Abstract
The T-cell dependency of B-cell responses to variant surface glycoprotein (VSG) epitopes exposed in their native surface conformation on Trypanosoma brucei rhodesiense clone LouTat 1 was investigated. T-cell requirements were examined by analyses of gamma globulin preparations derived from trypanosome-infected BALB/c nude (nu/nu) and thymus-intact (nu/+) mice. A radioimmunoassay was used to selectively quantitate antibody binding to native VSG 1 epitopes present on the surface of viable trypanosomes. Such analyses of VSG-specific antibody in infected mice demonstrated that in the absence of T cells there was a significant B-cell response to exposed VSG epitopes; however, in the presence of T cells these surface epitope-specific responses were greatly enhanced. In contrast to infection, immunization of mice with purified VSG 1 or paraformaldehyde-fixed parasites elicited significant VSG surface epitope-specific responses only in the presence of T cells (i.e., in nu/+ mice only). VSG-specific antibody responses in mice infected with three other clonal T. brucei rhodesiense populations (LouTat 1.2, 1.5, and 1.9) were found to be similar in this pattern, although not identical, to the anti-LouTat 1 responses. An important exception was that mice infected with LouTat 1.8 required T cells to produce VSG surface-specific antibody. Thus, the VSG surface epitope-specific B-cell responses in trypanosome-infected mice represent composite T-cell-independent and T-cell-dependent processes, and a significantly stronger response is made in the presence of T cells. However, immunization with VSG in the absence of infection elicited only T-cell-dependent responses. Since the relative contribution of T-cell-independent and T-cell-dependent processes to the total VSG-specific antibody produced during infection was variable (as seen with the absence of a T-cell-independent response to LouTat 1.8), this may reflect differences in the primary structure or display of VSG molecules on the trypanosome membrane or may represent active parasite interference with some epitope-specific B-cell responses.
Full text
PDF

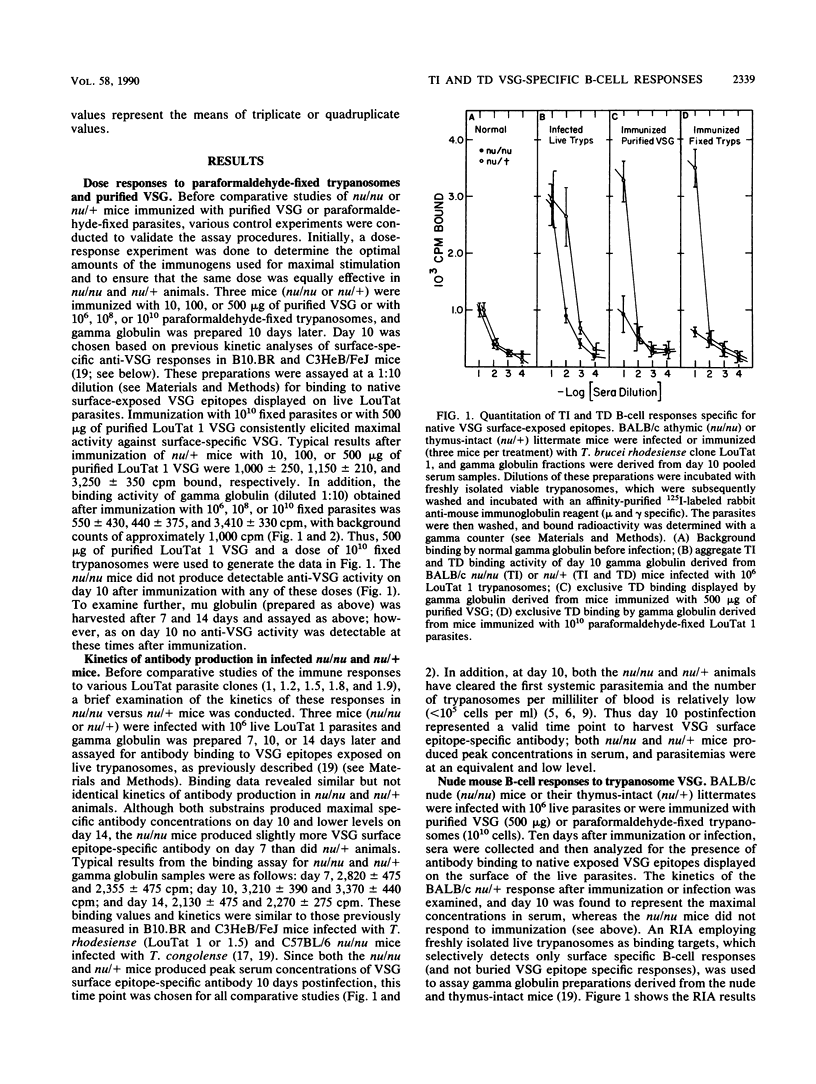
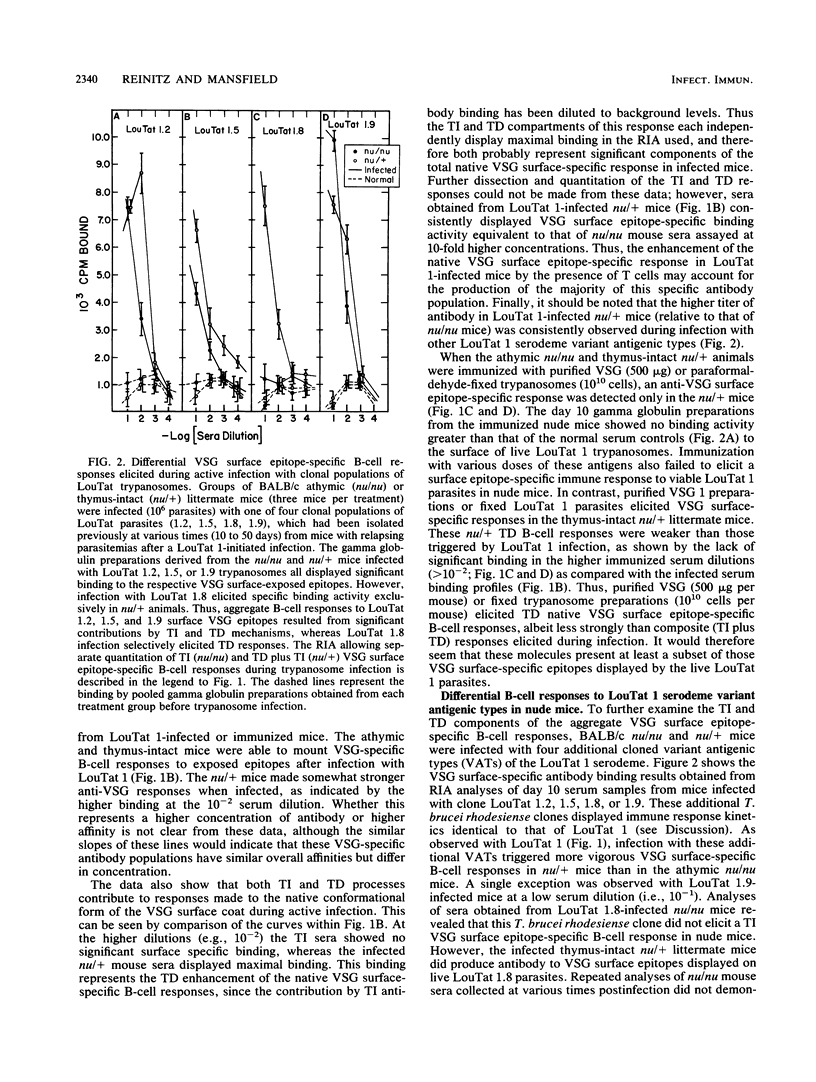


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell G. H., Esser K. M., Phillips S. M. Trypanosoma rhodesiense infection in congenitally athymic (nude) mice. Infect Immun. 1978 Jun;20(3):714–720. doi: 10.1128/iai.20.3.714-720.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke M. W., Barbet A. F., Pearson T. W. Structural features of antigenic determinants on variant surface glycoproteins from Trypanosoma brucei. Mol Immunol. 1987 Jul;24(7):707–713. doi: 10.1016/0161-5890(87)90052-6. [DOI] [PubMed] [Google Scholar]
- Clayton C. E., Ogilvie B. M., Askonas B. A. Trypanosoma brucei infection in nude mice: B lymphocyte function is suppressed in the absence of T lymphocytes. Parasite Immunol. 1979 Spring;1(1):39–48. doi: 10.1111/j.1365-3024.1979.tb00694.x. [DOI] [PubMed] [Google Scholar]
- Cross G. A. Release and purification of Trypanosoma brucei variant surface glycoprotein. J Cell Biochem. 1984;24(1):79–90. doi: 10.1002/jcb.240240107. [DOI] [PubMed] [Google Scholar]
- Donelson J. E., Rice-Ficht A. C. Molecular biology of trypanosome antigenic variation. Microbiol Rev. 1985 Jun;49(2):107–125. doi: 10.1128/mr.49.2.107-125.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvao-Castro B., Hochmann A., Lambert P. H. The role of the host immune response in the development of tissue lesions associated with African trypanosomiasis in mice. Clin Exp Immunol. 1978 Jul;33(1):12–24. [PMC free article] [PubMed] [Google Scholar]
- Johnson P. J., Kooter J. M., Borst P. Inactivation of transcription by UV irradiation of T. brucei provides evidence for a multicistronic transcription unit including a VSG gene. Cell. 1987 Oct 23;51(2):273–281. doi: 10.1016/0092-8674(87)90154-1. [DOI] [PubMed] [Google Scholar]
- Langhorne J., Rollwagen F. M., Finerty J. F. Induction of T cell activity in athymic (nu/nu) mice infected with Trypanosoma rhodesiense. Cell Immunol. 1983 Oct 1;81(1):180–186. doi: 10.1016/0008-8749(83)90224-1. [DOI] [PubMed] [Google Scholar]
- Lanham S. M., Godfrey D. G. Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp Parasitol. 1970 Dec;28(3):521–534. doi: 10.1016/0014-4894(70)90120-7. [DOI] [PubMed] [Google Scholar]
- Levine R. F., Mansfield J. M. Genetics of resistance to the African trypanosomes. III. Variant-specific antibody responses of H-2-compatible resistant and susceptible mice. J Immunol. 1984 Sep;133(3):1564–1569. [PubMed] [Google Scholar]
- Mansfield J. M., Levine R. F., Dempsey W. L., Wellhausen S. R., Hansen C. T. Lymphocyte function in experimental African trypanosomiasis. IV. Immunosuppression and suppressor cells in the athymic nu/nu mouse. Cell Immunol. 1981 Sep 1;63(1):210–215. doi: 10.1016/0008-8749(81)90043-5. [DOI] [PubMed] [Google Scholar]
- Miller E. N., Allan L. M., Turner M. J. Mapping of antigenic determinants within peptides of a variant surface glycoprotein of Trypanosoma brucei. Mol Biochem Parasitol. 1984 Nov;13(3):309–322. doi: 10.1016/0166-6851(84)90122-1. [DOI] [PubMed] [Google Scholar]
- Pearson T. W., Kar S. K., McGuire T. C., Lundin L. B. Trypanosome variable surface antigens: studies using two-dimensional gel electrophoresis and monoclonal antibodies. J Immunol. 1981 Mar;126(3):823–828. [PubMed] [Google Scholar]
- Pinder M., Chassin P., Fumoux F. Mechanisms of self-cure from Trypanosoma congolense infection in mice. J Immunol. 1986 Feb 15;136(4):1427–1434. [PubMed] [Google Scholar]
- Rank R. G., Roberts D. W., Weidanz W. P. Chronic infection with Trypanosoma musculi in congenitally athymic nude mice. Infect Immun. 1977 May;16(2):715–716. doi: 10.1128/iai.16.2.715-716.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinitz D. M., Mansfield J. M. Independent regulation of B cell responses to surface and subsurface epitopes of African trypanosome variable surface glycoproteins. J Immunol. 1988 Jul 15;141(2):620–626. [PubMed] [Google Scholar]
- Reinitz D. M., Voss E. W., Jr Identification of recurrent idiotypes within the unrestricted anti-fluorescein immune response. J Immunol. 1985 Nov;135(5):3365–3371. [PubMed] [Google Scholar]
- Robinett J. P., Rank R. G. Splenomegaly in murine trypanosomiasis: T cell-dependent phenomenon. Infect Immun. 1979 Feb;23(2):270–275. doi: 10.1128/iai.23.2.270-275.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelants G. E., Pinder M. Immunobiology of African trypanosomiasis. Contemp Top Immunobiol. 1984;12:225–274. doi: 10.1007/978-1-4684-4571-8_7. [DOI] [PubMed] [Google Scholar]


