Abstract
Objective
To evaluate the surgical outcomes of ventral interbody grafting and anterior or posterior spinal instrumentation for the treatment of advanced spondylodiscitis in patients who had failed medical management.
Methods
A total of 28 patients were evaluated for associated medical illness, detected pathogen, level of involved spine, and perioperative complications. Radiological evaluation including the rate of bony union, segmental Cobb angle, graft- and instrumentation-related complications, and clinical outcomes by mean Frankel scale and VAS score were performed.
Results
There are 14 pyogenic spondylodiscitis, 6 postoperative spondylodiscitis, and 8 tuberculous spondylodiscitis. There were 21 males and 7 females. Mean age was 51 years, with a range from 18 to 77. Mean follow-up period was 10.9 months. Associated medical illnesses were 6 diabetes, 3 pulmonary tuberculosis, and 4 chronic liver diseases. Staphylococcus was the most common pathogen isolated (25%), and Mycobacterium tuberculosis was found in 18% of the patients. Operative approaches, either anterior or posterior spinal instrumentation, were done simultaneously or delayed after anterior aggressive debridement, neural decompression, and structural interbody bone grafting. All patients with neurological deficits improved after operation, except only one who died from aggravation as military tuberculosis. Mean Frankel scale was changed from 3.78±0.78 preoperatively to 4.78±0.35 at final follow up and mean VAS score was improved from 7.43±0.54 to 2.07±1.12. Solid bone fusion was obtained in all patients except only one patient who died. There was no need for prolongation of duration of antibiotics and no evidence of secondary infection owing to spinal instrumentations.
Conclusion
According to these results, debridement and anterior column reconstruction with ventral interbody grafting and instrumentation is effective and safe in patients who had failed medical management and neurological deficits in advanced spondylodiscitis.
Keywords: Advanced spondylodiscitis, Ventral interbody grafting, Spinal instrumentation
INTRODUCTION
Spine is one of the most common site for hematogenous bone infections. Spondylodiscitis is rare, but represent a serious medical condition. Most patients with spondylodiscitis can be treated conservatively with long periods of intravenous antibiotic therapy and immobilization with bracing. Although early stage of spondylodiscitis is well responsive to conservative treatment, advanced or complicated cases should be considered for more aggressive therapy such as surgical management. Surgical management is usually indicated for patients in whom medical management of the disease has failed, those with progressive neurological compromise, spinal instability and deformity due to significant endplate and vertebral destruction, or intractable pain. Roles of surgery for these complicated spondylodiscitis are debridement of affected tissues, neural decompression, ventral vertebral body reconstruction, and spinal stabilization.
Surgical treatment options are numerous and a controversy concerning the role of spinal instrumentation in the presence of active infection is still remained. Several authors have suggested bed rest and prolonged external bracing rather than placing spinal instrumentation1,12). Others have advocated a staged instrumented operation with a period of antibiotics therapy after debridement only surgery4,17,19). In this study, anterior debridement and decompression, ventral interbody grafting, and anterior or posterior instrumentation for stabilization were performed in order to increase the spinal stability and to maintain spinal alignment for more rapid ambulation.
Therefore, the purpose of this study was to evaluate the clinical outcomes and radiographical results of 28 patients with advanced spondylodiscitis treated with these operations.
MATERIALS AND METHODS
Of the 45 patients who were admitted with spinal infection between September 1997 and March 2004, 28 patients who had advanced spondylodiscitis and failed medical management were managed with anterior debridement, strut-autologous iliac bone grafting and anterior or posterior instrumentation. Complete removal of the infected, necrotic tissue was attempted with extensive irrigation with antibiotic solution. Mean follow-up period was 10.9 months, with a range from 6 to 24 months. Diagnosis of spondylodiscitis was made through physical examination, neuroimaging study, and laboratory findings. Fever and leukocytosis were found in most of patients. An elevated value of erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were considered to be common laboratory abnormality10,20). Of these, CRP level was a more valuable serum marker during follow-up study because of its temporal pattern in the blood stream and comparatively quicker normalization with effective treatment20). Type of spondylodiscitis was classified as pyogenic, tuberculosis, and postoperative spondylodiscitis.
Pyogenic and tuberculosis spondylodiscitis were diagnosed according to pathogen of disease. Postoperative spondylodiscitis was suspected when the mean CRP level had not returned to a value below 50% of the peak value on the second postoperative day23), or when these markers fail to show a decline from preoperative values with aggravated clinical symptoms and signs such as fever, intractable pain, or neurological deficit.
Hospital records were reviewed for all available demographic data, level of involvement, neurological status, and presence of associated medical illness, organism isolated, types of surgical approach, and any perioperative complications. Radiological results were evaluated by bony fusion rate, segmental Cobb angle, and graft with instrumentation related complications. Clinical outcomes were evaluated with Frankel scale and VAS score.
RESULTS
Of the 28 patients, 21 were males and 7 females. Mean age was 51 years (range 18-77 years). The mean duration of symptoms before admission was 2.7 months with a range from 1 to 6 months. Fourteen patients presented with pyogenic spondylodiscitis, 8 patients with tuberculosis spondylodiscitis and 6 with postoperative spondylodiscitis. There were 4 cervical, 9 thoracic, and 15 lumbar lesions. Medical comorbidities were present in 13 patients (46%). Six patients had previous diabetes, 4 patients chronic liver disease, and 3 patients with pulmonary tuberculosis.
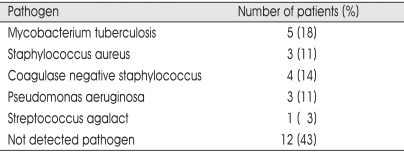
The most common identified organism was staphylococcus species (25%) including 3 staphylococcus aureus and 4 coagulase-negative staphylococcus. Next common pathogen was Mycobacterium tuberculosis (18%). Twelve patients (43%) showed negative cultures (Table 1). Anterior interbody fusion with anterior instrumentation were performed in 13 patients. Anterior interbody fusion with posterior instrumentation (one-staged operation was done in 9 patients, delayed two-staged operation was done in 6 patients) were performed in 15 patients. The two-stage surgical treatment for pyogenic or tuberculotic spondylitis used in patients who were in poor general condition. After operation, most of these patients were ambulated with the brace and several paralytic patients were mobilized with regular bedside physical therapy within 4 postoperative days. Average duration of postoperative intravenous antibiotic therapy in pyogenic and postoperative spondylodiscitis was six weeks and oral anti-tuberculosis drug was given fifteen months in tuberculosis spondylodiscitis. After a significant decrease in C-reactive protein and confirmation of no pathogen, the regimen was stopped. Initial antibiotic treatment consisted of a combination of aminoglycoside with second generation cephalosporin and modified depending on the sensitivity of the isolated organisms. Infection was successfully controlled in all patients except one patient who died from aggravation of miliary pulmonary tuberculosis and no recurrence was noted. ESR, CRP and leukocyte counts returned to normal within 6 months. It was also stressed that aggressive parenteral and enteral nutritional support was very important in theses patients and systemic illness should be controlled and any underlying focus of infection must be treated concurrently with the spine infection.
Table 1.
Pathogens isolated by perioperative cultures in 28 patients with spondylodiscitis
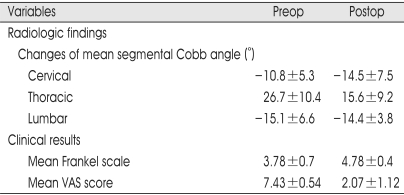
Successful interbody bony fusion rate was observed in 27 patients (96% fusion rate, except one patient who died of miliary tuberculosis aggravation). Radiolographically, mean segmental Cobb angle was determined preoperatively and postoperatively. For cervical lesions, average preoperative Cobb angle was -10.8±5.3˚, improving by a -14.5±7.5˚. For thoracic lesions, there was an average preoperative segmental Cobb angle was 26.7±10.4˚, and was improved to 15.6±9.2˚. For lumbar lesions (below L2), there was no improvement of average Cobb angle. In all patients, mean Frankel scale in preoperative state was 3.78±0.70, and this was improved to 4.78±0.35 at final follow-up. Mean VAS score at preoperative period was 7.43±0.54, and was 2.07±1.12 at final follow-up (Table 2).
Table 2.
Summary of surgical results of 28 patients with spondylodiscitis
Of the 28 patients, one patient died from acute respiratory distress syndrome 3 months postoperatively. In another patient, the screw had to be revised because there was posterior screw loosening. Two patients had superficial wound infection at surgical site but they did not require removal of the implanted material for spondylodesis. Two patients suffered from pleural effusion (Table 3). There were no needs for prolongation of antibiotics therapy and hospitalization. There have been no recurrence of infection and no evidence of secondary infection due to spinal instrumentations.
Table 3.
Perioperative complications
llustrative Cases
Case 1
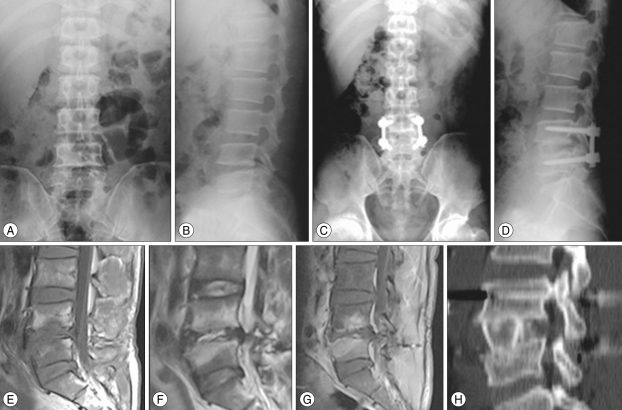
A 44-year-old male was transferred to our hospital with progressive, severe back pain with both leg radiating pain and weakness. Magnetic resonance imaging studies and X-ray films revealed findings consistent with spondylodiscitis at L4-L5. Patient was treated as one-stage anterior structural bone grafting and posterior transpedicular stabilization from L4 to L5. Intraoperative culture was obtained, and the result of the intraoperative culture was methicillin-sensitive Staphylococcus aureus. Final follow-up CT scans and X-ray films demonstrated successful fusion and maintained excellent lumbar lordotic curvature (Fig. 1).
Fig. 1.
A 44-year-old male with pyogenic spondylodiscitis and destruction of L4 and L5 endplate (A, B) treated with one stage anterior strut grafting at L4-L5 and posterior transpedicular stabilization of L4-L5. Spinal canal encroachment by infectious debris and L4, L5 endplate destruction with ring enhancement at the disc space can be seen (E-G). Postoperative X-rays (C, D, H) demonstrate a good fusion state and lumbar lordotic curvature.
Case 2
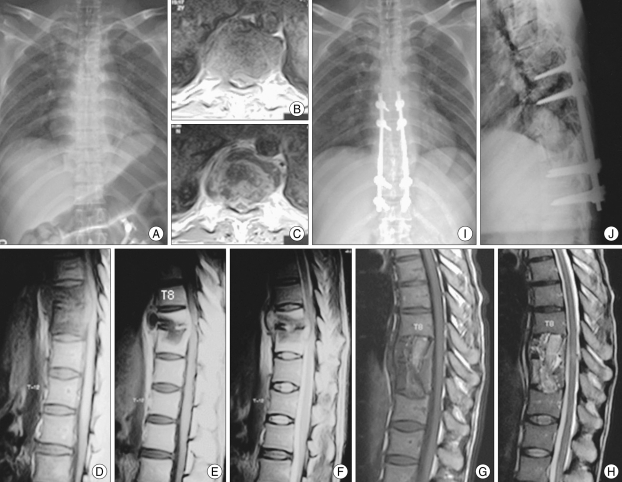
A 40-year-old male was admitted to the hospital with severe back pain and lower extremity motor weakness. Magnetic resonance images and X-ray films were obtained, which revealed a bony destruction of T9 and T10 vertebral bodies and extensive paravertebral and epidural abscess with cord compression from T8-T10 level. Two-stage operation was performed with T9 and T10 corpectomy and T8 to T11 autograft fusion initially followed by posterior transpedicular instrumentation from T7 to T12 level after 7 days. Follow-up Magnetic resonance images and plain X-ray films demonstrated excellent union and sagittal alignment (Fig. 2).
Fig. 2.
A 40-year-old male with tuberculosis spondylodiscitis with bony destruction of T9 and T10, and an extensive perivertebral abscess from T8 to T10 (A-F). T9 and T10 corpectomy and anterior interbody fusion with rib and iliac autograft via right side transthoracic approach (G, H), and 1 week later posterior transpedicular instrumentation on T7-T12 were performed. Postoperative X-ray follow-up demonstrates solid interbody fusion and eradication of infection (I, J).
DISCUSSION
The diagnosis of spondylodiscitis should be considered in patients who present with insidious-onset, progressive, severe back or neck pain associated with fever and other systemic symptoms. Suspicion must be increased in patients of advanced age, with diabetes mellitus, who are immunocompromised (chemotherapy or chronic alcoholism), or who have undergone prior surgery or have an established focus of infection. Neurological deficits associated with spondylodiscitis have been reported in 17 to 60% of cases5).
Well-known pathophysiological mechanisms of spondylodiscitis are hematogenous metastasis of microbial organisms into the richly vascularized vertebral metaphyses or through paraspinous venous plexus secondary to bacteremia16). The genitourinary tract, upper respiratory tract, oral cavity, cutaneous ulcer, traumatic wounds, and surgical site are common sources of the infection. The most commonly isolated organisms in the literature are staphylococcus species, followed in frequency by streptococcus species and gram-negative organisms including Escherichia coli22). For accurate identification of pathogen, percutaneous needle biopsy sampling of the infected vertebral body or disc space under fluoroscopic or CT guidance, or blood cultures should be considered. In this study, commonly cultured pathogens were staphylococcus species (25%), specific bacteria such as Mycobacterium tuberculosis (18%), pseudomonas aeruginosa (11%) but there were 12 patients (43%) whom had no isolated pathogens.
Because of relatively large vertebral body and disc space, the majority of spondylodiscitis reported to involve the lumbar and thoracic spines. The most common location in this study was lumbar spine (54%), followed by the thoracic spine (32%) and cervical spine (14%).
Imaging examination consists of plain radiographs, MRI, and CT scans. Plain radiographs generally demonstrates distinct endplate erosions suggestive of infection. MRI exhibits high sensitivity in the early identification of spondylodiscitis in all patients. MRI also clearly demonstrates the presence and location of any retropharyngeal, paravertebral, psoas, and epidural abscess. When there is spondylitis or discitis, edema and purulent material in the marrow or disc space will appear as a low signal with enhancement on T1-weighted and as a bright signal on T2-weighted images. CT scans with sagittal reconstructions can be used to delineate the degree of osseous destruction and determine the extent of the bone graft needed to reconstruct the vertebral defect. MRI was the diagnostic tool of choice in this study.
There are some controversies on operative or conservative treatment of spondylodiscitis despite modern medical management with antibiotics13,18,20). Conservative medical management in these patients is usually accompanied by a long period of immobilization as well as incomplete bony fusion. If bone destruction exists, the rate of pseudoarthrosis and instability is reported to be as high as 50%6). The indications for surgical treatment in this study were : 1) intractable pain, 2) failed medical management, 3) the presence of a neurological deficit, 4) significant endplate and vertebral destruction, 5) spinal instability or developing deformity.
Several surgical modalities have been reported for advanced staged spondylodiscitis via anterior approach : 1) anterior decompression with bone graft; 2) anterior decompression and fusion with posterior instrumentation8,9,20). and 3) anterior debridement and interbody grafting with anterior instrumentation2,3,6,11,24). Although, most spine surgeons are generally unwilling to place implant in infected area when the debridement of infected tissue is complete and an appropriate antibiotic therapy is followed, reconstruction of anterior vertebral column and instrumentation could be reasonable in active spondylodiscitis. Anterior debridement and interbody grafting with anterior instrumentation, anterior debridement and bone grafting, one-stage or two-stage posterior instrumentation did not reveal the recurrence of infection in this study. A retrospective review by Levi et al.14) found that no infections occurred after anterior spinal instrumentation procedures. Recurrence of infection in the presence of instrumentation is similar to that in its absence, indicating that infection may not be a contraindication for its use21). Furthermore, insertion of titanium interbody cage after debridement have gained wide acceptance in the setting of concomitant infection. Recently, several retrospective studies revealed greater improvement in sagittal alignment in patients with titanium cages and in patients with posterior instrumentation than for patients without such instrumentations7,9,15).
Anterior surgical approach was used to allow direct access to the focus of infection for aggressive debridement because spondylodiscitis primarily involved anterior vertebral body and adjacent disc spaces. These surgical procedures had provided several advantages such as correction of the deformed spine, complete decompression of spinal compression, and immediate spine stabilization. In this study, successful interbody bony fusion rate was achieved in 27 patients (96% fusion rate). Radiolographically, for cervical lesions, there was an average preoperative Cobb angle (-10.8±5.3˚) was improved to -14.5±7.5˚ postoperatively. For thoracic lesions, there was an average preoperative segmental Cobb angle in 26.7±10.4˚, improving by a 15.6±9.2˚. For lumbar lesions (below L2), there was no improvement of average Cobb angle. This study also revealed that the aggressive debridement and strut bone grafting to allow for adequate pathogen isolation for culture, neural decompression, and anterior spinal column support. Most patients improved significantly not only their general conditions such as fever, pain, and general infection markers, but also neurological status after surgery without any serious complications. In this study, mean Frankel scale at preoperative period 3.78±0.70, was improved to be 4.78±0.35 at final follow-up. Mean VAS score also improved from 7.43±0.54 at preoperative period to 2.07±1.12 at final follow-up. This technique has provided excellent relief of pain and demonstrated a high recovery rate of neurological functions and a high fusion rate for advanced staged spondylodiscitis.
CONCLUSION
Ventral interbody grafting and anterior or posterior instrumentation method after aggressive debridement is highly effective in the treatment of advanced staged spondylodiscitis. If the debridement of infected tissue is complete, instrumentation shows no occurrence of secondary infection and does not prolong the usage of antibiotics and hospitalization in the treatment of any types of advanced spondylodiscitis who had failed medical management without any serious complications.
References
- 1.Asamoto S, Doi H, Kobayashi N, Endoh T, Sakagawa H, Iwanaga Y, et al. Spondylodiscitis: diagnosis and treatment. Surg Neurol. 2005;64:103–108. doi: 10.1016/j.surneu.2004.11.034. discussion 108. [DOI] [PubMed] [Google Scholar]
- 2.Benli IT, Acaroglu E, Akalin S, Kis M, Duman E, Un A. Anterior radical debridement and anterior instrumentation in tuberculosis spondylitis. Eur Spine J. 2003;12:224–234. doi: 10.1007/s00586-002-0403-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benli IT, Kaya A, Acaroglu E. Anterior Instrumentation in Tuberculous Spondylitis: Is it Effective and Safe? Clin Orthop Relat Res. 2007;460:108–116. doi: 10.1097/BLO.0b013e318065b70d. [DOI] [PubMed] [Google Scholar]
- 4.Dimar JR, Carreon LY, Glassman SD, Campbell MJ, Hartman MJ, Johnson JR. Treatment of pyogenic vertebral osteomyelitis with anterior debridement and fusion followed by delayed posterior spinal fusion. Spine. 2004;29:326–332. doi: 10.1097/01.brs.0000109410.46538.74. discussion 332. [DOI] [PubMed] [Google Scholar]
- 5.Eismont FJ, Bohlman HH, Soni PL, Goldberg VM, Freehafer AA. Pyogenic and fungal vertebral osteomyelitis with paralysis. J Bone Joint Surg Am. 1983;65:19–29. [PubMed] [Google Scholar]
- 6.Eysel P, Hopf C, Vogel I, Rompe JD. Primary stable anterior instrumentation or dorsoventral spondylodesis in spondylodiscitis? Results of a comparative study. Eur Spine J. 1997;6:152–157. doi: 10.1007/BF01301428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fayazi AH, Ludwig SC, Dabbah M, Bryan Butler R, Gelb DE. Preliminary results of staged anterior debridement and reconstruction using titanium mesh cages in the treatment of thoracolumbar vertebral osteomyelitis. Spine J. 2004;4:388–395. doi: 10.1016/j.spinee.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Hadjipavlou AG, Mader JT, Necessary JT, Muffoletto AJ. Hematogenous pyogenic spinal infections and their surgical management. Spine. 2000;25:1668–1679. doi: 10.1097/00007632-200007010-00010. [DOI] [PubMed] [Google Scholar]
- 9.Hee HT, Majd ME, Holt RT, Pienkowski D. Better treatment of vertebral osteomyelitis using posterior stabilization and titanium mesh cages. J Spinal Disord Tech. 2002;15:149–156. doi: 10.1097/00024720-200204000-00010. discussion 156. [DOI] [PubMed] [Google Scholar]
- 10.Hsieh PC, Wienecke RJ, O'Shaughnessy BA, Koski TR, Ondra SL. Surgical strategies for vertebral osteomyelitis and epidural abscess. Neurosurg Focus. 2004;17:E4. doi: 10.3171/foc.2004.17.6.4. [DOI] [PubMed] [Google Scholar]
- 11.Jung SW CB, Rhee DY. Surgical treatment of tuberculous spondylitis. J Korean Neurosurg Soc. 1997;26:384–393. [Google Scholar]
- 12.Krodel A, Sturz H. Differentiated surgical and conservative treatment of spondylitis and spondylodiscitis. Z Orthop Ihre Grenzgeb. 1989;127:587–596. doi: 10.1055/s-2008-1040296. [DOI] [PubMed] [Google Scholar]
- 13.Lehovsky J. Pyogenic vertebral osteomyelitis/disc infection. Baillieres Best Pract Res Clin Rheumatol. 1999;13:59–75. doi: 10.1053/berh.1999.0006. [DOI] [PubMed] [Google Scholar]
- 14.Levi AD, Dickman CA, Sonntag VK. Management of postoperative infections after spinal instrumentation. J Neurosurg. 1997;86:975–980. doi: 10.3171/jns.1997.86.6.0975. [DOI] [PubMed] [Google Scholar]
- 15.Liljenqvist U, Lerner T, Bullmann V, Hackenberg L, Halm H, Winkelmann W. Titanium cages in the surgical treatment of severe vertebral osteomyelitis. Eur Spine J. 2003;12:606–612. doi: 10.1007/s00586-003-0614-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mann S, Schutze M, Sola S, Piek J. Nonspecific pyogenic spondylodiscitis: clinical manifestations, surgical treatment, and outcome in 24 patients. Neurosurg Focus. 2004;17:E3. doi: 10.3171/foc.2004.17.6.3. [DOI] [PubMed] [Google Scholar]
- 17.McGuire RA, Eismont FJ. The fate of autogenous bone graft in surgically treated pyogenic vertebral osteomyelitis. J Spinal Disord. 1994;7:206–215. doi: 10.1097/00002517-199407030-00002. [DOI] [PubMed] [Google Scholar]
- 18.Osenbach RK, Hitchon PW, Menezes AH. Diagnosis and management of pyogenic vertebral osteomyelitis in adults. Surg Neurol. 1990;33:266–275. doi: 10.1016/0090-3019(90)90047-s. [DOI] [PubMed] [Google Scholar]
- 19.Ozuna RM, Delamarter RB. Pyogenic vertebral osteomyelitis and postsurgical disc space infections. Orthop Clin North Am. 1996;27:87–94. [PubMed] [Google Scholar]
- 20.Przybylski GJ, Sharan AD. Single-stage autogenous bone grafting and internal fixation in the surgical management of pyogenic discitis and vertebral osteomyelitis. J Neurosurg. 2001;94:1–7. doi: 10.3171/spi.2001.94.1.0001. [DOI] [PubMed] [Google Scholar]
- 21.Rath SA, Neff U, Schneider O, Richter HP. Neurosurgical management of thoracic and lumbar vertebral osteomyelitis and discitis in adults: a review of 43 consecutive surgically treated patients. Neurosurgery. 1996;38:926–933. doi: 10.1097/00006123-199605000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Sapico FL. Microbiology and antimicrobial therapy of spinal infections. Orthop Clin North Am. 1996;27:9–13. [PubMed] [Google Scholar]
- 23.Steffen KR, Alireza G, Peter MZ, Madjid S. Monirotiring of blood parameters following anterior cervical fusion. J Neurosurg (Spine 2) 2000;92:169–174. doi: 10.3171/spi.2000.92.2.0169. [DOI] [PubMed] [Google Scholar]
- 24.Yilmaz C, Selek HY, Gurkan I, Erdemli B, Korkusuz Z. Anterior instrumentation for the treatment of spinal tuberculosis. J Bone Joint Surg Am. 1999;81:1261–1267. doi: 10.2106/00004623-199909000-00007. [DOI] [PubMed] [Google Scholar]