Abstract
The syndrome of the sinking skin flap was introduced to explain the phenomenon of neurological deterioration after decompressive craniectomy. A 37-year-old man was admitted with acute subdural hematoma and traumatic intraparenchymal hematoma. After decompressive craniectomy, the patient suffered from hydrocephalus for which a ventriculoperitoneal (V-P) shunt was inserted. Following this procedure, the depression of the skin flap became remarkable and his mentation was deteriorated. The patient recovered uneventfully after temporary elevating of valve pressure and cranioplasty. We present a patient who was successfully managed with elevation of valve pressure and cranioplasty for the syndrome of the sinking scalp flap with review of a pertinent literature.
Keywords: Craniectomy, Cranioplasty, Syndrome of sinking scalp flap, Ventriculoperitoneal shunt
INTRODUCTION
Decompressive craniectomy is widely used in neurosurgical field for the relief of intractable intracranial hypertension in patients with head injury, acute stroke, and severe brain edema after intracranial procedure7,11). After craniectomy, some patients may suffer from hydrocephalus for which cerebrospinal fluid (CSF) diversion, including V-P shunt, lumbar drainage, and extraventricular drainage are needed9,10).
However, this procedure paradoxically can cause to neurological deterioration and marked depression of craniectomy site. The syndrome of the sinking skin flap was introduced to explain neurological deterioration after decompressive craniectomy15). This phenomenon may result from CSF hypovolemia, atmospheric pressure gradient that may be aggravated by CSF diversion, dehydration, and position change4,7). Therefore, it is important to understand pathophysiologic mechanism and treatment of this condition.
We present a patient with neurological deterioration and severe depression of the skull bone defect area resulting from decompressive craniectomy against traumatic brain swelling followed by V-P shunt for hydrocephalus with therapeutic suggestion.
CASE REPORT
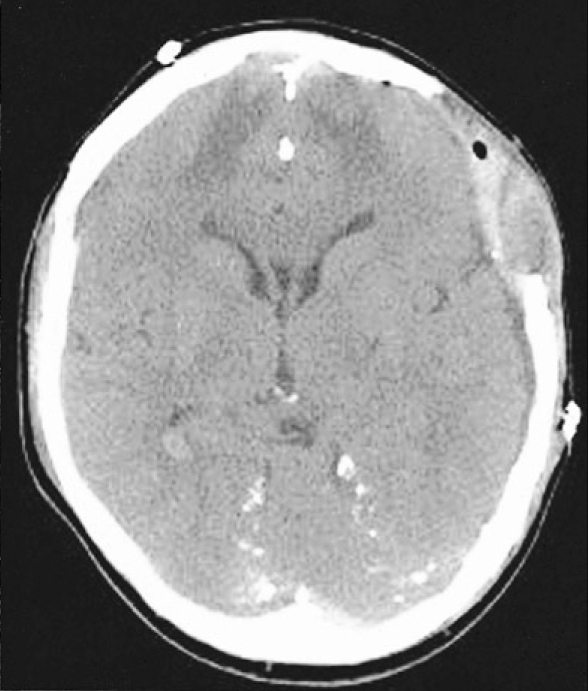
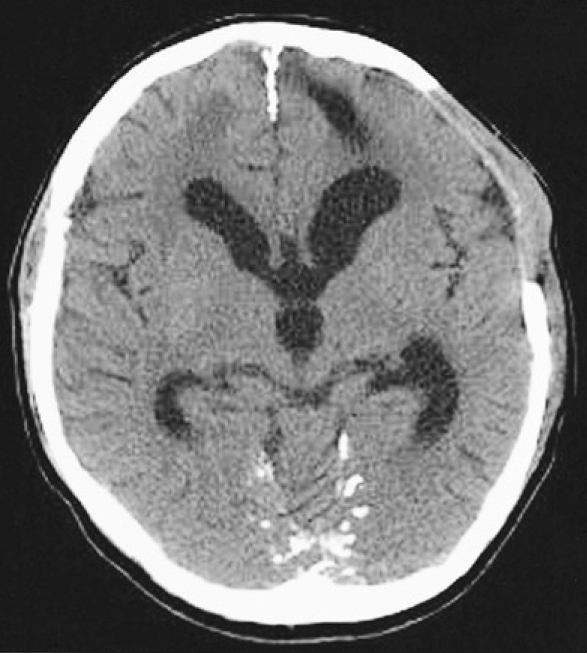
A 37-year-old man was transferred to our neurosurgical department through a local hospital with semicomatose mentality that had developed due to head injury at work. The brain computed tomographic (CT) scans showed acute subdural hematoma on the left frontotemporoparietal region and traumatic intracerebral hematoma on the left frontal region with significant mass effect and extensive midline shift to the right (Fig. 1). An emergency left frontotemporoparietal decompressive craniectomy for hematoma evacuation was performed. The patient gradually recovered and was nearly alert enough to obey command. Postoperative follow-up brain CT scans one week later showed a resolution of previous subdural hematoma and restoration of significant midline shift (Fig. 2). A follow-up brain CT scans four weeks after craniectomy demonstrated enlargement of the ventricular system and persistent bulging of the brain through the defect site of the craniectomy since previous study (Fig. 3). A V-P shunt via right Keen's point with the HAKIM programmable pressure valve device was performed for the communicating hydrocephalus. The opening pressure of V-P shunt device was set to 120 mmH2O. However, one day after the V-P shunt, the patient was deteriorated not to contact eye, obey command and unresponsive to noxious stimuli. The follow-up emergency CT scans revealed marked concavity of the brain at the defect site of the craniectomy associated with midline shift to the right (Fig. 4).
Fig. 1.
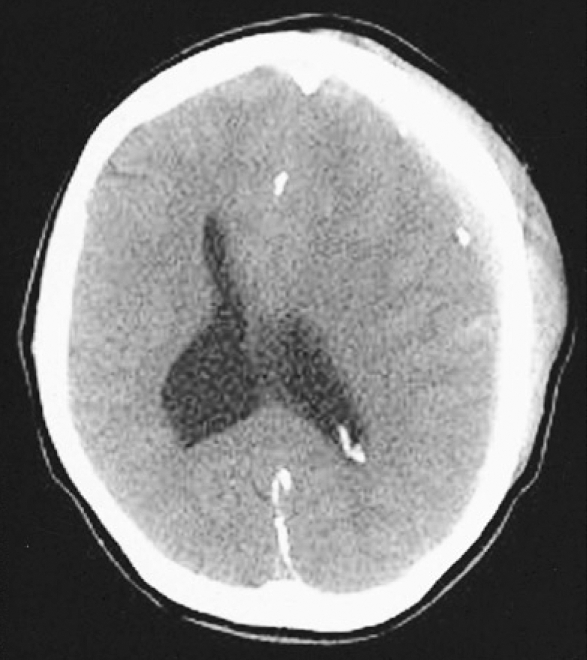
Preoperative axial computed tomographic scan showing the acute subdural hematoma on the left frototemporoparietal region and traumatic intraparenchymal hematoma on the left frontal region with extensive midline shift to the right.
Fig. 2.
Postcraniectomy axial computed tomographic scan one week later showing the resolved hematoma on the left frototemporoparietal region and the restoration of significant midline shift.
Fig. 3.
Postcraniectomy axial computed tomographic scan four weeks later showing the enlargement of the ventricular system and the persistent bulging of craniectomy site.
Fig. 4.
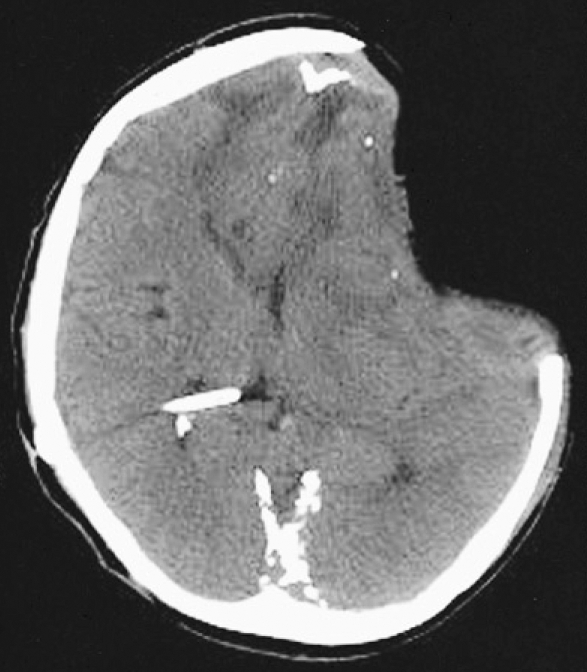
Post ventriculoperitoneal shunt axial computed tomographic scan one day later showing the sinking at the craniectomy site with extensive midline shift and a proximal tube of the ventroculoperitoneal shunt.
The patient was placed in the head-down position in conjunction with aggressive intravenous fluid replacement. The pressure of a programmable pressure valve was increased to allow the expansion of depressed brain area by 20 mmH2O.
The cranioplasty was performed, using the previous original sterilized bone flap three days after the V-P shunt. After operation, the patient mentality improved gradually to alert state and achieved good functional recovery. The follow-up CT scans images showed the restoration of midline shift without significant complications of the underlying brain (Fig. 5).
Fig. 5.
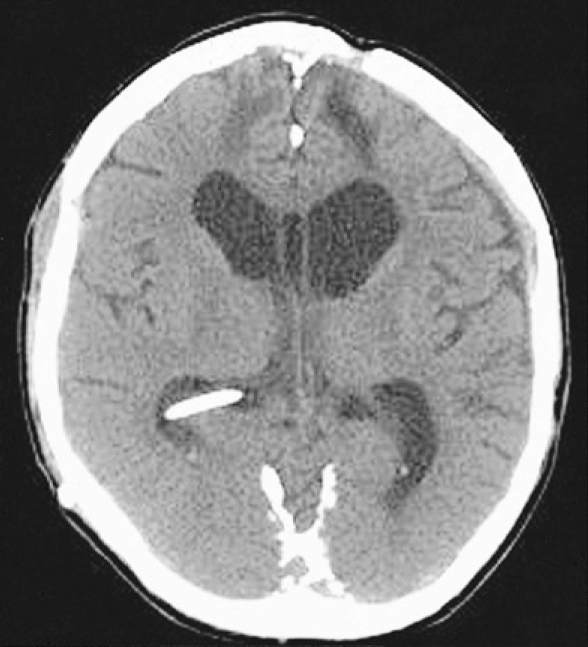
Postcranioplasty axial computed tomographic scan showing the restoration of midline shift without significant complications of underlying brain and subdural space.
DISCUSSION
Since Yamamura et al.15) reported in 1977, the syndrome of the sinking skin flap with neurological deterioration has been reported in the literature2,5,7,8,11,12). Many investigators have sought to explain the pathophysiology of this phenomenon. One had theorized that atmospheric pressure to be directly transmitted to the intracranial cavity, causing inward shifting of the scalp over craniectomy site6). According to this theory, George et al.3) showed that there was a correlation between the restoration of the midline shift and the clinical improvement following cranioplasty in a series of angiographies. Recently, several authors8,12) proposed that a negative gradient between atmospheric- and intracranial pressure, which is aggravated by changes in the CSF compartment following CSF hypovolemia to be the mechanism of neurological deterioration after craniectomy.
The CSF drainage in a patient suffering from hydrocephalus, or meningitis exacerbates this effect by creating a pressure gradient through craniectomy site. Also, prolonged dehydration and up-right position may precipitate this phenomenon. We postulated that our patient had multiple calcification around superior sagittal sinus that disturbed CSF absorption causing hydrocephalus. After V-P shunt, the increased negative pressure gradient between atmospheric-and intracranial pressure may have caused neurological deterioration such as the syndrome of the sinking skin flap.
The post-traumatic hydrocephalus occasionally develop in patients with undergoing decompressive craniectomy after head injury, accounting for 10.1% to 28.6%9,10). Although the obvious causal relationship between decompressive craniectomy and hydrocephalus remains unclear, the disturbance of CSF flow around the cerebral convexities may be the possible mechanism7). In the context of hydrocephalus after decompressive craniectomy, it is difficult for neurosurgeon to decide whether CSF diversion can be safely performed. Accordingly, it is important to recognize the presence of craniectomy with extreme caution to perform a continuous CSF diversion7).
The goal of treatment in patient with the syndrome of the sinking skin flap is restoration of the pressure exerted by depression of craniectomy site. Several authors4,5) suggested that severe CSF hypovolemia after craniotomy may produce a dramatic herniation syndrome that is completely reversed by the Trendelenberg position. Also, it was reported that intrathecal saline infusion reverses impending transtentorial herniation in patient with decline of mental status due to intracranial hypotnesion1). However, conservative management of the syndrome of the sinking skin flap with neurological deterioration is largely ineffective. The cranioplasty is the cornerstone of surgical treatment. Segal et al.13) suggested that cranioplasty improves the neurological deficits by an decrease in local intracranial pressure, and correction of abnormal CSF dynamics, reporting a case of neurological recovery after delayed cranioplasty. Another report suggested that the cranioplasty may affect postural blood flow regulation, cerebrovascular reserve capacity and cerebral glucose metabolism14). However, the cranioplasty for severe sinking at the skull defect may result in the dysfunction of the underlying brain, risk of fluid collection and hematoma formation in the subdural space due to the large dead space. The safe and effective surgical methods to expand the scalp depression and to eliminate the dead space in the context of V-P shunt are temporary occlusion or removal of shunt device before cranioplasty7). Additionally, the authors used a temporary increase of pressure valve to restore the scalp depression and midline shift.
CONCLUSION
We report an unusual case of patient who was treated by increasing the pressure of a programmable pressure valve and cranioplasty without complications in case of syndrome of sinking skin flap. Further clinical study will be needed to investigate clinical courses, causal relationship, and management of severe sinking problem after decompressive craniectomy.
References
- 1.Binder DK, Dillon WP, Fishman RA, Schmidt MH. Intrathecal saline infusion in the treatment of obtundation associated with spontaneous intracranial hypotension: technical case report. Neurosurgery. 2002;51:830–837. [PubMed] [Google Scholar]
- 2.Cho H, Kim CH, Kim JH, Kim JM. Paradoxical herniation after decompressive craniectomy for acute subdural hematoma. J Korean Neurosurg Soc. 2006;40:51–53. [Google Scholar]
- 3.George AE, Morantz RA, Abad RM, Rovit RL, Chase N. Neuroradiology of the posthemicraniectomy patient with special emphasis on the radiology of unilateral atrophy. Radiology. 1974;111:627–631. doi: 10.1148/111.3.627. [DOI] [PubMed] [Google Scholar]
- 4.Kelley GR, Johnson PL. Sinking brain syndrome: craniotomy can precipitate brainstem herniation in CSF hypovolemia. Neurology. 2004;62:157. doi: 10.1212/wnl.62.1.157. [DOI] [PubMed] [Google Scholar]
- 5.Komotar RJ, Mocco J, Ransom ER, Mack WJ, Zacharia BE, Wilson DA, et al. Herniation secondary to critical postcraniotomy cerebrospinal fluid hypovolemia. Neurosurgery. 2005;57:286–292. doi: 10.1227/01.neu.0000166661.96546.33. [DOI] [PubMed] [Google Scholar]
- 6.Langfitt TW. Increased intracranial pressure. Clin Neurosurg. 1968;16:436–471. doi: 10.1093/neurosurgery/16.cn_suppl_1.436. [DOI] [PubMed] [Google Scholar]
- 7.Liao CC, Kao MC. Cranioplasty for patients with severe depressed skull bone defect after cerebrospinal fluid shunting. J Clin Neurosci. 2002;9:553–555. doi: 10.1054/jocn.2002.1116. [DOI] [PubMed] [Google Scholar]
- 8.Oyelese AA, Steinberg GK, Huhn SL, Wijman CA. Paradoxical herniation secondary to lumbar puncture after decompressive craniectomy for a large space-occupying hemisphere stroke: case report. Neurosurgery. 2005;57:594. doi: 10.1227/01.neu.0000170437.79760.df. [DOI] [PubMed] [Google Scholar]
- 9.Phuenpathom N, Ratanalert S, Saeheng S. Post-traumatic hydrocephalus: experience in 17 consecutive cases. J Med Assoc Thai. 1999;82:46–53. [PubMed] [Google Scholar]
- 10.Polin RS, Shaffrey ME, Bogaev CA, Tisdale N, Germanson T, Bocchicchio B, et al. Decompressive bifrontal craniectomy in the treatment of severe refractory posttraumatic cerebral edema. Neurosurgery. 1997;41:84–94. doi: 10.1097/00006123-199707000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Schiffer J, Gur R, Nisim U, Pollak L. Symptomatic patients after craniectomy. Surg Neurol. 1997;47:231–237. doi: 10.1016/s0090-3019(96)00376-x. [DOI] [PubMed] [Google Scholar]
- 12.Schwab S, Erbuguth F, Aschoff A, Orberk E, Spranger M, Hacke W. Paradoxical herniation after decompressive trephining [in German] Nervenarzt. 1998;69:896–900. doi: 10.1007/s001150050360. [DOI] [PubMed] [Google Scholar]
- 13.Segal DH, Oppenheim JS, Murovic JA. Neurosurgical recovery after cranioplasty. Neurosurgery. 1994;34:729–731. doi: 10.1227/00006123-199404000-00024. [DOI] [PubMed] [Google Scholar]
- 14.Winkler PA, Stummer W, Linke R, Krishnan KG, Tatsch K. Influence of craniaoplasty on postural blood flow regulation, cerebrovascular reserve capacity, and cerebral glucose metabolism. J Neurosurg. 2000;93:53–61. doi: 10.3171/jns.2000.93.1.0053. [DOI] [PubMed] [Google Scholar]
- 15.Yamamura A, Sato M, Meguro K, Nakamura T, Uemura K, Makino H. Cranioplasty following decompressive craniectomy Analysis of 300 cases. No Shinkei Geka. 1977;5:345–353. [PubMed] [Google Scholar]


