Abstract
Objective
Burr hole drainage has been widely used to treat chronic subdural hematoma (CSDH). However, the incidence of recurrent CSDH varies from 3.7 to 30% after surgery. The authors attempted to elucidate the risk factors associated with the recurrence of CSDH in one burr hole drainage technique.
Methods
A total of 255 consecutive cases who underwent one burr hole drainage for CSDH were included in this study. Twenty-four patients (9.4%) underwent a repeated operation because of the recurrence of CSDH. We analyzed retrospectively the demographic, clinical and radiologic factors associated with the recurrence of CSDH.
Results
In this study, two risk factors were found to be independently associated with the recurrence of CSDH. The incidence of CSDH recurrence in the high- and mixed-density groups was significantly higher than those in the low- and iso-density groups (p<0.001). Bleeding tendency such as in leukemia, liver disease and chronic renal failure was also significantly associated with recurrence of CSDH (p=0.037).
Conclusion
These results suggest that high- and mixed- density shown on computed tomographic scan was closely relates with a high incidence of recurrence. Therefore, the operation could be delayed in those cases unless severe symptoms or signs are present. Reoperation using the previous burr hole site is a preferred modality to treat the recurrent CSDH.
Keywords: Chronic subdural hematoma (CSDH), Recurrence, Computed tomography
INTRODUCTION
Chronic subdural hematoma (CSDH) is one of the most frequent types of intracranial hemorrhage that is still associated with significant morbidity7,16,20). The CSDH is a common disease in elderly patients, and its incidence is highest in persons older than 70 years of age17). Although minor previous head injury is sometimes unrecognized, a mild traumatic event has preceded the hemorrhage in 60 to 80% of reported cases2,17). Three principal techniques have been used for resolution of CSDH : burr hole with or without irrigation and/or a closed drainage system, twist drill trephination and craniotomy8,14,19). Burr hole trephination with drainage is the most common procedure for treatment of a patient with CSDH. The burr hole drainage is relatively less invasive with higher cure rate, even for older or high-risk patients2,5,20). However, some patients suffer from the recurrence after surgery, with an incidence as variable as 3.7 to 30%2,11,13,14). Numerous factors influencing recurrence have been reported in the literature, but controversial findings are not uncommonly reported1,2,12,17,19). In our institute, one burr hole with closed system drainage has been the operative technique of choice over the last 20 years. The aim of this study is to analyze the potential risk factors in the recurrent CSDH treated with one burr hole with a closed drainage system retrospectively.
MATERIALS AND METHODS
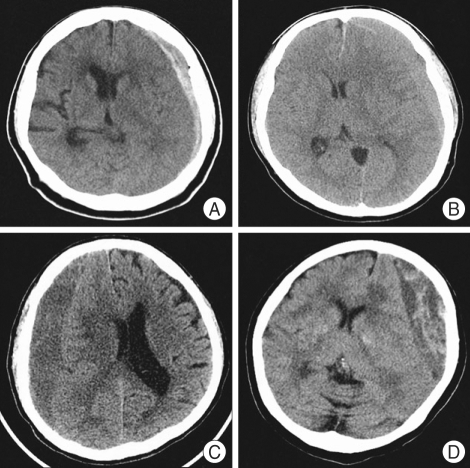
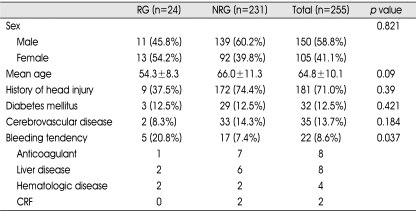
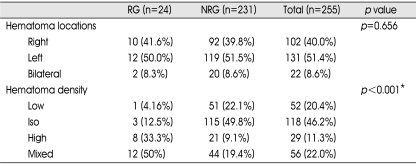
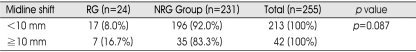
The medical and radiographic records of 255 consecutive cases with CSDH who underwent surgery for CSDH at our hospital between January 2001 and December 2006 were retrospectively reviewed. We defined CSDH as a SDH surrounded by a thin capsule and consisted of dark reddish liquefied blood found at operation. If the date of head trauma was clear, a CSDH was defined as a hematoma that had persisted more than three weeks after head trauma. The clinical features, computed tomography (CT) findings, surgical results, and postoperative complications were statistically analyzed to elucidate the factors affecting the recurrence of CSDH. Recurrence of CSDH was defined as re-accumulation of the hematoma in the postoperative hematoma cavity on follow-up CT scans within 3 months postoperatively and the reappearance of neurologic symptoms such as dementia, hemiparesis, headache or aphasia. In this study, hygroma, infantile CSDH, calcified or ossified CSDH were excluded because they were considered to be clinically different entities. On admission, neurologic examination was performed using the Glasgow Coma Scale score, and diagnosis was based on brain CT in all patients. Various informations such as history of head injury, onset of initial symptoms, alcohol abuse, and the use of anticoagulant or antiplatelet drugs were collected from the patient or family members. The correlation of recurrence was evaluated with personal and clinical factors such as age, sex, history of head injury, and interval between onset of initial symptoms and surgery; laboratory findings such as bleeding tendency and liver function; CT findings such as hematoma density and brain atrophy, the site of the hematoma (unilateral or bilateral), and the degree of midline shift. Hematoma density was classified into four types : low-, iso-, and high- and mixed density relative to the brain parenchymal density (Fig. 1). The liver function was estimated by the examination of SGOT/SGPT/total bilirubin, protein and albumin in initial serum of the patients. For the bleeding tendency, PT/aPTT examined in initial serum were investigated. Brain atrophy was classified into three stages : no or mild atrophy, definite atrophy such as dilated sulci, and severe atrophy such as widely dilated sulci and subdural space.
Fig. 1.
Chronic subdural hematoma is classified according to its density on brain CT scans. A: High-type, B: Iso-type, C: Low-type, D: Mixed-type.
A single burr hole was made at the site of its maximal hematoma thickness under local anesthesia. After dural incision, a small hole was opened in the outer membrane. The silicon tube was inserted into the hematoma cavity immediately, and then it was connected to a closed drainage system. No evacuation or irrigation of the hematoma was performed. The drainage tube was usually removed 2 days after surgery with the confirmation of residual hematoma amount on the follow up brain CT scan. Patients were kept in bed with hydration until the drain was removed. Patients were discharged as soon as symptoms related to the CSDH had disappeared and a follow-up CT scan had shown a total or significant reduction in the thickness of the CSDH. All patients were followed-up for more than three months until the clinically regarded as in remission.
Statistical analysis was performed with chi-square test and the student t-test to assess the relationship between each parameter and the recurrence of CSDH. For all analyses, a p-value of <0.05 was considered statistically significant.
RESULTS
The clinical data of 255 consecutive cases were summarized in Table 1. There were 150 men (58.8%) and 105 women (41.1%). Twenty-four patients (9.4%) experienced recurrences. There were 11 men (45.8%) and 13 women (54.2%). Reoperation was required in all 24 recurrent cases, and the third operation was performed in 3 cases. Mean age (± standard deviation) of patients in the recurrence group (RG) (54.3±8.3) was not significantly different from that in the non-recurrence group (NRG) (66.0±11.3). One-hundred-eighty-one out of 255 patients had remembered their initial head trauma. It ranged from 3 weeks to 3 months (42.9 days on average) for the patients with the history of initial trauma to be made as CSDH. Diabetes and cerebrovascular disease were the common underlying disease in 255 patients, but they were not related to the recurrence of CSDH. Bleeding tendency such as in leukemia, liver disease and chronic renal failure was significantly associated with recurrence of CSDH (p=0.037).
Table 1.
Characteristics and clinical findings in the recurrence group and the nonrecurrence group
RG: recurrence group, NRG: non-recurrence group, CRF: chronic renal failure
Preoperative CT findings were summarized in Table 2. The recurrence of CSDH occurred on the right side in 10 patients (41.6%), left side in 12 (50.0%), and bilateral in 2 cases (8.3%). There was no significant difference in relation to location between two groups (p<0.656). However, hematoma density was significantly different between two groups (p<0.001). The incidence of recurrence in the high-density group (33.3%) and mixed-density group (50%) was higher than that in any other groups. Mixed density consisted of a low- or iso-density at upper layer and a high-density lower layer, so could be considered as closely related with the high-density category. Classification of the hematomas into two groups combining the high-density and mixed-density groups, and the low-density and iso-density groups showed that the incidence of CSDH recurrence in the former group (23.5%) was significantly higher than those in the latter group (2.4%); p<0.001 between the two groups.
Table 2.
Preoperative computed tomographic findings and incidences of recurrence
*p-value reveals the relationship between low/iso-density group and high/mixed-density group, RG: recurrence group, NRG: non-recurrence group
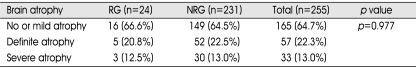
Degrees of midline shift of preoperative CT scan are shown in Table 3. Mean midline shift (±standard deviation) of patients in the RG (11.6±3.0 mm) was greater than that in the NRG (7.9±1.7 mm). Patients who had midline shift of less than 10 mm (8.0%) had a lesser chance of recurrence in comparison with patients with more than 10 mm (16.7%). However, the difference in both groups was not statistically significant (p=0.772). There was no correlation between the incidence of recurrence and severity of brain atrophy (Table 4).
Table 3.
Midline shift and incidences of recurrence
*RG: recurrence group, NRG: non-recurrence group
Table 4.
Types of brain atrophy and incidences of recurrence
RG: recurrence group, NRG: non-recurrence group
DISCUSSION
Burr hole drainage could be the best modality as the initial treatment for CSDH. Recently, lower recurrence rates using a burr hole and closed drainage system without irrigation have been reported11,17). The intention of irrigation is to remove the hematoma completely or at least to dilute its content. However, repeated irrigation induces the abrupt decrease in intracranial pressure, resulting in complications such as convulsions, brain edema and cerebral hemorrhage17). In this study, the recurrence rate of CSDH was 8.7%. It is generally considered that craniotomy is mandatory if burr hole drainage fails. However, craniotomy has a greater associated morbidity and mortality3). We performed repeated burr hole drainage at the previous operation site, which provided good results. Our study suggests that burr hole drainage using the previous burr hole appears to be effective treatment in the recurred CSDH. Craniotomy should be saved in a patient with a solid hematoma or who suffers multiple recurrences4).
Risk factors for CSDH recurrence are divided into three categories. Factors associated with patient characteristics, such as old age, brain atrophy, poor performance status on admission, bleeding tendency, kidney diseases, liver dysfunction, chronic alcoholism, diabetes, epilepsy, dementia, hemodialysis, use of anticoagulants, antiplatelet and chemotherapeutic agents, intracranial hypotension (cerebrospinal fluid shunt surgery), have commonly been reported1,16,20). Factors associated with the pathogenesis of CSDH include large hematoma, bilateral CSDHs, septum formation or multiple membranes in the hematoma cavity, mixed-density and high-density hematoma on the CT scan2,9,10,12,14,20). Factors associated with surgery such as 1) insufficient drainage intraoperatively and postoperatively, 2) air collection in the hematoma cavity, 3) early surgery when the hematoma has a poorly developed capsule are thought to increase the risk of recurrence17). We therefore attempted to confirm the risk factors in our series, and finally following variables were found to be correlated with the recurrence of CSDH : 1) preoperative bleeding tendency, 2) high- or mixed-density group on the preoperative CT scan.
The pathogenesis of the formation and development of CSDH is complex. Membrane formation around the hematoma and secondary enlargement of the hematoma are characteristic features of CSDH. Repeated microhemorrhages from the neocapillary network in the outer membrane or abnormally high vascular permeability is thought to be responsible for hematoma enlargement in CSDH21). Neomembrane in recurrent cases is vulnerable to repeat microbleeding into hematoma cavity over the short term. The density of CSDH reflects the proportion of fresh blood clots in hematoma cavity, and high density indicates that the hematoma involves in much fresh bleeding16). High proportion of fresh blood clots means the active growth of blood vessels into the membrane of CSDH. Several reports have shown the variation of rebleeding tendency in different hematoma stages13,15). Nakaguchi et al.13) advocated three different phases of hematoma development based on the neuroimaging : a homogenous stage (stage 1), a seprated or multilayered stage (stage 2), and a trabecular stage (stage 3). During the stage 1 (homogenous type on CT scans), the rebleeding tendency is low because the balance between fibrinolysis and coagulation is maintained. The neuroimaging appearance of the hematoma is predominantly homogenous, hypo- or isodense according to whether the bleeding is more or less recent. During stage 2 (separated or multilayered stage), hyperfibrinolysis and the rebleeding from the neomembrane is high. This process ceases in stage 3 (trabecular stage) because the membrane has a large fibrous component, and therefore, bleeding tendency is extremely low2,13). In this study, the incidence of CSDH recurrence in the high-density and mixed-density groups was significantly higher than that in the low-density and iso-density groups (p<0.001). These results suggest the CSDH with rapid progression in the relatively acute phase appears as high density on CT have a high incidence of recurrence. It is clear that the phase of initial brain CT and neurologic condition are the most important factors in the treatment of CSDH. The possibility of recurrence is high in the case of the short period of time between the onset of symptoms and the operation. Early surgery when the hematoma has a poorly developed capsule could increase the risk of recurrence. Therefore, if the CSDH with rapid progression in the acute phase as high density on CT scan is likely to recur, the operation could be delayed unless severe symptoms are present. The surgical procedure may be performed in the later stage when the density of CSDH changes to be iso- or low-density on CT scan.
In most case of CSDH, the origin of hematoma is the torn bridging vein. Owing to the venous bleeding, the initial subdural hemorrhage is small and does not usually induce a sudden increase in intracranial pressure. Additionally, the wide sulci and atrophic brain allows for some enlargement before the onset of significant neurololgical symptoms. Large hematoma, older age and brain atrophy have been reported as risk factors for the recurrence4). There are many occasions when hematoma progresses for a long time after its occurrence without the effect of elevated intracranial pressure in old patients due to severe brain atrophy. In the present study, older age, brain atrophy and midline shift were not associated with recurrent CSDH. It seems that the older the patient, the longer it takes for the brain to be restored. It is possible that prolonged reaccumulations of blood within the hematoma cavity might have been misunderstood as unnecessarily subjective to reoperation6,18). Additional studies are needed to confirm those factors associated with the recurrence in the future.
CONCLUSION
In the present study, two major risk factors, hematoma density and underlying bleeding tendency, might be helpful in predicting the recurrence of CSDH after burr hole drainage. In case of high-density on CT scan, the operation could be delayed unless severe symptoms are present. In addition, burr hole drainage using the previous burr hole is preferred for recurrent CSDH.
References
- 1.El-Kadi H, Miele VJ, Kaufman HH. Prognosis of chronic subdural hematomas. Neurosurg Clin N Am. 2000;11:553–567. [PubMed] [Google Scholar]
- 2.Frati A, Salvati M, Mainiero F, Ippoliti F, Rocchi G, Raco A, et al. Inflammation markers and risk factors for recurrence in 35 patients with a posttraumatic chronic subdural hematoma: a prospective study. J Neurosurg. 2004;100:24–32. doi: 10.3171/jns.2004.100.1.0024. [DOI] [PubMed] [Google Scholar]
- 3.Gazzeri R, Galarza M, Neroni M, Canova A, Refice GM, Esposito S. Continuous subgaleal suction drainage for the treatment of chronic subdural haematoma. Acta Neurochir (Wien) 2007;149:487–493. doi: 10.1007/s00701-007-1139-8. [DOI] [PubMed] [Google Scholar]
- 4.Gelabert-Gonzalez M, Iglesias-Pais M, Garcia-Allut A, Martinez-Rumbo R. Chronic subdural haematoma: surgical treatment and outcome in 1000 cases. Clin Neurol Neurosurg. 2005;107:223–229. doi: 10.1016/j.clineuro.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Gonugunta V, Buxton N. Warfarin and chronic subdural haematomas. Br J Neurosurg. 2001;15:514–517. doi: 10.1080/02688690120097822. [DOI] [PubMed] [Google Scholar]
- 6.Jeong CA, Kim TW, Park KH, Chi MP, Kim JO, Kim JC. Retrospective analysis of re-operated patients after chronic subdural hematoma surgery. J Korean Neurosurg Soc. 2005;38:116–120. [Google Scholar]
- 7.Kang HL, Shin HS, Kim TH, Hwang YS, Park SK. Clinical analysis of recurrent chronic subdural hematoma. J Korean Neurosurg Soc. 2006;40:262–266. [Google Scholar]
- 8.Kang MS, Koh HS, Kwon HJ, Choi SW, Kim SH, Youm JY. Factors Influencing recurrent chronic subdural hematoma after surgery. J Korean Neurosurg Soc. 2007;41:11–15. [Google Scholar]
- 9.Kim HY, Kwon SC, Kim TH, Shin HS, Hwang YS, Park SK. Analysis of management according to CT findings in chronic subdural Hematoma. J Korean Neurosurg Soc. 2005;37:96–100. [Google Scholar]
- 10.Kostanian V, Choi JC, Liker MA, Go JL, Zee CS. Computed tomographic characteristics of chronic subdural hematomas. Neurosurg Clin N Am. 2000;11:479–489. [PubMed] [Google Scholar]
- 11.Kuroki T, Katsume M, Harada N, Yamazaki T, Aoki K, Takasu N. Strict closed-system drainage for treating chronic subdural haematoma. Acta Neurochir (Wien) 2001;143:1041–1044. doi: 10.1007/s007010170010. [DOI] [PubMed] [Google Scholar]
- 12.Murakami H, Hirose Y, Sagoh M, Shimizu K, Kojima M, Gotoh K, et al. Why do chronic subdural hematomas continue to grow slowly and not coagulate? Role of thrombomodulin in the mechanism. J Neurosurg. 2002;96:877–884. doi: 10.3171/jns.2002.96.5.0877. [DOI] [PubMed] [Google Scholar]
- 13.Nakaguchi H, Tanishima T, Yoshimasu N. Factors in the natural history of chronic subdural hematomas that influence their postoperative recurrence. J Neurosurg. 2001;95:256–262. doi: 10.3171/jns.2001.95.2.0256. [DOI] [PubMed] [Google Scholar]
- 14.Nakaguchi H, Tanishima T, Yoshimasu N. Relationship between drainage catheter location and postoperative recurrence of chronic subdural hematoma after burr-hole irrigation and closed-system drainage. J Neurosurg. 2000;93:791–795. doi: 10.3171/jns.2000.93.5.0791. [DOI] [PubMed] [Google Scholar]
- 15.Nomura S, Kashiwagi S, Fujisawa H, Ito H, Nakamura K. Characterization of local hyperfibrinolysis in chronic subdural hematomas by SDS-PAGE and immunoblot. J Neurosurg. 1994;81:910–913. doi: 10.3171/jns.1994.81.6.0910. [DOI] [PubMed] [Google Scholar]
- 16.Oishi M, Toyama M, Tamatani S, Kitazawa T, Saito M. Clinical factors of recurrent chronic subdural hematoma. Neurol Med Chir (Tokyo) 2001;41:382–386. doi: 10.2176/nmc.41.382. [DOI] [PubMed] [Google Scholar]
- 17.Okada Y, Akai T, Okamoto K, Iida T, Takata H, Iizuka H. A comparative study of the treatment of chronic subdural hematoma-burr hole drainage versus burr hole irrigation. Surg Neurol. 2002;57:405–409. doi: 10.1016/s0090-3019(02)00720-6. discussion 410. [DOI] [PubMed] [Google Scholar]
- 18.Tsutsumi K, Maeda K, Iijima A, Usui M, Okada Y, Kirino T. The relationship of preoperative magnetic resonance imaging findings and closed system drainage in the recurrence of chronic subdural hematoma. J Neurosurg. 1997;87:870–875. doi: 10.3171/jns.1997.87.6.0870. [DOI] [PubMed] [Google Scholar]
- 19.Voelker JL. Nonoperative treatment of chronic subdural hematoma. Neurosurg Clin N Am. 2000;11:507–513. [PubMed] [Google Scholar]
- 20.Yamamoto H, Hirashima Y, Hamada H, Hayashi N, Origasa H, Endo S. Independent predictors of recurrence of chronic subdural hematoma: results of multivariate analysis performed using a logistic regression model. J Neurosurg. 2003;98:1217–1221. doi: 10.3171/jns.2003.98.6.1217. [DOI] [PubMed] [Google Scholar]
- 21.Yamashima T, Yamamoto S. Clinicopathological classification of chronic subdural hematoma. Zentralbl Neurochir. 1985;46:304–314. [PubMed] [Google Scholar]