Abstract
The transient actions of gonadal steroids on the adult brain facilitate social behaviors, including reproduction. In male rodents, testosterone acts in the posterior medial amygdala (MeP) and medial preoptic area (MPOA) to promote mating. Adult neurogenesis occurs in both regions. The current study determined if testosterone and/or sexual behavior promote cell proliferation and survival in MeP and MPOA. Two experiments were conducted using the thymidine analog BrdU. First, gonad-intact and castrated male hamsters (n=6/group) were compared 24 hours or 7 weeks after BrdU. In MeP, testosterone stimulated cell proliferation 24 hours after BrdU (intact: 22.8±3.9 cells/mm2, castrate: 13.2±1.4cells/mm2). Testosterone did not promote cell proliferation in MPOA. Seven weeks after BrdU, cell survival was sparse in both regions (MeP: 2.5±0.6 and MPOA: 1.7±0.2 cells/mm2), and was not enhanced by testosterone. In Experiment 2, gonad-intact sexually-experienced animals were mated weekly to determine if regular neural activation enhances cell survival 7 weeks after BrdU in MeP and MPOA. Weekly mating failed to increase cell survival in MeP (8.1±1.6 vs. 9.9±3.2 cells/mm2) or MPOA (3.9±0.7 vs. 3.4±0.3 cells/mm2). Furthermore, mating at the time of BrdU injection did not stimulate cell proliferation in MeP (8.9±1.7 vs. 8.1±1.6 cells/mm2) or MPOA (3.6±0.5 vs. 3.9±0.7 cells/mm2). Taken together, our results demonstrate a limited capacity for neurogenesis in the mating circuitry. Specifically, cell proliferation in MeP and MPOA are differentially influenced by testosterone, and the birth and survival of new cells in either region are not enhanced by reproductive activity.
Keywords: cell proliferation, cell survival, testosterone, medial amygdala, medial preoptic area, hamster, BrdU, sexual behavior, animal
Introduction
Gonadal steroid hormones exert activational effects on the adult brain that sculpt neural circuits for expression of adult behavior. Hormones act in steroid-responsive brain regions, where they exert neurotrophic effects to enhance neuronal morphology and synaptic connectivity (Cooke and Woolley, 2005). In addition, gonadal hormones stimulate neurogenesis in adult mammals (Fowler, Liu, and Wang, 2007; Galea, Spritzer, Barker, and Pawluski, 2006). These structural changes are thought to be a principal mechanism through which hormones promote social behaviors, including mating.
Adult mammalian neurogenesis includes both cell proliferation and cell survival. It is notably demonstrated in the hippocampus (Gould, 2007). In male rodents, testosterone promotes the survival of new neurons in the dentate gyrus (Spritzer and Galea, 2007). Cell proliferation has also been demonstrated elsewhere in the male brain, including the olfactory bulb (Peretto, Giachino, Panzica, and Fasolo, 2001), posterior medial nucleus of the amygdala (MeP) (Fowler, Freeman, and Wang, 2003), medial preoptic area (MPOA) and bed nucleus of the stria terminalis (BST) (Huang and Bittman, 2002). MeP, BST, and MPOA are essential for male rodent sexual behavior. Although this mating circuit exhibits a lower capacity for neurogenesis, it serves as an excellent model to study hormone-driven cell proliferation and survival. The actions of testosterone throughout the mating circuit are essential for the expression of reproductive behavior in the presence of an appropriate sexual partner (reviewed in Wood and Swann, 2000).
Both MeP and medial MPOA transduce testosterone via androgen and estrogen receptors to stimulate mating (reviewed in Wood and Newman, 1995a). Neurogenesis occurs in both regions (Fowler et al., 2003; Huang and Bittman, 2002). In MeP, testosterone increases cell proliferation (Fowler et al., 2003). It is unknown whether testosterone influences new cell birth in MPOA. Therefore, it is important to determine whether testosterone-enhanced cell proliferation extends to MPOA. Additionally, we examined whether the survival of newly-born cells parallels testosterone's long term effects on behavior. Gonadal hormones exert lasting effects throughout the male brain. For example, mating behavior in male rodents is not immediately abolished after castration (reviewed in Wood and Newman, 1995a). Conversely, the full recovery of sexual behavior in long-term castrates requires weeks of testosterone exposure. Thus, newly-born cells are more likely to be functionally important if they persist through the time course known to influence behavior. Using the cell proliferation marker BrdU, the first experiment determined if testosterone-driven cell proliferation is similar in MeP and MPOA. Additionally, we determined if testosterone promotes cell survival in MeP and MPOA.
In a second experiment, we determined if the regular activation of reproductive circuits enhances the survival of cells in MeP and MPOA by comparing male hamsters that were allowed to mate on a weekly basis with males that did not mate. Several investigators have put forth the hypothesis that the birth and/or survival of new cells in the adult brain are enhanced in regions that are regularly activated by experience or environment (Gould, Tanapat, Rydel, and Hastings, 2000; Lledo, Alonso, and Grubb, 2006; Prickaerts, Koopmans, Blokland, and Scheepens, 2004). Animals engaged in hippocampal-dependent learning tasks exhibit higher levels of cell proliferation and survival in the dentate gyrus compared to animals that have not engaged in these tasks (Dalla, Bangasser, Edgecomb, and Shors, 2007; Shors, 2004). Additionally, cell proliferation is enhanced in the olfactory bulb after rodents are repeatedly exposed to novel olfactory stimuli (Rochefort, Gheusi, Vincent, and Lledo, 2002). If the activity-dependent hypothesis applies to the hamster mating behavior circuit, then regular sexual activity should enhance cell proliferation and survival in MeP and MPOA.
Materials & Methods
Subjects
Forty-four adult male Syrian hamsters (Mesocricetus auratus, 130-150g) were obtained from Charles River Laboratories (Wilmington, MA). Hamsters were singly-housed on a long day photoperiod (14:10 LD) with access to food and water ad libitum. Experimental procedures were approved by USC's Institutional Animal Care and Use Committee and conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals (DHEW Publication 80-23, revised 1985, Office of Science and Health reports, DRR/NIH, Bethesda, MD 20205).
Experiment 1
Gonad-intact and castrated male hamsters were compared at 24 hours and 7 weeks post-BrdU to determine if testosterone promotes cell proliferation and survival in MeP and MPOA. Twenty-eight animals were used. Three weeks prior to BrdU injections, half of the animals were castrated. Castration causes a profound loss in circulating androgens, and is accompanied by the elimination of sexual behavior (reviewed in Siegel, 1985; Wood and Newman, 1995a). The other half remained gonad intact. Proliferating cell populations were labeled 3 weeks following castration, when mating behavior is severely diminished (reviewed in Wood and Newman, 1995a).
All animals received a single injection of BrdU [300mg/kg BW ip (Sigma, St. Louis, MO) in 0.9% saline with 0.007N NaOH] to maximally incorporate BrdU into dividing cells (Cameron and McKay, 2001; Fowler et al., 2003). Half of the hamsters in each group (n=7 each) were sacrificed 24h later to determine if testosterone promotes cell proliferation in MPOA and MeP. The remaining males were sacrificed 7 wk post-BrdU to determine if testosterone enhances the survival of newly-born cells. The remaining animals were sacrificed 7 wk post-BrdU to determine if testosterone enhances the survival of newly-born cells. Seven weeks is sufficient for testosterone to restore mating behavior in long-term castrates and for newly-born olfactory neurons to express Fos in response to estrous females (Huang and Bittman, 2002; Morin and Zucker, 1978). By examining cell survival after seven weeks, the current study seeks to bridge short-term (Fowler et al., 2003) and long-term (Huang and Bittman, 2002) studies. The hypothesis is that testosterone enhances long-term cell survival at seven weeks similar to its effects on short-term cell proliferation, as measured at 24 hours.
Experiment 2
Gonad-intact, sexually-experienced male hamsters (n=18) were mated weekly or left unstimulated to determine if regular sexual activity enhances cell proliferation and survival in MeP and MPOA. Sexual interactions acutely stimulate testosterone in male hamsters (Pfeiffer and Johnston, 1992). Accordingly, we hypothesize that the consistent activation of mating circuits will increase cell survival in MeP and MPOA. Males were included in the study only if they mated to ejaculation in two of three preliminary tests for sexual experience.
Mating behavior tests were conducted during the first hours of the dark phase under dim light. Eight female hamsters were used as stimulus animals for mating behavior. All females were ovariectomized via bilateral dorsal flank incision, and received a 4-mm Silastic estradiol implant sc (id: 1.98 mm, od: 3.18 mm; Dow Corning, MI) to maintain chronic physiologic levels of estrogen. To stimulate lordosis, females received 250 ug progesterone in cottonseed oil sc 4 hours prior to testing (see Carter, 1985). For testing, an estrous female was introduced into the male's home cage for 10 minutes. Behavior of the test male was recorded including mounts, intromissions, and ejaculations. For weekly mating experience after BrdU injection in Experiment 2, males were required to ejaculate at least once during each test. If necessary, males were given extra time and/or placed with a different stimulus female to facilitate ejaculation. The week-long period between mating exposures ensured that males would successfully copulate at each mating test without becoming sexually satiated (Arteaga, Motte-Lara, and Velazquez-Moctezuma, 2000; Beach and Rabedeau, 1959).
As in Experiment 1, all males received a single injection of BrdU (300mg/kg BW ip). The hamsters were separated into three groups (n=6/group). Males in the Control group did not mate after BrdU administration. The remaining hamsters were allowed to mate weekly for 7 weeks. However, to determine whether reproduction stimulates cell proliferation, hamsters in the Immediate Mating (IM) group were allowed to mate 10 minutes after BrdU administration. Animals in the Delayed Mating (DM) group mated 24 hours later. Thus, males in the IM and DM groups received the same amount of sexual activity. However, only males in the IM group mated during BrdU incorporation, which occurs over 2 hours following BrdU administration (Takahashi, Nowakowski, and Caviness, 1992). Therefore, BrdU-labeling in IM hamsters should reflect mating-induced cell proliferation and survival, while BrdU-labeling in DM hamsters should indicate activity-dependent cell survival only. After the final sexual behavior test, all hamsters were sacrificed.
Perfusion
Hamsters were deeply anesthetized with sodium pentobarbital (150mg/kg BW) and perfused intracardially with 150mL of 0.1M sodium phosphate buffer (PB, pH=7.4) containing 0.9% NaCl and 0.1% NaNO3, followed by 250mL 4% paraformaldehyde in PB. The brains were removed, post-fixed in the perfusion fixative for 1h and cryoprotected for 5 days at 4°C with 20% sucrose in PB. The brains were rapidly frozen and sectioned coronally at 40um. Sections were stored in PB with 0.01% sodium azide at 4°C until processed for BrdU immunocytochemistry.
Immunocytochemistry for BrdU
Every fourth section was stained for BrdU according to the methods of Fowler, et al. (2003). Sections from males in different groups of the same experiment were stained at the same time. To denature the DNA, free-floating brain sections were pre-treated with 2N HCl for 30 min at 37°C and then washed to neutralize the acid with 0.1M borate buffer (pH=8.5) at room temperature (RT) for 20 min. After additional washing with PB, sections were incubated overnight at RT in monoclonal rat anti-BrdU antibody (1:500; AbD Serotec, Raleigh, NC) with 4% normal donkey serum and 0.3% Triton X-100 in PB. The following day, sections were incubated in biotinylated donkey-anti-rat secondary antibody (1:200, Jackson Immunoresearch, West Grove, PA) and the avidin-biotin-horseradish peroxidase complex, each for 1 hour at RT with extensive washes in between. BrdU-labeled cells were visualized using NiCl-enhanced 3′3-diaminobenzidine tetrahydrochloride (DAB) with 0.25% hydrogen peroxide. Sections were mounted onto gelatin-coated slides, air-dried, cleared in xylenes, and coverslipped. Adjacent sections from each brain were Nissl-stained to verify the boundaries of MeP and MPOA.
Data Analysis
Sections from each brain region were matched across animals to ensure that MeP and MPOA were sampled consistently. BrdU-labeled cells were counted bilaterally on coded slides by an observer blind to the treatment group using an Olympus BH-2 microscope equipped with a drawing tube. Labeled cells were counted in a 0.4 mm2 triangular region of MeP lateral to the optic tract that included both dorsal and ventral subdivisions (Plate 27 in Morin and Wood, 2001). MPOA cell counts were completed in a 1.5 mm2 rectangular area adjacent to the third ventricle, extending dorsally to the border of BST and laterally to the edge of the optic chiasm (Plate 22 in Morin and Wood, 2001). Figure 1 illustrates BrdU labeling in MeP and MPOA.
Figure 1.
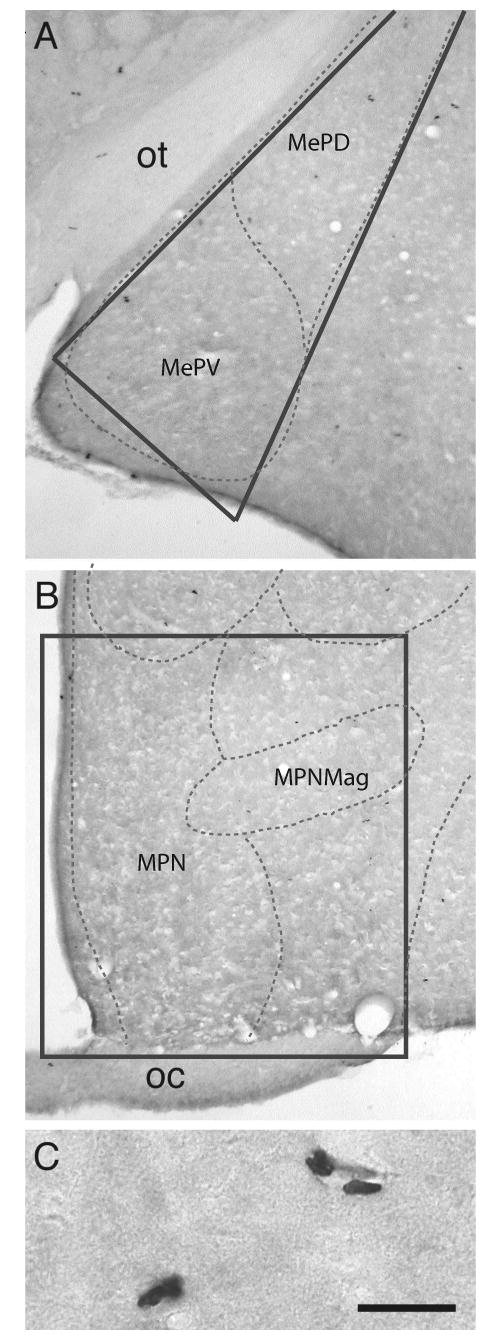
Photographs of BrdU-labeled cells in MeP (A) and MPOA (B) at 24h post BrdU. (C) High magnification photograph of BrdU-labeling in MeP. Labeled cells were observed as individual cells or in 2-cell clusters. Scale bar = (A-B) 250um, (C) 25um. Dotted lines indicate atlas boundaries. Solid lines indicate counting regions. Abbreviations: MPN, medial preoptic nucleus; MPNmag, medial preoptic nucleus, magnocellular part; MePD, medial amygdaloid nucleus, posterodorsal part; MePV, medial amygdaloid nucleus, posteroventral part; oc, optic chiasm; ot, optic tract.
BrdU-labeled cells were identified at 10× and verified at 40×. Because there was no left-right asymmetry in MeP or MPOA, cell counts from both hemispheres were combined. The total number of BrdU-positive cells per section was averaged for each animal and the density of BrdU-labeled cells per mm2 was calculated. For each region, group differences were analyzed by a fully factorial analysis of variance (ANOVA). Post-hoc comparisons using the Fisher's LSD test were conducted when statistically significant differences (p < 0.05) were found.
Results
BrdU labeling was observed as dark punctuate nuclear staining (Fig. 1a-c). Labeled nuclei had an irregular shape and were distributed throughout MeP and MPOA. The specific subnuclear distribution of BrdU-labeled cells has implications for the origin of newly-born cells in the brain. Adult neurogenesis may arise by migration of proliferating cells from the subventricular zone or by division of neuronal precursors in situ (Lois and Alvarez-Buylla, 1994; Zhao, Momma, Delfani, Carlen, Cassidy, Johansson, Brismar, Shupliakov, Frisen, and Janson, 2003). This latter mechanism is thought to give rise to pairs of labeled daughter cells (Cameron and McKay, 2001). In MeP, labeled cells were observed near the lateral ventricle. In MPOA, BrdU labeling was present near the ependymal layer of the third ventricle. Labeled cells were also present at the lateral edges of both cell counting regions. In addition, BrdU-labeled cells commonly appeared in 2-cell clusters throughout MeP and MPOA (Fig. 1c).
Experiment 1
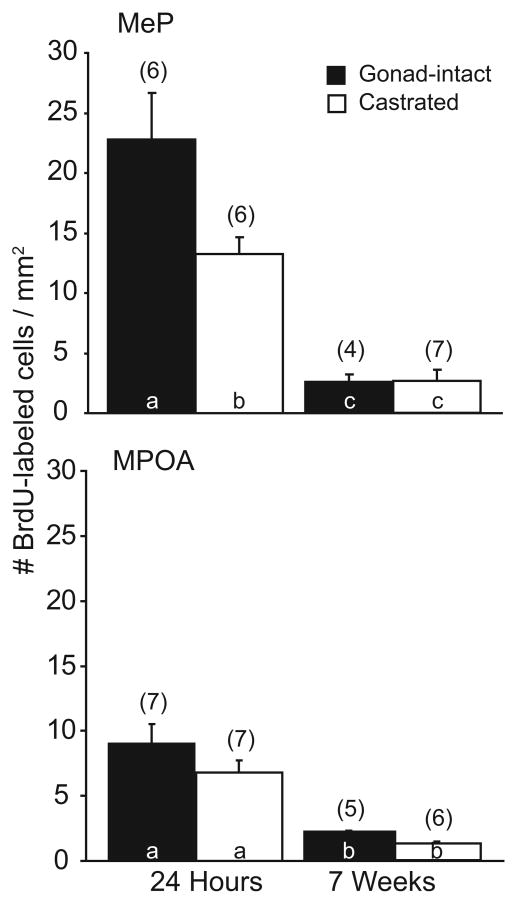
Twenty four hours after injection, newly-born cells were observed in MeP, as determined by the presence of BrdU-labeled cells (18.0±2.4 cells/mm2, mean±SEM of gonad-intact and castrated males). Furthermore, cell proliferation in MeP was enhanced by testosterone. Gonad-intact males had a significantly higher density of BrdU-labeled cells (22.8±3.9 cells/mm2) than did castrated males (13.2±1.4 cells/mm2, p<0.05, Fig. 2a). However, after 7 weeks, the density of BrdU-labeled cells in MeP was drastically reduced in both gonad-intact and castrated hamsters (overall mean 2.5±0.6 cells/mm2, p<0.05). In addition, testosterone did not promote the survival of cells in MeP (gonad-intact 2.5±0.6 vs. castrate 2.5±1.0 cells/mm2, n.s., Fig. 2a).
Figure 2.
The effects of testosterone on cell proliferation and survival in the mating circuitry. Mean±SEM number of labeled cells per mm2 tissue at 24 hours and 7 weeks post-BrdU in gonad-intact (black bars) and castrated (white bars) male hamsters (n=4-7/group) in MeP (top) and MPOA (bottom). Numbers in parentheses indicate n for each group. Bars with different letter subscripts are significantly different.
Cell proliferation in MPOA was substantially less than in MeP. Gonad-intact and castrated males sacrificed 24h post-injection had an average of 7.9±1.0 BrdU-labeled cells/mm2 (p<0.05, vs. MeP by paired t-test). Moreover, testosterone did not promote cell proliferation in MPOA. The density of BrdU-labeled cells in gonad-intact males (9.0±1.5 cells/mm2) was not different from that in castrated males (6.8±0.9 cells/mm2, n.s., Fig. 2b). As with MeP, survival of newly-born cells was poor in both gonad-intact and castrated males sacrificed 7 weeks post BrdU (1.7±0.2 cells/mm2, p<0.05). Similarly, testosterone had no effect on cell survival in MPOA (gonad-intact: 2.2±0.1 cells/mm2 vs. castrate: 1.3±0.1 cells/mm2, n.s., Fig. 2b). Despite the reduced proliferation of cells in MPOA relative to MeP, the survival of BrdU-labeled cells at 7 weeks was equivalent in both regions.
Experiment 2
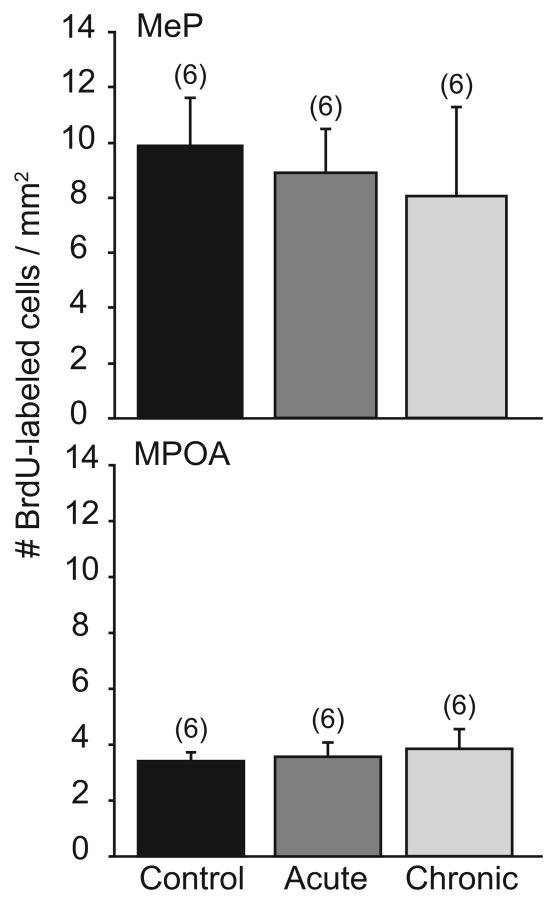
BrdU-labeled cells were observed in both MeP and MPOA of gonad-intact sexually-experienced male hamsters. Nonetheless, the regular activation of mating circuits did not enhance cell survival measured 7 weeks after BrdU. Likewise, individual aspects of mating behavior (mounts, intromissions, or ejaculations) did not correlate with cell survival in MeP or MPOA.
In MeP, DM hamsters that mated weekly had a similar density of BrdU-labeled cells (8.1±1.6 cells/mm2) to Control males that did not mate (9.9±3.2 cells/mm2, Fig. 3a). Likewise, mating did not enhance cell survival in MPOA (3.9±0.7 vs. 3.4±0.3 cells/mm2, n.s., Fig. 3b). Furthermore, mating at the time of BrdU injection did not promote cell proliferation in male hamsters. In MeP, BrdU-labeling was not different between IM (8.9±1.7 cells/mm2) and DM males (8.1±1.6 cells/mm2, n.s., Fig. 3a). The same pattern was observed in MPOA (3.6±0.5 vs. 3.9±0.7 cells/mm2, Fig. 3b).
Figure 3.
The effects of reproductive activity on cell proliferation and survival in MeP and MPOA. Mean±SEM number of BrdU-labeled cells per mm2 tissue in reproductively inactive (Control, black bars) or reproductively active hamsters (n=6/group) in MeP (top) and MPOA (bottom). Reproductively active hamsters mated either during BrdU incorporation (IM, dark gray) or 24h later (DM, light gray). Numbers in parentheses indicate n for each group. There were no significant differences between groups.
Interestingly, unlike cell survival in Experiment 1, the survival of newly-born cells was not equivalent in MeP and MPOA. Across the IM, DM, and Control groups, male hamsters had a higher density of BrdU-labeled cells in MeP (8.9±1.3 cells/mm2) than in MPOA (3.6±0.3 cells/mm2, p<0.05).
Discussion
The current study used BrdU labeling to determine the effects of testosterone and reproductive activity on cell proliferation and survival in MeP and MPOA of the male Syrian hamster. Cell proliferation occurs throughout the mating circuitry in a site-specific manner. Specifically, more cells arise in MeP than in MPOA. We also found that cell proliferation in MeP, but not MPOA, is sensitive to testosterone. Nonetheless, long-term (7 wk) cell survival was limited in both regions and testosterone did not promote cell survival in MeP or MPOA. When sexually-experienced animals were allowed to mate on a regular basis, repeated activation of the sexual behavior circuitry did not enhance cell survival. Likewise, acute mating experience did not further facilitate cell survival in MeP or MPOA. Overall, our results demonstrate a limited capacity for cell proliferation and survival in the mating circuitry that is differentially influenced by testosterone.
The comparison of hamsters with (gonad-intact) and without (castrate) testosterone is relevant to the normal physiologic environment of male hamsters. Syrian hamsters are seasonal breeders. During long day photoperiods, male hamsters are sexually active and fertile under the stimulatory actions of testicular androgens (Miernicki, Pospichal, and Powers, 1990; Morin and Zucker, 1978). In contrast, testosterone levels approach those of castrated males during short days. Therefore, it is realistic to assume that hormone-dependent cell proliferation and/or survival also varies with season and that MeP and MPOA are influenced by natural fluctuations of testosterone that occur across the lifespan.
Because MeP and MPOA contain abundant steroid hormone receptors (Wood, Brabec, Swann, and Newman, 1992) and play important roles in mating behavior, it would appear that gonadal hormones might act similarly in both regions to stimulate cell proliferation. The action of testosterone in either MeP or MPOA is sufficient to stimulate mating (Wood and Newman, 1995b). Correspondingly, lesions to either region impair sexual activity (Lehman and Winans, 1982; Powers, Newman, and Bergondy, 1987) In addition, MeP and MPOA are heavily interconnected (reviewed in Wood and Newman, 1995a). However, the results of the current study indicate that the capacity for cell proliferation is greater in MeP than in MPOA. Furthermore, testosterone exerted regionally-specific effects on the birth of new cells. In MeP, cell proliferation was sensitive to testosterone. In MPOA, testosterone did not stimulate new cell birth.
Although MPOA displays a low level of cell proliferation, it demonstrates a large capacity for hormone-driven structural plasticity. Testosterone enhances dendritic morphology in MPOA neurons (Cherry, Tobet, DeVoogd, and Baum, 1992). Interestingly, this pattern is also observed in the female ventromedial hypothalamus (VMH), the major organizing center for female rodent sexual behavior (Mathews and Edwards, 1977; Pfaff and Sakuma, 1979). In VMH, estrogen does not stimulate cell proliferation (Fowler, Johnson, and Wang, 2005) but drastically remodels dendritic morphology in VMH neurons (Calizo and Flanagan-Cato, 2000; Frankfurt, Gould, Woolley, and McEwen, 1990). This argues that the hypothalamic centers for sexual behavior in both sexes demonstrate hormone-driven plasticity in cell remodeling rather than cell proliferation.
However, in both MeP and MPOA, the long-term survival of newly-born cells in the mating circuitry is limited. Few studies have examined cell survival after 7 weeks. Instead, cell survival is commonly measured only up to 30 days after BrdU injection. This time frame is relevant for the study of memory formation in the hippocampus (Gould, Beylin, Tanapat, Reeves, and Shors, 1999). However, hormone-induced changes in reproductive behavior occur over a much longer time course (reviewed in Wood and Newman, 1995a). Castrated male hamsters do not show obvious impairments in sexual performance immediately following gonadectomy. Rather, mating behavior declines slowly after castration with the complete elimination of sexual behavior occurring 7-10 weeks later (Park, Takasu, Alvarez, Clark, Aimaq, and Zucker, 2004; Siegel, 1985). Conversely, testosterone administration to long-term castrates results in the full recovery of mating after 8 weeks (Sachs and Meisel, 1988; Siegel, 1985; Wood and Newman, 1995a). In the olfactory bulb, the maturation and subsequent activation of newly-proliferated cells occurs across a similar time frame. Longer time periods (7 weeks) are sufficient to detect odor-induced Fos in substantial populations of olfactory neurons that have migrated appropriately into the olfactory bulb (Huang and Bittman, 2002). Thus, the analysis of cell survival at 7 weeks allowed us to follow hormone-influenced cell proliferation and survival over a functionally-relevant time course.
However, few newly-born cells remained in the mating circuit after 7 weeks. Previous work in MeP and MPOA of gonad-intact males has shown similar findings (Huang and Bittman, 2002). In the present study, we found that the stimulatory effects of testosterone on cell proliferation in MeP were not sustained over seven weeks. Additionally, we used a high dose of BrdU, designed to label a much larger population of proliferating cells without inducing toxicity (Cameron and McKay, 2001; Fowler et al., 2003). Nonetheless, the results indicate that cell survival is extremely limited in the mating circuitry and is not enhanced by testosterone. It seems that the small number of surviving cells in MeP and MPOA would be unlikely to have substantial consequences on reproductive behavior.
Our observation that testosterone stimulated cell proliferation selectively in MeP but did not enhance long-term cell survival stands in contrast to the hippocampus, where testosterone enhances cell survival but does not promote cell proliferation in the dentate gyrus (Spritzer and Galea, 2007). This difference may be mediated, in part, through the specific actions of testosterone's metabolites. In the male, testosterone can be aromatized to estrogen or reduced to the androgen dihydrotestosterone (DHT). Behavioral and morphologic evidence suggests that the actions of testosterone in MeP are mediated through its conversion to estradiol (Fowler et al., 2003; Gomez and Newman, 1991; Wood, 1996). In contrast, DHT mediates the effects of testosterone on hippocampal morphology and neurogenesis (Leranth, Hajszan, and MacLusky, 2004) and on cell survival (Spritzer and Galea, 2007). Taken together, these observations suggest that different brain regions are tuned to preferentially respond to androgenic or estrogenic metabolites of testosterone, thereby producing different effects on cell proliferation and survival.
Because cell survival in MeP and MPOA in Experiment 1 was limited, it was important in Experiment 2 to test the role of experience and neuronal activity in maintaining newly-born cells. There is precedent for this in both hippocampus and olfactory bulb, where neurogenesis is influenced by experience. In the olfactory bulb, odor-enriching and odor-depriving environments increase (Rochefort et al., 2002) and decrease (Petreanu and Alvarez-Buylla, 2002) granule cell survival, respectively. In the hippocampus, the acquisition of a trace conditioning response increases the survival of granule cells in the rodent dentate gyrus (Gould et al., 1999). On the other hand, animals that experience uncontrollable stressors have decreased cell proliferation in DG (Malberg and Duman, 2003).
By contrast, sexual activity had no significant effect on cell survival or proliferation in MeP and MPOA. First, when animals were mated on a weekly basis, cell survival was not enhanced in either region. If activation enhances cell survival, then regular mating experience should have increased BrdU labeling in MeP and MPOA. Second, BrdU labeling was not different between animals that mated during BrdU incorporation and animals that mated 24 hours post-BrdU. Male hamsters experience a pulse of testosterone immediately following mating (Pfeiffer and Johnston, 1992). If mating-induced testosterone stimulated cell proliferation, more cells would have been present in IM hamsters than in DM males.
Interestingly, cell survival in sexually-experienced hamsters was selectively-enhanced, compared with sexually-naïve hamsters. In gonad-intact but sexually-naïve hamsters from Experiment 1, the survival of new cells was equivalent in MeP and in MPOA. In sexually-experienced Control hamsters from Experiment 2 that did not mate following BrdU, cell survival was significantly greater in MeP than in MPOA. Thus, experience before, but not after, BrdU may have increased the capacity for cell survival in MeP.
Differences between sexually-naïve and experienced male hamsters have been previously reported, and suggest that permanent physiological changes occur after the acquisition of sexual behavior in male hamsters. First, sexually-experienced male hamsters exposed to vaginal secretions from female hamsters (FHVS) show increased neuronal activation compared to sexually-naïve hamsters presented with FHVS (Westberry and Meredith, 2003). Second, sexually-experienced male hamsters retain copulatory behaviors for longer periods of time after castration than do sexually-naïve hamsters (Lisk and Heimann, 1980). Nonetheless, given the relatively small number of surviving neurons, the selective increase in BrdU labeling in sexually-experienced hamsters is unlikely to have a substantial impact on mating behavior.
Overall, our results are in keeping with earlier studies demonstrating a limited capacity for cell proliferation and survival in the mating circuitry. However, our results extend the findings of earlier studies by demonstrating that testosterone-stimulated cell proliferation does not occur evenly throughout the mating circuitry. We also report that neither testosterone nor regular neural activation improves cell survival in the mating circuit. This suggests that although male hamsters exhibit dramatic behavioral changes across their lifespan, the low survival of newly-born cells in MeP and MPOA occurs over a restricted time course and is unlikely to account for seasonal changes in reproductive response across the lifespan.
Acknowledgments
The authors thank Ruby E. Jong for her assistance with tissue analysis of BrdU-labeling. This work is supported by NIH RO1-MH55034.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arteaga M, Motte-Lara J, Velazquez-Moctezuma J. Temporal pattern of recovery from sexual satiety in male golden hamster (Mesocricetus auratus) Physiol Behav. 2000;68(4):591–4. doi: 10.1016/s0031-9384(99)00200-0. [DOI] [PubMed] [Google Scholar]
- Beach FA, Rabedeau RG. Sexual exhaustion and recovery in the male hamster. J Comp Physiol Psychol. 1959;52(1):56–61. doi: 10.1037/h0041896. [DOI] [PubMed] [Google Scholar]
- Calizo LH, Flanagan-Cato LM. Estrogen selectively regulates spine density within the dendritic arbor of rat ventromedial hypothalamic neurons. J Neurosci. 2000;20(4):1589–96. doi: 10.1523/JNEUROSCI.20-04-01589.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435(4):406–17. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Carter CS. Female Sex Behavior. In: Siegel HI, editor. The Hamster: Reproduction and Behavior. Plenum Press; New York: 1985. pp. 173–189. [Google Scholar]
- Cherry JA, Tobet SA, DeVoogd TJ, Baum MJ. Effects of sex and androgen treatment on dendritic dimensions of neurons in the sexually dimorphic preoptic/anterior hypothalamic area of male and female ferrets. J Comp Neurol. 1992;323(4):577–85. doi: 10.1002/cne.903230410. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Woolley CS. Gonadal hormone modulation of dendrites in the mammalian CNS. J Neurobiol. 2005;64(1):34–46. doi: 10.1002/neu.20143. [DOI] [PubMed] [Google Scholar]
- Dalla C, Bangasser DA, Edgecomb C, Shors TJ. Neurogenesis and learning: acquisition and asymptotic performance predict how many new cells survive in the hippocampus. Neurobiol Learn Mem. 2007;88(1):143–8. doi: 10.1016/j.nlm.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Freeman ME, Wang Z. Newly proliferated cells in the adult male amygdala are affected by gonadal steroid hormones. J Neurobiol. 2003;57(3):257–69. doi: 10.1002/neu.10273. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Johnson F, Wang Z. Estrogen regulation of cell proliferation and distribution of estrogen receptor-alpha in the brains of adult female prairie and meadow voles. J Comp Neurol. 2005;489(2):166–79. doi: 10.1002/cne.20638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Liu Y, Wang Z. Estrogen and adult neurogenesis in the amygdala and hypothalamus. Brain Res Rev. 2007 doi: 10.1016/j.brainresrev.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankfurt M, Gould E, Woolley CS, McEwen BS. Gonadal steroids modify dendritic spine density in ventromedial hypothalamic neurons: a Golgi study in the adult rat. Neuroendocrinology. 1990;51(5):530–5. doi: 10.1159/000125387. [DOI] [PubMed] [Google Scholar]
- Galea LA, Spritzer MD, Barker JM, Pawluski JL. Gonadal hormone modulation of hippocampal neurogenesis in the adult. Hippocampus. 2006;16(3):225–32. doi: 10.1002/hipo.20154. [DOI] [PubMed] [Google Scholar]
- Gomez DM, Newman SW. Medial nucleus of the amygdala in the adult Syrian hamster: a quantitative Golgi analysis of gonadal hormonal regulation of neuronal morphology. Anat Rec. 1991;231(4):498–509. doi: 10.1002/ar.1092310412. [DOI] [PubMed] [Google Scholar]
- Gould E. How widespread is adult neurogenesis in mammals? Nat Rev Neurosci. 2007;8(6):481–8. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2(3):260–5. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P, Rydel T, Hastings N. Regulation of hippocampal neurogenesis in adulthood. Biol Psychiatry. 2000;48(8):715–20. doi: 10.1016/s0006-3223(00)01021-0. [DOI] [PubMed] [Google Scholar]
- Huang L, Bittman EL. Olfactory bulb cells generated in adult male golden hamsters are specifically activated by exposure to estrous females. Horm Behav. 2002;41(3):343–50. doi: 10.1006/hbeh.2002.1767. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Winans SS. Vomeronasal and olfactory pathways to the amygdala controlling male hamster sexual behavior: autoradiographic and behavioral analyses. Brain Res. 1982;240(1):27–41. doi: 10.1016/0006-8993(82)90641-2. [DOI] [PubMed] [Google Scholar]
- Leranth C, Hajszan T, MacLusky NJ. Androgens increase spine synapse density in the CA1 hippocampal subfield of ovariectomized female rats. J Neurosci. 2004;24(2):495–9. doi: 10.1523/JNEUROSCI.4516-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisk RD, Heimann J. The effects of sexual experience and frequency of testing on retention of copulatory behavior following castration in the male hamster. Behav Neural Biol. 1980;28(2):156–71. doi: 10.1016/s0163-1047(80)91503-4. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7(3):179–93. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264(5162):1145–8. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28(9):1562–71. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- Mathews D, Edwards DA. The ventromedial nucleus of the hypothalamus and the hormonal arousal of sexual behaviors in the female rat. Horm Behav. 1977;8(1):40–51. doi: 10.1016/0018-506x(77)90019-8. [DOI] [PubMed] [Google Scholar]
- Miernicki M, Pospichal MW, Powers JB. Short photoperiods affect male hamster sociosexual behaviors in the presence and absence of testosterone. Physiol Behav. 1990;47(1):95–106. doi: 10.1016/0031-9384(90)90046-7. [DOI] [PubMed] [Google Scholar]
- Morin LP, Wood RI. A stereotaxic atlas of the golden hamster brain. Academic Press; San Diego: 2001. [Google Scholar]
- Morin LP, Zucker I. Photoperiodic regulation of copulatory behaviour in the male hamster. J Endocrinol. 1978;77(2):249–58. doi: 10.1677/joe.0.0770249. [DOI] [PubMed] [Google Scholar]
- Park JH, Takasu N, Alvarez MI, Clark K, Aimaq R, Zucker I. Long-term persistence of male copulatory behavior in castrated and photo-inhibited Siberian hamsters. Horm Behav. 2004;45(3):214–21. doi: 10.1016/j.yhbeh.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Peretto P, Giachino C, Panzica GC, Fasolo A. Sexually dimorphic neurogenesis is topographically matched with the anterior accessory olfactory bulb of the adult rat. Cell Tissue Res. 2001;306(3):385–9. doi: 10.1007/s00441-001-0471-1. [DOI] [PubMed] [Google Scholar]
- Petreanu L, Alvarez-Buylla A. Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. J Neurosci. 2002;22(14):6106–13. doi: 10.1523/JNEUROSCI.22-14-06106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff DW, Sakuma Y. Facilitation of the lordosis reflex of female rats from the ventromedial nucleus of the hypothalamus. J Physiol. 1979;288:189–202. [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer CA, Johnston RE. Socially stimulated androgen surges in male hamsters: the roles of vaginal secretions, behavioral interactions, and housing conditions. Horm Behav. 1992;26(2):283–93. doi: 10.1016/0018-506x(92)90048-z. [DOI] [PubMed] [Google Scholar]
- Powers JB, Newman SW, Bergondy ML. MPOA and BNST lesions in male Syrian hamsters: differential effects on copulatory and chemoinvestigatory behaviors. Behav Brain Res. 1987;23(3):181–95. doi: 10.1016/0166-4328(87)90019-2. [DOI] [PubMed] [Google Scholar]
- Prickaerts J, Koopmans G, Blokland A, Scheepens A. Learning and adult neurogenesis: survival with or without proliferation? Neurobiol Learn Mem. 2004;81(1):1–11. doi: 10.1016/j.nlm.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Rochefort C, Gheusi G, Vincent JD, Lledo PM. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J Neurosci. 2002;22(7):2679–89. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs BD, Meisel RI. The physiology of male sexual behavior. In: K E, N J, editors. The physiology of reproduction. Raven Press; New York: 1988. pp. 1393–1485. [Google Scholar]
- Shors TJ. Memory traces of trace memories: neurogenesis, synaptogenesis and awareness. Trends Neurosci. 2004;27(5):250–6. doi: 10.1016/j.tins.2004.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel HI. Male Sexual Behavior. In: Siegel HI, editor. The Hamster: Reproduction and Behavior. Plenum Press; New York: 1985. pp. 191–204. [Google Scholar]
- Spritzer MD, Galea LA. Testosterone and dihydrotestosterone, but not estradiol, enhance survival of new hippocampal neurons in adult male rats. Dev Neurobiol. 2007;67(10):1321–33. doi: 10.1002/dneu.20457. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Nowakowski RS, Caviness VS., Jr BUdR as an S-phase marker for quantitative studies of cytokinetic behaviour in the murine cerebral ventricular zone. J Neurocytol. 1992;21(3):185–97. doi: 10.1007/BF01194977. [DOI] [PubMed] [Google Scholar]
- Westberry J, Meredith M. The influence of chemosensory input and gonadotropin releasing hormone on mating behavior circuits in male hamsters. Brain Res. 2003;974(12):1–16. doi: 10.1016/s0006-8993(03)02535-6. [DOI] [PubMed] [Google Scholar]
- Wood RI. Estradiol, but not dihydrotestosterone, in the medial amygdala facilitates male hamster sex behavior. Physiol Behav. 1996;59(45):833–41. doi: 10.1016/0031-9384(95)02204-x. [DOI] [PubMed] [Google Scholar]
- Wood RI, Brabec RK, Swann JM, Newman SW. Androgen and estrogen concentrating neurons in chemosensory pathways of the male Syrian hamster brain. Brain Res. 1992;596(12):89–98. doi: 10.1016/0006-8993(92)91536-n. [DOI] [PubMed] [Google Scholar]
- Wood RI, Newman SW. Hormonal Influences on Neurons of the Mating Behavior Pathway in Male Hamsters. In: Micevych PE, Hammer RP, editors. Neurobiological Effects of Sex Steroid Hormones. Cambridge University Press; New York: 1995a. [Google Scholar]
- Wood RI, Newman SW. The medial amygdaloid nucleus and medial preoptic area mediate steroidal control of sexual behavior in the male Syrian hamster. Horm Behav. 1995b;29(3):338–53. doi: 10.1006/hbeh.1995.1024. [DOI] [PubMed] [Google Scholar]
- Wood RI, Swann JM. Neuronal Integration of Chemosensory and Hormonal Signals in the Control of Male Sexual Behavior. In: Wallen K, Schneider JE, editors. Reproduction in Context: Social and Environmental Influences on Reproduction. MIT Press; Cambridge, MA: 2000. pp. 423–444. [Google Scholar]
- Zhao M, Momma S, Delfani K, Carlen M, Cassidy RM, Johansson CB, Brismar H, Shupliakov O, Frisen J, Janson AM. Evidence for neurogenesis in the adult mammalian substantia nigra. Proc Natl Acad Sci U S A. 2003;100(13):7925–30. doi: 10.1073/pnas.1131955100. [DOI] [PMC free article] [PubMed] [Google Scholar]