endocrine system unveiled in recent years, understanding the mechanism underlying the development of polyuria would have novel physiologic and pathologic implications.
This rationale prompted us to investigate the mechanism in this study. Our study demonstrated relatively normal renal water-handling and urine-concentrating capacity in VDR(−/−) mice in response to water restriction and AVP, strongly suggesting that the polyuric phenotype is unlikely caused by impaired renal functions. The normal expression of AQP in the kidney further supports this assessment. Our data, however, point to overstimulation of the central regulation of water drinking as the main cause of polyuria in VDR(−/−) mice. The initial molecular event that leads to the development of polyuria is the upregulation of renin in the kidney as well as in the central nervous system, caused by VDR inactivation. We recently demonstrated that the molecular basis for renin upregulation is that 1,25-dihydroxyvitamin D suppresses renin gene transcription by blocking the cAMP response element (CRE)-mediated promoter activity.8 Renin upregulation in the kidney and brain leads to hyperreninemia and AngII overproduction in the circulation as well as in local areas of the brain. AngII, a potent dipsogenic hormone, stimulates the brain to increase water intake, leading to polyuria in a context of normal fluid handling by the kidney.
It is long established that the SFO in the brain is critically involved in the regulation of water intake in response to AngII.15,16 The SFO lies outside the blood-brain barrier and is thus accessible to the circulating AngII, and AngII receptors were also localized within the SFO.22 Indeed, previous studies demonstrated that the SFO mediates water drinking in response to systemic administration of AngII.15,16,23 In VDR(−/−) mice, the upregulation of renin expression in the kidney sustains a high level of circulating AngII, which acts on the SFO to stimulate water drinking. This notion is supported by the enhanced expression of c-Fos in SFO in VDR(−/−) mice. The high circulating renin in VDR(−/−) mice can also reach to the SFO to increase the local AngII level (see Figure 6Db) from angiotensinogen highly expressed in the SFO.24 Increased production of AngII in SFO has been co-related to elevated water drinking and salt appetite in mice recently.25
The effect of blood-borne AngII on the brain is limited by the blood-brain barrier, which separates the systemic RAS from the brain RAS. Interestingly, in VDR(−/−) mice, c-Fos expression, which indicates activation of neurons, is also increased in the PVN, a region known to be involved in regulation of water drinking.14,26 Because PVN is located inside the blood-brain barrier, the PVN neurons are likely activated by AngII locally generated inside the brain. One interesting finding of this study is the upregulation of renin in the brain of VDR(−/−) mice, indicating that vitamin D regulation of renin expression is not limited to the kidney. As reported previously by others,27,28 renin mRNA levels in the brain were low and hard to detect in wild-type mice; however, renin mRNA is readily detectable in VDR(−/−) brain by RT-PCR. Immunostaining localized increased renin protein in the MHb and PVN regions in VDR(−/−) mice. Consistent with the notion that high renin will lead to high local production of AngII, increased AngII staining was co-localized in the MHb and PVN regions in VDR(−/−) mice. Whereas the effect of AngII elevation in the MHb neurons is unknown, the increase of AngII in the PVN most likely contributes to the development of polyuria in VDR(−/−) mice through stimulation of water drinking in an autocrine or a paracrine manner.
The critical role of AngII in the development of polyuria was supported by several lines of evidence. AngII was previously shown to induce c-Fos expression in multiple regions of the brain, including the SFO and PVN26; consistently, c-Fos was markedly increased in the SFO and PVN regions in VDR(−/−) mice. Furthermore, disruption of AngII signaling by AT1R antagonist losartan, which is able to cross the blood-brain barrier,29,30 reduced c-Fos expression as well as normalized water drinking in the mutant mice; therefore, both the systemic and brain-derived AngII contributes to the overdrinking behavior of VDR(−/−) mice, leading to polyuria.
Our data suggest that VDR(−/−) mice have increased intestinal salt absorption to balance their high salt excretion from the kidney. At present, however, we do not have sufficient data to explain how VDR(−/−) mice maintain an elevated salt transport across the intestinal epithelia. Plasma AngII is known to increase salt absorption in the small intestine; it also enhances sodium transport in the large intestine through stimulation of aldosterone,4 which targets the epithelial sodium channel. Because AngII and aldosterone levels are elevated in VDR(−/−) mice,21 they may play a major role in stimulating intestinal salt transport in the mutant mice, which partly explains the high salt excretion seen in VDR(−/−) mice; however, the mechanism of AngII-stimulated salt absorption remains unclear. Some studies suggested the involvement of the sympathetic nervous system in the stimulation.31 In this study, we found that NHE3 was upregulated in the small intestine of VDR(−/−) mice, which may explain in part the increase in intestinal sodium transport; however, we do not know whether the increased NHE3 expression actually leads to increased salt absorption in VDR(−/−) mice. AngII has been shown to upregulates NHE-3 expression.32 Intestinal water uptake may also increase accompanying with the increased intestinal sodium transport. It is unclear whether other epithelial sodium transporters or channels are also involved. Potentially, the tubular epithelial salt transport in the kidney may be another site of regulation in the development of high salt excretion. More investigations are needed to elucidate the mechanism underlying the increased salt absorption and excretion in VDR(−/−) mice.
Given the high salt excretion in the VDR(−/−) mouse urine, one question needed to be addressed is whether the polyuria is driven by solute diuresis or water diuresis. Data presented in Figure 1 suggest that both solute and water diuresis might be involved in the development of polyuria. Early studies demonstrated that intracerebroventricular infusion of AngII or increased local production of AngII in the brain stimulates water intake and sodium appetite.10–12,25 Under this condition, animals produced high urinary volume with increased salt excretion; however, the driving force is increased water drinking.25 VDR(−/−) mice have an increase in AngII production not only in the brain but also in the circulation; therefore, the brain effect of AngII is compounded by the intestinal effect of circulating AngII on salt absorption, leading to water intake–induced polyuria in a context of high salt excretion. Under this condition, the urine is osmotically normal. When the mice were placed on the Na+-, K+-, and Cl−-deficient diet, which eliminated the effect of intestinal salt absorption, VDR(−/−) mice still exhibited an increase in water intake and urinary volume, even though their urine was osmotically dilute, with osmolality reduced by >80% (Figure 9). These data provide compelling evidence that polyuria seen in VDR(−/−) mice is mostly driven by increased water intake–induced water diuresis, and the intestinal salt absorption–induced solute diuresis plays only a minor role, if any, in the development of polyuria.
In summary, we have demonstrated that, although VDR(−/−) mice develop polyuria, their urine-concentrating capacity is intact. Polyuria is unlikely caused by impaired renal functions, and increased intestinal salt transport might only play a minor role. VDR inactivation leads to upregulation of renin expression in both the kidney and the brain, leading to an increase in both systemic and brain AngII production. AngII stimulates central regulation of water intake, which plays the leading role in the development of polyuria. Unveiling the mechanism of vitamin D regulation of water drinking underscores the novel role of the vitamin D endocrine system in the regulation of extracellular volume homeostasis in animals.
CONCISE METHODS
Animal Studies
Two- to 3-mo-old C57BL/6 VDR(+/+) and VDR(−/−) mice were used in the study. VDR(−/−) mice were originally generated in 129/sv-C57BL/6 mixed background,33 and were backcrossed into C57BL/6 background for at least 10 generations. Urine was collected using metabolic cages. For water restriction, mice were placed in metabolic cages without water access, and urine was collected at 0, 24, and 48 h. In some experiments, mice were given one intraperitoneal injection of desamino d-arginine vasopressin (Sigma Co, St. Louis, MO) at a dosage of 1 μg/kg, and urine was collected after 4 h. In some experiments, mice were treated with losartan in drinking water at 30 mg/kg body wt per d for 7 d as previously reported,21 and urine was collected before and after the treatment. To determine salt intake, we simultaneously gave mice tap water and 0.3 M NaCl saline in separate burettes. In some experiments, we placed mice on a Na+-, K+-, and Cl−-deficient diet (TD.08251; Harlan Teklad, Madison, WI) for 2 wk before determining their water drinking and urinary volume and salt output. Mice were killed by exsanguination to obtain the plasma, and the kidney and brain were immediately harvested for protein and RNA extraction or for histology analyses. Mouse food intake was determined by averaging three consecutive measurements, with each measurement lasting for 2 to 3 d, and the food intake was normalized to body weight per day. The animal studies were approved by the Institutional Animal Care and Use Committee at the University of Chicago.
Plasma Renin Activity and AngII Concentration
Plasma renin activity (PRA) was determined using a commercial kit from DiaSorin (Stillwater, MN). Briefly, first we determined the renin activity by measuring the conversion of angiotensinogen to angiotensin I, using plasma from bilaterally nephrectomized rats as the source for angiotensinogen substrate as described previously,34 then the amount of AngI generated was determined by RIA. Plasma AngII levels were determined using a commercial AngII RIA kit (Phoenix Pharmaceuticals, Mountain View, CA) as described previously.7
Urinary Osmolality and Salt Concentrations
Urinary osmolality was measured using a 5500 Vapor Pressure Osmometer (Wescor, Logan, UT). Urinary Na+, K+, Cl−, and creatinine concentrations were determined using a Beckman CX5 Autoanalyzer as described previously.21
Immunohistochemistry
Mice were perfused with 4% paraformaldehyde made in PBS (pH 7.2). we isolated the whole brain intact by carefully removing the bone. The brain was placed in an 1-mm Mouse Brain Slicer (Zivic Instruments, Pittsburgh, PA) and cut with a razor blade at −1 and −4 mm from the bregma. The portion between −1 and −4 mm was placed in OCT and frozen in −80°C or used for Western blot as described in the next section. Brain frozen sections (40 μm) were cut using a cryostat. Before staining, the brain sections were washed three times with 0.1 M phosphate buffer (pH 7.3) containing 0.1% Triton X100 (PBTX), incubated in PBTX containing 0.1 M glycine for 30 min, followed by PBTX-0.5% H2O2 for 10 min, and then blocked with PBTX-5% FBS for 1 h. The sections were incubated with 1:1000 anti–c-Fos antibody (1:1000) or renin antiserum (1:6000) at 4°C overnight. The sections were then stained with horseradish peroxidase–conjugated or fluorescein-conjugated second antibody and the antigen was visualized with a peroxidase substrate diaminobenzidine kit (Vector Laboratories, Burlingame, CA)21 or with a fluorescence microscope.
Western Blot
The kidney, intestinal mucosa, and brain tissues were homogenized in Laemmli buffer (Boston Bioproducts, Worcester, MA), followed by 5 min of boiling. Protein concentrations were determined using a BioRad Protein Assay kit (BioRad, Hercules, CA). Proteins were separated by SDS-PAGE and transferred onto Immobilon membranes. Western blotting was carried out as described previously35 using antibody against renin, c-Fos, NHE-2, or NHE-3.
Northern Blot
Total cellular RNA were isolated using TRIzol reagents (Invitrogen, Grand Island, NY). The RNA were separated on 0.8% agarose gels containing 0.6 M formaldehyde, and Northern blotting was carried out as described previously.7
RT-PCR and Real-Time RT-PCR
First-strand cDNA were synthesized from 2 μg of total RNA in 20-μl reaction using MML-V reverse transcriptase (Invitrogen, Carlsbad, CA) and hexanucleotide random primers. The first-strand cDNA served as the template for the PCR, which was carried out using a BioRad DNA Engine. The PCR primers used in this study are listed in Table 1. Glyceraldehyde-3-phosphate dehydrogenase and β-2 microglobulin served as the internal controls. Real-time RT-PCR was performed in Applied Biosystems 7900 Real-Time PCR System using a SYBR green PCR reagent kit (Applied Biosystems, Foster City, CA) as described previously.36
Table 1.
Primers used in RT-PCR amplificationa
| Gene | Primer Nucleotide Sequences |
|---|---|
| AQP-1 | Forward 5′-TGT ACA TCA TCG CCC AGT GT-3′ |
| Reverse 5′-TGC AGA GTG CCA ATG ATC TC-3′ | |
| AQP-2 | Forward 5′-CAT CAA CCC TGC TGT GAC TG-3′ |
| Reverse 5′-GGT CAG GAA GAG CTC CAC AG-3′ | |
| AQP-3 | Forward 5′-GGC TTC TTT GAT CAG TTC AT-3′ |
| Reverse 5′-AGT CGT GAA GAC TTC TGA GC-3′ | |
| AQP-4 | Forward 5′-AGC AAT TGG ATT TTC CGT TG-3′ |
| Reverse 5′-TGA GCT CCA CAT CAG GAC AG-3′ | |
| AQP-6 | Forward 5′-GTG GTC CAC AAC AGC ACA TC-3′ |
| Reverse 5′-AGA TCC AAT GGA CTG CGA AC-3′ | |
| AQP-7 | Forward 5′-CTT CAG GTC CAC CCA CAA CT-3′ |
| Reverse 5′-TAC ATG GAC TCC CAT GGT CA-3′ | |
| AQP-11 | Forward 5′-AGC CTC ACA GGA GCA TTG TT-3′ |
| Reverse 5′-GTT CGA GTC TTT GGG AGT GG-3′ | |
| Renin | Forward 5′-GAG GCC TTC CTT GAC CAA TC-3′ |
| Reverse 5′-TGT GAA TCC CAC AAG CAA GG-3′ | |
| Angiotensinogen | Forward 5′-ACG TTC ACT TCC AAG GAA CGA-3′ |
| Reverse 5′-TCA CTC CAG TGC TGG AAG TT-3′ | |
| ACE | Forward 5′-CCC ATC TGC TAG GGA ACA TGT-3′ |
| Reverse 5′-GGT GTC CAT CCC TGC TTT ATC A-3′ | |
| AT1R | Forward 5′-CTG CTC TCC CGG ACT TAA CA-3′ |
| Reverse 5′-TGG GGC AGT CAT CTT GAA TTC T-3′ | |
| GAPDH | Forward 5′-ACA CAT TGG GGG TAG GAA CAC-3′ |
| Reverse 5′-CAA CTT TGG CAT TGT GGA AGG-3′ | |
| β2 microglobulin | Forward 5′-ACC GGC CTG TAT GCT ATC CAG AAA-3′ |
| Reverse 5′-ATT TCA ATG TGA GGC GGG TGG AAC-3′ |
All primers were designed according to the cDNA sequence of each gene deposited in the GenBank database. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Statistical Analysis
Data are presented as means ± SD. Data were analyzed with t test and ANOVA to assess significance. P ≤ 0.05 was considered statistically significance.
DISCLOSURES
None.
Acknowledgments
This work was supported by National Institutes of Health grants R01HL085793 and R21DK073183 (to Y.C.L.).
We thank Dr. Eugene Chang (University of Chicago) for kindly providing the anti-NHE2 and anti-NHE3 antibodies and Dr. Yunmin Ding (University of Chicago) for expert assistance in brain immunostaining.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Valenti G, Procino G, Tamma G, Carmosino M, Svelto M: Minireview: Aquaporin 2 trafficking. Endocrinology 146: 5063–5070, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Inagami T: The renin-angiotensin system. Essays Biochem 28: 147–164, 1994 [PubMed] [Google Scholar]
- 3.Antunes-Rodrigues J, de Castro M, Elias LL, Valenca MM, McCann SM: Neuroendocrine control of body fluid metabolism. Physiol Rev 84: 169–208, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Levens NR: Control of intestinal absorption by the renin-angiotensin system. Am J Physiol 249: G3–G15, 1985 [DOI] [PubMed] [Google Scholar]
- 5.Scheinman SJ, Guay-Woodford LM, Thakker RV, Warnock DG: Genetic disorders of renal electrolyte transport. N Engl J Med 340: 1177–1187, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Brenner BM, Ballermann BJ, Gunning ME, Zeidel ML: Diverse biological actions of atrial natriuretic peptide. Physiol Rev 70: 665–699, 1990 [DOI] [PubMed] [Google Scholar]
- 7.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP: 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 110: 229–238, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan W, Pan W, Kong J, Zheng W, Szeto FL, Wong KE, Cohen R, Klopot A, Zhang Z, Li YC: 1,25-Dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter. J Biol Chem 282: 29821–29830, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Fitzsimons JT: Angiotensin, thirst, and sodium appetite. Physiol Rev 78: 583–686, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Bryant RW, Epstein AN, Fitzsimons JT, Fluherty SJ: Arousal of a specific and persistent sodium appetite in the rat with continuous intracerebroventricular infusion of angiotensin II. J Physiol (Lond) 301: 365–382, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buggy J, Fisher AE: Evidence for a dual central role for angiotensin in water and sodium intake. Nature 250: 733–735, 1974 [DOI] [PubMed] [Google Scholar]
- 12.Epstein AN, Fitzsimons JT, Rolls BJ: Drinking induced by injection of angiotensin into the rain of the rat. J Physiol 210: 457–474, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bookstein C, DePaoli AM, Xie Y, Niu P, Musch MW, Rao MC, Chang EB: Na+/H+ exchangers, NHE-1 and NHE-3, of rat intestine expression and localization. J Clin Invest 93: 106–113, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sunn N, Egli M, Burazin TC, Burns P, Colvill L, Davern P, Denton DA, Oldfield BJ, Weisinger RS, Rauch M, Schmid HA, McKinley MJ: Circulating relaxin acts on subfornical organ neurons to stimulate water drinking in the rat. Proc Natl Acad Sci U S A 99: 1701–1706, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simpson JB, Routtenberg A: Subfornical organ: Site of drinking elicitation by angiotensin II. Science 181: 1172–1175, 1973 [DOI] [PubMed] [Google Scholar]
- 16.Simpson JB, Routtenberg A: Subfornical organ: A dipsogenic site of action of angiotensin II. Science 201: 379–381, 1978 [DOI] [PubMed] [Google Scholar]
- 17.Paul M, Poyan Mehr A, Kreutz R: Physiology of local renin-angiotensin systems. Physiol Rev 86: 747–803, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Dzau VJ, Ingelfinger J, Pratt RE, Ellison KE: Identification of renin and angiotensinogen messenger RNA sequences in mouse and rat brains. Hypertension 8: 544–548, 1986 [DOI] [PubMed] [Google Scholar]
- 19.Schelling P, Meyer D, Loos HE, Speck G, Phillips MI, Johnson AK, Ganten D: A micromethod for the measurement of renin in brain nuclei: its application in spontaneously hypertensive rats. Neuropharmacology 21: 455–463, 1982 [DOI] [PubMed] [Google Scholar]
- 20.Ekker M, Tronik D, Rougeon F: Extra-renal transcription of the renin genes in multiple tissues of mice and rats. Proc Natl Acad Sci U S A 86: 5155–5158, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kong J, Li YC: Effect of angiotensin II type I receptor antagonist and angiotensin-converting enzyme inhibitor on vitamin D receptor null mice. Am J Physiol Regul Integr Comp Physiol 285: R255–R261, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Mendelsohn FA, Quirion R, Saavedra JM, Aguilera G, Catt KJ: Autoradiographic localization of angiotensin II receptors in rat brain. Proc Natl Acad Sci U S A 81: 1575–1579, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson AK, Thunhorst RL: The neuroendocrinology of thirst and salt appetite: Visceral sensory signals and mechanisms of central integration. Front Neuroendocrinol 18: 292–353, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Pickel VM, Chan J, Ganten D: Dual peroxidase and colloidal gold-labeling study of angiotensin converting enzyme and angiotensin-like immunoreactivity in the rat subfornical organ. J Neurosci 6: 2457–2469, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakai K, Agassandian K, Morimoto S, Sinnayah P, Cassell MD, Davisson RL, Sigmund CD: Local production of angiotensin II in the subfornical organ causes elevated drinking. J Clin Invest 117: 1088–1095, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oldfield BJ, McKinley MJ: Distribution of Fos in rat brain resulting from endogenously-generated angiotensin II. Kidney Int 46: 1567–1569, 1994 [DOI] [PubMed] [Google Scholar]
- 27.Tada M, Fukamizu A, Seo MS, Takahashi S, Murakami K: Renin expression in the kidney and brain is reciprocally controlled by captopril. Biochem Biophys Res Commun 159: 1065–1071, 1989 [DOI] [PubMed] [Google Scholar]
- 28.Iwai N, Inagami T: Quantitative analysis of renin gene expression in extrarenal tissues by polymerase chain reaction method. J Hypertens 10: 717–724, 1992 [PubMed] [Google Scholar]
- 29.Li Z, Bains JS, Ferguson AV: Functional evidence that the angiotensin antagonist losartan crosses the blood-brain barrier in the rat. Brain Res Bull 30: 33–39, 1993 [DOI] [PubMed] [Google Scholar]
- 30.Polidori C, Ciccocioppo R, Nisato D, Cazaubon C, Massi M: Evaluation of the ability of irbesartan to cross the blood-brain barrier following acute intragastric treatment. Eur J Pharmacol 352: 15–21, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Levens NR, Peach MJ, Carey RM: Interactions between angiotensin peptides and the sympathetic nervous system mediating intestinal sodium and water absorption in the rat. J Clin Invest 67: 1197–1207, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li XC, Zhuo JL: Selective knockdown of AT1 receptors by RNA interference inhibits Val5-ANG II endocytosis and NHE-3 expression in immortalized rabbit proximal tubule cells. Am J Physiol Cell Physiol 293: C367–C378, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R, Demay MB: Targeted ablation of the vitamin D receptor: An animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci U S A 94: 9831–9835, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurtz A, Pfeilschifter J, Hutter A, Buhrle C, Nobiling R, Taugner R, Hackenthal E, Bauer C: Role of protein kinase C in inhibition of renin release caused by vasoconstrictors. Am J Physiol 250: C563–C571, 1986 [DOI] [PubMed] [Google Scholar]
- 35.Li YC, Bolt MJ, Cao L-P, Sitrin MD: Effects of vitamin D receptor inactivation on the expression of calbindins and calcium metabolism. Am J Physiol Endocrinol Metab 281: E558–E564, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Zhang Z, Sun L, Wang Y, Ning G, Minto AW, Kong J, Quigg RJ, Li YC: Renoprotective role of the vitamin D receptor in diabetic nephropathy. Kidney Int 73: 163–171, 2008 [DOI] [PubMed] [Google Scholar]