Abstract
Calcineurin inhibitors (CNI) are used to prevent inflammatory diseases and allograft rejection. However, little is known about the mechanism(s) underlying their ability to promote the development and recurrence of cancer. Recent studies suggested that the chemokine receptor CXCR3 may play important roles in tumorigenesis. CXCR3 has two splice variants with opposite functions: CXCR3-A promotes cell proliferation, and CXCR3-B inhibits cell growth. Here, we explored the effects of CNI on the expression and function of CXCR3 splice variants. Compared with normal renal tissues and renal epithelial cells, human renal cancer tissues and renal cancer cell lines demonstrated higher expression of CXCR3-A and markedly lower expression of CXCR3-B. In human renal cancer cells (786-0 and Caki-1) and renal epithelial cells, CNI markedly downregulated the expression of CXCR3-B, whereas expression of CXCR3-A was unchanged. This CNI-mediated downregulation of CXCR3-B resulted in increased proliferation and migration of renal cancer cells; CNI-mediated cell proliferation involved signaling through Gi proteins, perhaps via CXCR3-A. Finally, it was observed that CNI treatment increased the growth of human renal tumors in vivo, and the expression of CXCR3-B was significantly decreased in these tumors. In summary, these observations suggest that CNI may mediate the progression of human renal cancer by downregulating CXCR3-B and by promoting proliferative signals, likely through CXCR3-A. Targeting CXCR3 splice variants or the signaling pathways downstream of CXCR3 receptors may provide a therapeutic strategy for the prevention of CNI-mediated renal cancer progression.
Calcineurin is a serine/threonine phosphatase that is activated by cellular calcium.1,2 In T cells, it has been demonstrated that calcineurin activity is necessary for the expression of several cytokines through the dephosphorylation of a family of transcription factors known as nuclear factor of activated T cells (NFAT).3–5 A major function of calcineurin inhibitors (CNI) is to prevent the activation of NFAT and thereby to suppress T cell activation.6 This immunosuppressive activity of CNI established their use in the treatment of a variety of inflammatory diseases.7,8 In the field of organ transplantation, the therapeutic introduction of these inhibitors revolutionized the treatment and prevention of acute allograft rejection.7,9
Nevertheless, recent studies clearly identified a major association between the use of CNI and the development and recurrence of different types of cancer in both transplant and nontransplant patients.10–14 The immunosuppressive effect of CNI may result in impaired immune surveillance for neoplastic cells15,16 and may increase susceptibility to oncogenic viral infections.12,17 CNI may also directly cause DNA damage to cells and interfere with normal DNA repair mechanisms18; however, independent of these effects, it has been found that commonly used CNI cyclosporine (CsA) and FK506 may promote tumor growth through the induced expression of different genes, including TGF-β, IL-10, and angiogenic cytokines (e.g., vascular endothelial growth factor).10,19–23 Thus, understanding the mechanism of CNI-mediated tumor development may allow a safer and effective use of these inhibitors in patients.
Chemokines are small, cytokine-like, secreted proteins (8 to 11 kD) that have chemoattractant properties and are well established to function in the recruitment of leukocytes into inflamed tissues.24–26 The biologic effects of chemokines are mediated through specific G-protein–coupled receptors, primarily expressed on leukocytes, but also on nonlymphoid cells, including endothelial and epithelial cells.27,28 The interaction between chemokines and their receptors has been found to be important in various acute and chronic inflammatory processes27,29; however, over the past few years, it has been demonstrated that chemokines and their receptors may also be functional in tumor development.28,30,31
The chemokine CXCL10 (also known as IP-10) is a T cell chemoattractant and is classically thought to have tumor inhibitory properties via its effects to elicit T cell–dependent mechanisms of tumor destruction as well as its effects to inhibit tumor angiogenesis.32 Nevertheless, paradoxically, some recent reports have indicated that CXCL10 can also promote tumor growth.33–36 This led to a controversy whether this chemokine may function as an anti- or protumorigenic agent. We recently reported that this controversy in the function of CXCL10 is in part related to alternative splicing of its receptor (CXCR3).28 CXCR3 exists as two novel variants called CXCR3-A and CXCR3-B, each of which mediates different intracellular signals, and has select functions37–39: CXCR3-A promotes chemotaxis and cell proliferation, whereas CXCR3-B signals for growth inhibition.28,37,40 Therefore, any changes in the relative balance in the expression of CXCR3 splice variants may play a critical role in regulating different cellular functions in response to CXCR3-A/B-binding chemokines.
In this study, we show that CNI can downregulate the expression of the growth-inhibitory CXCR3-B receptor in human renal cancer cells, without altering growth-promoting CXCR3-A. In the absence of CXCR3-B, renal cancer cells undergo increased proliferation and migration. These results suggest a mechanism underlying the association between CNI use in transplant patients and the progression of human renal cancer.
RESULTS
Expression Profiles of CXCR3-A and CXCR3-B in Human Renal Tumor Tissues and in Renal Cancer Cell Lines
We first analyzed the expression profiles of CXCR3-A and CXCR3-B in human renal cell carcinoma (RCC) tissues by real-time PCR using gene-specific primer–probe sets. We examined a total of 12 RCC tissues (six low-stage and six high-stage tumors), compared with normal renal tissues. We observed that the expression of the growth-promoting CXCR3-A was markedly higher (approximately 5- to 170-fold increase) in all tumors as compared with normal renal tissues (Figure 1A). In contrast, the expression of the growth-inhibitory CXCR3-B in renal tumor tissues (both low- and high-stage) was consistently at lower levels of expression (approximately 25 to 90% decrease) than those observed in normal renal tissues (Figure 1B).
Figure 1.
Differential expression of CXCR3-A and CXCR3-B in human renal cancer tissues. (A and B) Total RNA was isolated from renal cancer and normal renal tissues and reverse-transcribed. Either the fold increase of CXCR3-A (A) or the percentage decrease of CXCR3-B (B) mRNA expression in renal cancer tissues versus the mean level of expression of each gene in normal renal tissues (n = 4) was measured by real-time PCR. S1 through S6 represent low-stage (Robson stages I and II), whereas S7 through S12 represent high-stage (Robson stages III and IV) renal tumor tissues. Columns are the average of triplicate readings of the sample; bars are ±SD.
We next examined the expression profiles of the CXCR3 splice variants in two well-established human renal cancer cell lines (786-0 and Caki-1) compared with normal renal tubular epithelial cells (REC). We observed that CXCR3-A was markedly higher and CXCR3-B was lower in both cancer cell lines as compared with normal REC (Figure 2, A and B).
Figure 2.
Expression pattern of CXCR3 splice variants and CXCR3-binding ligands in human renal cancer and renal epithelial cell lines. (A through C) Total RNA was isolated from REC, 786-0, and Caki-1 cells and reverse-transcribed. Fold changes in mRNA expression of CXCR3-A (A), CXCR3-B (B), or CXCR3-binding ligands (C) was measured by real-time PCR. Data reflect three independent experiments. Columns are average of triplicate readings of the sample; bars are ±SD. In A and B, **P < 0.01 versus REC; in C, **P < 0.01 and *P < 0.05 versus REC.
We also examined the expression of known CXCR3-binding chemokines (CXCL9, CXCL10, CXCL11, and CXCL4)38 in 786-0 and Caki-1 cell lines compared with normal REC. It is known that CXCL9, CXCL10, and CXCL11 interact with both CXCR3-A and CXCR3-B splice variants; however, CXCL4 binds selectively to the growth-inhibitory CXCR3-B.37,38 Whereas CXCL9 was very low in expression (data not shown), we found that CXCL10 and CXCL11 were significantly higher in both 786-0 and Caki-1 cells compared with REC (Figure 2C). In contrast, the expression of CXCL4 was markedly lower in 786-0 cells (Figure 2C). Together, these observations demonstrate that there are completely different expression patterns of CXCR3 splice variants and CXCR3-binding ligands in human renal cancer cells, compared with normal REC.
CNI Downregulate the Expression of CXCR3-B in Human Normal REC and Renal Cancer Cells
We next wished to evaluate the effect of CNI on the expression of CXCR3-A and CXCR3-B in normal REC and in renal cancer cells (786-0 and Caki-1). The cells were treated with either CsA (0.1 and 1.0 μg/ml) or FK506 (0.01 and 0.10 μg/ml) or vehicle alone, and gene expression was measured by real-time PCR. As shown in Figure 3, A and B, both CsA and FK506 significantly downregulated the growth-inhibitory CXCR3-B (P < 0.05) in all cell lines as compared with vehicle-treated controls; however, there was no significant change in the expression of growth-promoting CXCR3-A in these cells after CNI treatment (Figure 3C). These observations suggest that CNI may differentially regulate CXCR3 splice variants and may have an impact on renal cancer progression.
Figure 3.
CNI downregulate the expression of CXCR3-B in human renal cancer cells. (A through C) REC, 786-0, and Caki-1 cells were treated with CsA (0.1 and 1.0 μg/ml), FK506 (0.01 and 0.10 μg/ml), or vehicle alone and incubated for 18 h. Total RNA was isolated from these cells and reverse-transcribed. Fold changes in mRNA expression of CXCR3-B in response to CsA (A) or FK506 (B) was measured by real-time PCR. Fold changes in mRNA expression of CXCR3-A in response to CsA was also measured by real-time PCR (C). Data reflect three independent experiments. Columns are average of triplicate readings of two different samples; bars are ±SD. In A and B, *P < 0.05 versus vehicle-treated cells.
Downregulation of CXCR3-B Is Associated with Increased Signals for Renal Cancer Cell Proliferation
We next questioned whether downregulation of growth-inhibitory CXCR3-B expression would result in a reciprocal increase in renal cancer cell proliferation. We transfected Caki-1 and 786-0 cells with CXCR3-B–specific small interfering RNA (siRNA), which selectively inhibited CXCR3-B mRNA expression; however, the efficiency of knockdown was greater in Caki-1 cells (approximately 80% knockdown; Supplemental Figure 1) versus 786-0 cells (approximately 50% knockdown, data not shown). Thus, for our siRNA transfection experiments, we used Caki-1 cells. As shown in Figure 4A, transfection of Caki-1 cells with CXCR3-B siRNA (25 and 50 nM) resulted in a marked increase in cell proliferation, as compared with control siRNA-transfected cells. Next, we transfected Caki-1 cells with a CXCR3-B overexpression plasmid, which resulted in an approximately 250-fold increase in the gene expression in these cells (Supplemental Figure 2). We found that the overexpression of CXCR3-B resulted in a decrease in cancer cell proliferation (Figure 4B). Similar results were obtained in 786-0 cells (data not shown). Together, these results suggest that CXCR3-B mediates a growth-inhibitory signal in renal cancer cells, and the inhibition of CXCR3-B expression may withdraw this inhibitory signal to promote cell proliferation.
Figure 4.
Inhibition of CXCR3-B expression is associated with increased proliferation of human renal cancer cells. (A) Caki-1 cells were transfected with either the control or the CXCR3-B siRNA (25 and 50 nM). (B) Caki-1 cells were transfected with either CXCR3-B overexpression plasmid (1.0 and 1.5 μg/ml) or the empty expression vector. All of the cells in A and B were incubated for 72 h and subjected to cell proliferation assay by measuring [3H]thymidine incorporation within the cells as described in the Concise Methods section. Data reflect three independent experiments. Columns are average of triplicate readings (cpm) of the samples; bars are ±SD. In A and B, *P < 0.05 versus either control siRNA–or empty vector–transfected cells.
CNI-Mediated Downregulation of CXCR3-B in Renal Cancer Cells Promotes Cell Proliferation and Migration
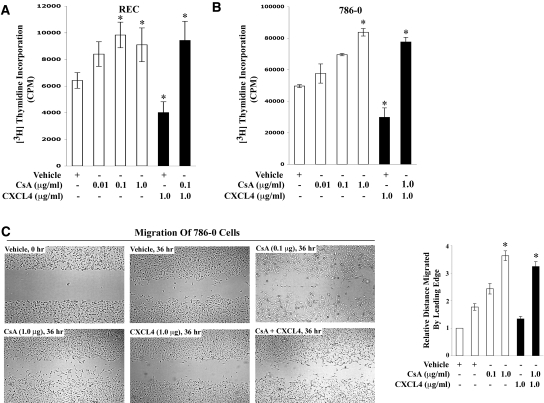
We next tested whether inhibition of CXCR3-B expression by CNI can induce renal cancer cell proliferation and migration. Normal REC or 786-0 cells were treated with the increasing concentrations (0.1 to 1.0 μg/ml) of either CsA or FK506 or vehicle alone, and a cell proliferation assay was performed. As shown in Figure 5, A and B, CsA treatment markedly induced cell proliferation in both REC and 786-0 cells, compared with vehicle-treated controls. Similar results were obtained using FK506 (data not shown). To examine further the role of CXCR3-B–mediated signaling in renal cancer cell proliferation, we made use of the ligand CXCL4, known to interact specifically with CXCR3-B.37,38 We observed that treatment of REC and 786-0 cells with CXCL4 markedly suppressed the proliferation of both cell types; however, CXCL4 did not inhibit CsA-mediated cell proliferation (Figure 5, A and B). These results suggest that CNI may promote cancer cell proliferation by downregulating CXCR3-B expression (as observed in Figure 3, A and B), and in absence of CXCR3-B, CXCL4 cannot inhibit CsA-mediated renal cancer cell proliferation.
Figure 5.
CNI-mediated downregulation of CXCR3-B is associated with increased proliferation and migration of human renal cancer cells. (A and B) REC and 786-0 cells were treated with either different concentrations (0.01 to 1.00 μg/ml) of CsA or vehicle alone for 72 h. Some cells from both CsA-treated and the vehicle-treated group were incubated with CXCL4 (1.0 μg/ml) in the last 24 h. All of the cells in A and B were subjected to cell proliferation assay by measuring [3H]thymidine incorporation within the cells as described in the Concise Methods section. Data reflect three independent experiments. Columns are average of triplicate readings (cpm) of the samples; bars are ±SD. (C) The effects of CsA and CXCL4 on renal cancer cell migration were tested by in vitro wound-healing assay, as described in the Concise Methods section. Confluent monolayers of 786-0 cells were scarred, and repair was monitored microscopically after 36 h of treatment with CsA (0.1 and 1.0 μg/ml), or CXCL4 (1.0 μg/ml), CsA (1.0 μg/ml) + CXCL4 (1.0 μg/ml), or vehicle alone. The right panel represents the quantification of distance migrated by the cells in 36 h relative to vehicle-treated control cells in 0 h. Representative and average readings of three independent experiments; bars are ±SD. In *P < 0.05 versus vehicle-treated cells.
Next, we evaluated whether CNI could promote the migration of renal cancer cells by altering the expression of CXCR3 splice variants. We treated 786-0 cells with CsA in the absence or presence of the CXCR3-B–specific ligand CXCL4 and then performed an in vitro wound-healing assay. As shown in Figure 5C, CsA treatment (0.1 and 1.0 μg/ml) induced the migration of renal cancer cells, compared with the vehicle-treated controls. In contrast, CXCL4 markedly inhibited cell migration, compared with the vehicle-treated controls; however, CXCL4 did not inhibit migration of cells treated with CsA (Figure 5C). These results again support our previous observation and suggest that CsA may promote renal cancer cell migration by inhibiting CXCR3-B; and in the absence of CXCR3-B, CXCL4 cannot inhibit CsA-mediated cell migration.
CNI-Mediated Renal Cancer Cell Proliferation Involves Gi Proteins
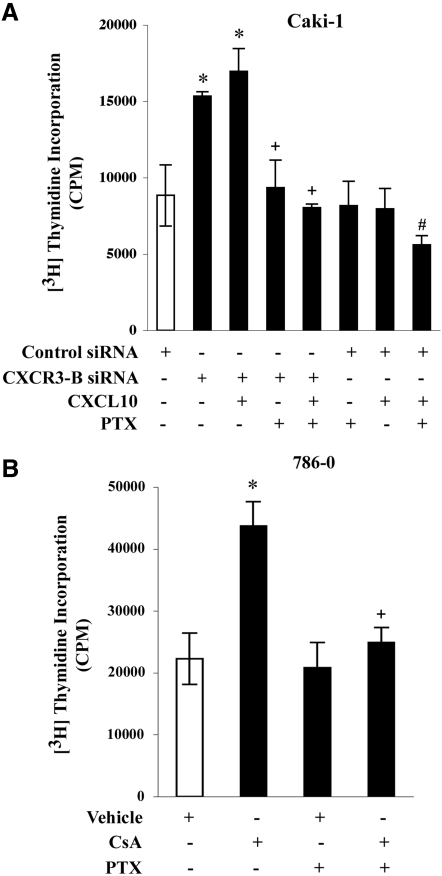
CXCR3-A–mediated signaling involves Gi proteins.28,37,40 Here, we first examined whether inhibition of CXCR3-B expression in renal cancer cells could promote cell proliferation via Gi protein–mediated responses. We transfected Caki-1 cells with either a control or CXCR3-B–specific siRNA and performed a cell proliferation assay in the absence or presence of the Gi protein inhibitor pertussis toxin (PTX). As shown in Figure 6A, the knockdown of CXCR3-B in these cells increased cell proliferation as compared with control siRNA-transfected cells, and in the presence of the CXCR3-A/B-binding ligand CXCL10, this proliferation was minimally increased; however, in the presence of PTX, the increased cell proliferation in CXCR3-B siRNA-transfected cells was significantly inhibited (Figure 6A). This suggests a role for Gi proteins in this proliferation process, likely involving CXCR3-A.
Figure 6.
CNI-mediated proliferation of renal cancer cells involves Gi proteins. (A) Caki-1 cells were transfected with either the CXCR3-B or control siRNA (50 nM) for 48 h, incubated with PTX (1.0 μg/ml)/vehicle for 16 h, and then either untreated or treated with CXCL10 (20 ng/ml) for 24 h. (B) 786-0 cells were incubated with PTX (1.0 μg/ml)/vehicle for 16 h and then treated with either CsA (1.0 μg/ml) or vehicle alone for 72 h. All of the cells in A and B were subjected to cell proliferation assay by measuring [3H]thymidine incorporation within the cells as described in the Concise Methods section. Data reflect three independent experiments. Columns are average of triplicate readings (cpm) of the samples; bars are ±SD. In A and B, *P < 0.05 versus either control siRNA-transfected or vehicle-treated cells; in A, +P < 0.05 versus CXCR3-B siRNA-transfected but PTX-untreated cells and #P < 0.05 versus control siRNA-transfected untreated cells; in B, +P < 0.05 versus CsA-treated but PTX-untreated cells.
Furthermore, when we treated the control siRNA-transfected Caki-1 cells (expressing both CXCR3-A and CXCR3-B) with CXCL10, there was no significant change in cell proliferation, compared with untreated controls (Figure 6A); however, when we treated the cells with PTX to inhibit CXCR3-A–mediated responses, we found that CXCL10 significantly inhibited cancer cell proliferation (Figure 6A). We suggest that this decreased cell proliferation is probably mediated via unopposed growth-inhibitory CXCR3-B, which does not involve Gi proteins.28,37,40
Next, we analyzed the role of Gi proteins in CNI-mediated renal cancer cell proliferation. We treated the 786-0 cells with CsA in the absence or the presence of PTX, and we performed a cell proliferation assay. We observed that CsA treatment promoted cell proliferation as compared with vehicle-treated controls, and CsA-mediated cell proliferation was significantly inhibited by PTX (Figure 6B). Thus, CNI may induce a Gi protein–dependent signaling mechanism, which may promote cell proliferation in the absence of CXCR3-B, and likely involves CXCR3-A.
CNI Promotes the Growth of Human Renal Tumors In Vivo and Downregulates the Expression of CXCR3-B in Tumor Tissues
We next examined whether CsA can promote human renal tumor growth in vivo, and we evaluated the expression profiles of CXCR3-A and CXCR3-B in these tumor tissues. Human renal cancer cells were injected subcutaneously into immunodeficient (nu/nu) mice, and the mice were treated either with CsA (10 mg/kg per d) or with vehicle alone for 30 d. We observed that CsA treatment markedly enhanced tumor volume compared with vehicle-treated controls (Figure 7A). Tumors were harvested on day 30, and we evaluated them for the expression of CXCR3-A and CXCR3-B. As shown in Figure 7B, CsA treatment significantly decreased the expression of CXCR3-B in the tumor compared with vehicle-treated controls. In contrast, there was no significant change in CXCR3-A expression in the tumor after CsA treatment. Together, these in vivo observations support our in vitro findings and suggest that in the presence of CNI, there may be an increased protumorigenic signal through unopposed CXCR3-A, allowing an accelerated progression of existing tumors.
Figure 7.
CNI CsA promotes the growth of human renal tumors in vivo with decreased expression of CXCR3-B in tumor tissues. (A) Human renal cancer cells (786-0; 1.0 × 106) were injected subcutaneously into immunodeficient (nu/nu) mice, which (n = 8 in each group) were treated either with CsA (10 mg/kg per d) or with vehicle. Treatment was continued for 30 d, and tumor volume was monitored at regular intervals using a digital caliper. Tumors were harvested 30 d after tumor injection. The data reflect three independent experiments. Columns are average value of tumor volume; bars are ±SD. *P < 0.05 versus vehicle-treated controls. (B) Total RNA was isolated from harvested tumor tissues (day 30) and reverse-transcribed. Fold change in CXCR3-B and CXCR3-A mRNA expression was measured by real-time PCR. Data reflect three independent experiments resulting from duplicate readings of three different samples. Columns are average value of CXCR3-B and CXCR3-A mRNA expression, bars are ±SD. *P < 0.05 versus vehicle-treated cells.
DISCUSSION
In this study, we show that CNI can promote the progression of human renal cancer by downregulating the expression of the growth-inhibitory receptor CXCR3-B in cancer cells, which leads to increased cell proliferation and migration. It is now established that immunosuppressed individuals, including patients with an organ transplant, are at increased risk for either de novo cancer development or recurrence/progression of cancer.12,13,15 Furthermore, cancers that develop in transplant recipients are often more aggressive than those in the general population.41 CNI, both CsA and FK506, are thought to play a critical role in the development/recurrence of cancer in immunosuppressed individuals. Apart from mediating immune escape of tumor cells, CNI may promote cancer through direct cellular effects involving different cytokines10,19–23 or by a reduction in p53-induced apoptosis42; however, the mechanism by which CNI promotes cancer is still unclear.
The chemokine receptor CXCR3 and its ligands are important players in the development and progression of human tumors by facilitating migration and proliferation of malignant cells.33–36,43,44 In this study, we showed that CXCR3 and its ligands are overexpressed in human renal cancer, one of the major cancers after solid organ transplantation.18,45 The two splice variants of CXCR3 mediate distinctly different signals and have completely opposite functions in tumor cells as well as in a variety of normal cell types, such as lymphocytes, endothelial cells, and airway epithelial cells28,37,39,40; CXCR3-A promotes chemotaxis and cell proliferation, whereas CXCR3-B mediates growth inhibition.28,37,40 Thus, it is likely that signaling through CXCR3-B in renal cancer cells may inhibit cell growth. We have observed that in the presence of CXCL4, the specific ligand for CXCR3-B,37 renal cancer cells undergo reduced proliferation; however, when the cancer cells are treated with CNI (CsA or FK506), the expression of CXCR3-B (but not CXCR3-A) is downregulated, which results in increased cell proliferation and migration. CNI-mediated downregulation of CXCR3-B may involve a factor(s) regulating mRNA splicing or mRNA stability or possibly could involve other novel signaling mechanisms that need to be investigated.
In a recent report, Petrai et al.46 showed that the growth-inhibitory signal through CXCR3-B is mediated by the p38 mitogen-activated protein kinase pathway. Although CXCR3-B is expressed at low levels in tumor tissues as compared with normal tissues, CXCR3-B–mediated negative signals may have a predominant effect over the growth-promoting positive signals through CXCR3-A and may thus prevent tumor progression. We suggest that with further reduction in the levels of CXCR3-B, these negative signals for growth inhibition are withdrawn, which may facilitate an accelerated progression of existing tumors through the interaction of CXCR3-A and its ligands.
It is established that CXCR3-A and CXCR3-B may involve differential G protein coupling.28,37,40 Our findings indicate that treatment with CNI inhibits CXCR3-B and induces Gi protein–mediated signals promoting renal cancer cell proliferation, likely through CXCR3-A. In addition, we suggest that in the absence of CXCR3-B, the CXCR3-binding ligands (CXCL9, CXCL10, and CXCL11) that are found to be overexpressed in renal cancer cell lines and renal cancer tissues (data not shown) can interact in an autocrine manner with the growth-promoting CXCR3-A with higher affinity. This may promote a positive signal for the recurrence and progression of renal cancer in CNI-treated immunosuppressed patients. We and others have shown that Gi protein–dependent and CXCR3-mediated signals can activate the phosphatidylinositol-3-kinase/Akt and extracellular signal–regulated kinase 1/2 signaling pathways to promote cell proliferation28,47; however, an alternative explanation is that there are other novel receptors that may also interact with CXCR3-binding ligands48,49 and may be involved in CNI-mediated renal cancer cell proliferation.
In summary, although the problem of cancer in immunosuppressed patients, including transplant recipients, has been documented for some time, new approaches to deal with neoplasms in these patients are difficult to implement. Treatment of metastatic renal cancer in transplant recipients is largely ineffective because of immunosuppressive therapy. Thus, a treatment regimen that monitors the risk for recurrence/progression of renal cancer in transplant patients would be of high value. Our studies clearly suggest that changes in the expression profile of CXCR3 splice variants in normal renal epithelial and renal cancer cells after CNI treatment may play a role in the progression of renal cancer likely through CXCR3-A. Furthermore, in previous studies, we observed that CXCR3-A–mediated cancer cell proliferation might involve the Akt signaling pathway.28 We suggest that a potent inhibitor of Akt-mediated signaling events, such as rapamycin, may block the growth-promoting responses through CXCR3-A. Thus, monitoring the relative expression and/or targeting CXCR3 splice variants or its signals or ligands might serve to prevent the progression of CNI-mediated renal cancer, particularly in transplant recipients.
CONCISE METHODS
Reagents
The CNI CsA (Novartis, East Hanover, NJ) and FK506 (tacrolimus; Astellas, Deerfield, IL) were purchased from the Children's Hospital Boston pharmacy. The Gi inhibitor PTX was purchased from Calbiochem (La Jolla, CA). The siRNA for CXCR3-B and its respective control were purchased from Invitrogen (Carlsbad, CA). Recombinant CXCL10 and CXCL4 (PF-4) were purchased from R&D Systems (Minneapolis, MN).
Tissue Samples
Twelve tissue samples of RCC were obtained from surgical specimens of patients who underwent surgery at the University Hospital (Würzburg, Germany). The protocol to obtain tissue samples was approved by the review board of the hospital. Tumor tissues were graded (stages I through IV) according to Robson staging system. Normal renal tissues were obtained from normal parts of the surgical specimens, and the normalcy of these tissues was confirmed by histology.
Cell Culture
The human renal cancer cell lines (786-0 and Caki-1) were obtained from American Type Culture Collection (Manassas, VA). The cells were grown in RPMI-1640 supplemented with 10% FBS (Hyclone Laboratories, Logan, UT). Human renal proximal tubular epithelial cells were purchased from Clonetics (Walkersville, MD) and were cultured in complete epithelial medium (REGM BulletKit, Walkersville, MD), as supplied, according to recommended instructions.
Plasmid
CXCR3-B overexpression plasmid (pcDNA3-CXCR3-B) was a gift from Paola Romagnani (University of Florence, Florence, Italy).46
Transfection Assays
The cells were transfected with the expression plasmids using the Effectene transfection reagent (Qiagen, Valencia, CA). The total amount of transfected plasmid DNA was normalized using a control empty expression vector. Transfection efficiency was determined by co-transfection of the β-galactosidase gene under the control of cytomegalovirus immediate early promoter and by measurement of β-galactosidase activity. The cells were transfected with the siRNA using Lipofectamine-2000 (Invitrogen).
RNA Isolation and Real-Time PCR
Total RNA was prepared using the RNeasy isolation kit (Qiagen), and cDNA was synthesized using cloned AMV first-strand synthesis kit (Invitrogen). To analyze mRNA expression, we performed real-time PCR using the Assays-on-Demand Gene Expression product (TaqMan, Mammalian Gene Collection probes) according to the manufacturer's instructions (Applied Biosystems, Foster City, CA). As an internal control, 18S mRNA was amplified and analyzed under identical conditions. Gene-specific primer–probe sets for human CXCR3-A/CXCR3-B/CXCR3-binding ligands/18S were obtained from Applied Biosystems.37 Ct value (the cycle number at which emitted fluorescence exceeded an automatically determined threshold) for gene of interest was corrected by the Ct value for 18S and expressed as ΔCt. Data were measured as fold changes of mRNA amount, which was calculated as follows: (fold changes) = 2X (where X = ΔCt for control group − ΔCt for experimental group).
Cell Proliferation Assay
Cells (5 × 103) were seeded and grown in 96-well plates. [3H]Thymidine (0.5 μCi/well) was added for the final 15 h before cell harvesting. [3H]Thymidine incorporation was measured using a microplate scintillation and luminescence counter (Perkin Elmer/Wallac, Boston, MA).
In Vitro Wound-Healing Cell Migration Assay
Cell migration assays were performed as described previously.50 Cultured cells were allowed to form a confluent monolayer in a six-well plate in the presence of 1% serum. The wound was made by scraping a conventional pipette tip across the monolayer. The cells were either vehicle-treated or treated with CsA/CXCL4 and allowed to heal for 36 h. Pictures of representative wells were taken under the microscope at 0 and 36 h after wounding. The relative distance traveled by the leading edge from 0 to 36 h was assessed using Photoshop software.
In Vivo Tumor Development
Human renal cancer cells (786-0) were injected subcutaneously in immunodeficient (nu/nu) mice. Either CsA (10 mg/kg per d) or the vehicle was then administered intraperitoneally to these mice. Tumor volume was measured using a digital caliper at regular intervals. The volume was estimated by following a standard method,21 using the formula V = Π / 6 × a2 × b, where a is the short and b is the long tumor axis. Mice were killed at designated times after injection or when complications occurred, which included signs of inactivity, cachexia, or decreased responsiveness. All animal works were approved by the animal care and use committee at Children's Hospital Boston and in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals.
Statistical Analysis
Statistical evaluation for data analysis was determined either by t test for two groups of data or by one-way ANOVA for three or more groups. Differences with P < 0.05 were considered statistically significant.
DISCLOSURES
None.
Acknowledgments
This work was supported by a John-Merrill Grant (American Society of Nephrology-American Society of Transplantation) and National Institutes of Health grant DK64182 to S.P. We wish to thank Dr. Paola Romagnani for the gift of CXCR3-B plasmid. We also thank Katiana Calzadilla for technical assistance.
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Klee CB, Crouch TH, Krinks MH: Calcineurin: A calcium- and calmodulin-binding protein of the nervous system. Proc Natl Acad Sci U S A 76: 6270–6273, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klee CB, Ren H, Wang X: Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J Biol Chem 273: 13367–13370, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Graef IA, Chen F, Chen L, Kuo A, Crabtree GR: Signals transduced by Ca(2+)/calcineurin and NFATc3/c4 pattern the developing vasculature. Cell 105: 863–875, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Crabtree GR, Olson EN: NFAT signaling: Choreographing the social lives of cells. Cell 109[Suppl]: S67–S79, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Hogan PG, Chen L, Nardone J, Rao A: Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev 17: 2205–2232, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Liu J, Farmer JD Jr, Lane WS, Friedman J, Weissman I, Schreiber SL: Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 66: 807–815, 1991 [DOI] [PubMed] [Google Scholar]
- 7.Ponticelli C: Cyclosporine: From renal transplantation to autoimmune diseases. Ann N Y Acad Sci 1051: 551–558, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Chow DK, Leong RW: The use of tacrolimus in the treatment of inflammatory bowel disease. Expert Opin Drug Saf 6: 479–485, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Kaufman DB, Shapiro R, Lucey MR, Cherikh WS, Bustami RT, Dyke DB: Immunosuppression: practice and trends. Am J Transplant 4[Suppl 9]: 38–53, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Hojo M, Morimoto T, Maluccio M, Asano T, Morimoto K, Lagman M, Shimbo T, Suthanthiran M: Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature 397: 530–534, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Guba M, Graeb C, Jauch KW, Geissler EK: Pro- and anti-cancer effects of immunosuppressive agents used in organ transplantation. Transplantation 77: 1777–1782, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Gutierrez-Dalmau A, Campistol JM: Immunosuppressive therapy and malignancy in organ transplant recipients: A systematic review. Drugs 67: 1167–1198, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Morath C, Mueller M, Goldschmidt H, Schwenger V, Opelz G, Zeier M: Malignancy in renal transplantation. J Am Soc Nephrol 15: 1582–1588, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Webster AC, Craig JC, Simpson JM, Jones MP, Chapman JR: Identifying high risk groups and quantifying absolute risk of cancer after kidney transplantation: A cohort study of 15,183 recipients. Am J Transplant 7: 2140–2151, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Bustami RT, Ojo AO, Wolfe RA, Merion RM, Bennett WM, McDiarmid SV, Leichtman AB, Held PJ, Port FK: Immunosuppression and the risk of post-transplant malignancy among cadaveric first kidney transplant recipients. Am J Transplant 4: 87–93, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Jamil B, Nicholls K, Becker GJ, Walker RG: Impact of acute rejection therapy on infections and malignancies in renal transplant recipients. Transplantation 68: 1597–1603, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Molmenti EP, Nagata DE, Roden JS, Squires RH, Molmenti H, Fasola CG, Winick N, Tomlinson G, Lopez MJ, D'Amico L, Dyer HL, Savino AC, Sanchez EQ, Levy MF, Goldstein RM, Anderson JA, Klintmalm GB: Post-transplant lymphoproliferative syndrome in the pediatric liver transplant population. Am J Transplant 1: 356–359, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Kasiske BL, Snyder JJ, Gilbertson DT, Wang C: Cancer after kidney transplantation in the United States. Am J Transplant 4: 905–913, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Maluccio M, Sharma V, Lagman M, Vyas S, Yang H, Li B, Suthanthiran M: Tacrolimus enhances transforming growth factor-beta1 expression and promotes tumor progression. Transplantation 76: 597–602, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, Bruns CJ, Zuelke C, Farkas S, Anthuber M, Jauch KW, Geissler EK: Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: Involvement of vascular endothelial growth factor. Nat Med 8: 128–135, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Koehl GE, Andrassy J, Guba M, Richter S, Kroemer A, Scherer MN, Steinbauer M, Graeb C, Schlitt HJ, Jauch KW, Geissler EK: Rapamycin protects allografts from rejection while simultaneously attacking tumors in immunosuppressed mice. Transplantation 77: 1319–1326, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Ohsawa I, Murakami T, Uemoto S, Kobayashi E: In vivo luminescent imaging of cyclosporin A-mediated cancer progression in rats. Transplantation 81: 1558–1567, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Basu A, Contreras AG, Datta D, Flynn E, Zeng L, Cohen HT, Briscoe DM, Pal S: Overexpression of vascular endothelial growth factor and the development of post-transplantation cancer. Cancer Res 68: 5689–5698, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zlotnik A, Yoshie O: Chemokines: A new classification system and their role in immunity. Immunity 12: 121–127, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Segerer S: The role of chemokines and chemokine receptors in progressive renal diseases. Am J Kidney Dis 41: S15–S18, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Balkwill F, Mantovani A: Inflammation and cancer: Back to Virchow? Lancet 357: 539–545, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Proudfoot AE: Chemokine receptors: Multifaceted therapeutic targets. Nat Rev Immunol 2: 106–115, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Datta D, Flaxenburg JA, Laxmanan S, Geehan C, Grimm M, Waaga-Gasser AM, Briscoe DM, Pal S: Ras-induced modulation of CXCL10 and its receptor splice variant CXCR3-B in MDA-MB-435 and MCF-7 cells: Relevance for the development of human breast cancer. Cancer Res 66: 9509–9518, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Viola A, Luster AD: Chemokines and their receptors: Drug targets in immunity and inflammation. Annu Rev Pharmacol Toxicol 48: 171–197, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Zlotnik A: Chemokines in neoplastic progression. Semin Cancer Biol 14: 181–185, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Strieter RM, Belperio JA, Phillips RJ, Keane MP: CXC chemokines in angiogenesis of cancer. Semin Cancer Biol 14: 195–200, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Luster AD, Leder P: IP-10, a -C-X-C- chemokine, elicits a potent thymus-dependent antitumor response in vivo. J Exp Med 178: 1057–1065, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zipin-Roitman A, Meshel T, Sagi-Assif O, Shalmon B, Avivi C, Pfeffer RM, Witz IP, Ben-Baruch A: CXCL10 promotes invasion-related properties in human colorectal carcinoma cells. Cancer Res 67: 3396–3405, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Walser TC, Rifat S, Ma X, Kundu N, Ward C, Goloubeva O, Johnson MG, Medina JC, Collins TL, Fulton AM: Antagonism of CXCR3 inhibits lung metastasis in a murine model of metastatic breast cancer. Cancer Res 66: 7701–7707, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Suyama T, Furuya M, Nishiyama M, Kasuya Y, Kimura S, Ichikawa T, Ueda T, Nikaido T, Ito H, Ishikura H: Up-regulation of the interferon gamma (IFN-gamma)-inducible chemokines IFN-inducible T-cell alpha chemoattractant and monokine induced by IFN-gamma and of their receptor CXC receptor 3 in human renal cell carcinoma. Cancer 103: 258–267, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Teichmann M, Meyer B, Beck A, Niedobitek G: Expression of the interferon-inducible chemokine IP-10 (CXCL10), a chemokine with proposed anti-neoplastic functions, in Hodgkin lymphoma and nasopharyngeal carcinoma. J Pathol 206: 68–75, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L, Sagrinati C, Mazzinghi B, Orlando C, Maggi E, Marra F, Romagnani S, Serio M, Romagnani P: An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med 197: 1537–1549, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romagnani P, Lasagni L, Annunziato F, Serio M, Romagnani S: CXC chemokines: The regulatory link between inflammation and angiogenesis. Trends Immunol 25: 201–209, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Kelsen SG, Aksoy MO, Yang Y, Shahabuddin S, Litvin J, Safadi F, Rogers TJ: The chemokine receptor CXCR3 and its splice variant are expressed in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 287: L584–L591, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Romagnani P, Maggi L, Mazzinghi B, Cosmi L, Lasagni L, Liotta F, Lazzeri E, Angeli R, Rotondi M, Fili L, Parronchi P, Serio M, Maggi E, Romagnani S, Annunziato F: CXCR3-mediated opposite effects of CXCL10 and CXCL4 on TH1 or TH2 cytokine production. J Allergy Clin Immunol 116: 1372–1379, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Dantal J, Pohanka E: Malignancies in renal transplantation: An unmet medical need. Nephrol Dial Transplant 22[Suppl 1]: i4–i10, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Sugie N, Fujii N, Danno K: Cyclosporin-A suppresses p53-dependent repair DNA synthesis and apoptosis following ultraviolet-B irradiation. Photodermatol Photoimmunol Photomed 18: 163–168, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Kawada K, Hosogi H, Sonoshita M, Sakashita H, Manabe T, Shimahara Y, Sakai Y, Takabayashi A, Oshima M, Taketo MM: Chemokine receptor CXCR3 promotes colon cancer metastasis to lymph nodes. Oncogene 26: 4679–4688, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Furuya M, Suyama T, Usui H, Kasuya Y, Nishiyama M, Tanaka N, Ishiwata I, Nagai Y, Shozu M, Kimura S: Up-regulation of CXC chemokines and their receptors: Implications for proinflammatory microenvironments of ovarian carcinomas and endometriosis. Hum Pathol 38: 1676–1687, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Wimmer CD, Rentsch M, Crispin A, Illner WD, Arbogast H, Graeb C, Jauch KW, Guba M: The janus face of immunosuppression: De novo malignancy after renal transplantation—The experience of the Transplantation Center Munich. Kidney Int 71: 1271–1278, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Petrai I, Rombouts K, Lasagni L, Annunziato F, Cosmi L, Romanelli RG, Sagrinati C, Mazzinghi B, Pinzani M, Romagnani S, Romagnani P, Marra F: Activation of p38(MAPK) mediates the angiostatic effect of the chemokine receptor CXCR3-B. Int J Biochem Cell Biol 40: 1764–1774, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Kouroumalis A, Nibbs RJ, Aptel H, Wright KL, Kolios G, Ward SG: The chemokines CXCL9, CXCL10, and CXCL11 differentially stimulate G alpha i-independent signaling and actin responses in human intestinal myofibroblasts. J Immunol 175: 5403–5411, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ, Wei K, McMaster BE, Wright K, Howard MC, Schall TJ: A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med 203: 2201–2213, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ehlert JE, Addison CA, Burdick MD, Kunkel SL, Strieter RM: Identification and partial characterization of a variant of human CXCR3 generated by posttranscriptional exon skipping. J Immunol 173: 6234–6240, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Connor KM, Hempel N, Nelson KK, Dabiri G, Gamarra A, Belarmino J, Van De Water L, Mian BM, Melendez JA: Manganese superoxide dismutase enhances the invasive and migratory activity of tumor cells. Cancer Res 67: 10260–10267, 2007 [DOI] [PubMed] [Google Scholar]