Abstract
Objective
The aim of this study was to identify the anatomical location and course of the facial nerve (FN) and their relationship to the tumor size in surgically treated vestibular schwannomas.
Methods
A retrospective study was conducted on 163 patients who had been treated by the microsurgical resection for a newly diagnosed vestibular schwannoma between 1995 and 2005 (mean age of 46.1 years; 108 females and 55 males). Surgery was carried out via retrosigmoid approach in all patients with the electromyographic monitoring for the FN function. The anatomical location and course of the FN along the tumor surface were verified in each patient during the microsurgery, and were classified into 4 groups : 1) the FN displaced along the ventral and superior surface of the tumor (VS); 2) the ventral and central (VC); 3) the ventral and inferior (VI); and 4) the dorsal (Do).
Results
The FN displacement was identified as the followings : VS in 91 patients (55.8%); VC in 57 (35.0%); VI in 14 (8.6%); and Do in 1 (0.6%). In the subgroup with tumors less than 2 cm in diameter (n=23), the FN was displaced along the ventral and central surface of the tumor in the majority (65.2%), whereas, in the patients with tumors larger than 2cm (n=140), it was displaced along the ventral and superior surface most frequently (59.3%).
Conclusion
The FN can be displaced variably in vestibular schwannomas, and most frequently along the ventral and superior surface of the tumor, especially in large ones.
Keywords: Vestibular schwannoma, Facial nerve, Microsurgery
INTRODUCTION
Vestibular schwannomas (VSc) are benign and slowly growing tumors which originate predominantly from the superior vestibular nerve sheath. These tumors comprise about 10% of the primary intracranial tumors, 85% of the tumors at the cerebellopontine angle, and 90% of the intracranial schwannomas1,3,6,15).
For the treatment of VSc, surgeons have three therapeutic modalities : conservative treatment, microsurgical resection, and radiosurgery. Treatment strategy may be tailored according to the clinical situation adopting one or combination of those modalities2,5). The advent of microsurgical techniques and intraoperative monitoring tools, and improvement in imaging studies made it feasible to remove the tumor completely with preservation of the cranial nerve functions. Although radiosurgery has recently been accepted as a valid therapeutic modality in terms of achieving good tumor control and functional preservation, surgery still stands the mainstay in the treatment of VSc, especially in large tumors7,13). During the microsurgical resection, it is important to recognize the facial nerve (FN) along the tumor surface to accomplish complete tumor excision while preserving the nerve.
The aim of this study was to identify the anatomical location and course of the FN and their relationship to the tumor size in a microsurgical series of VSc.
MATERIALS AND METHODS
Patients
Between 1995 and 2005, all of the 165 consecutive patients underwent the microsurgical resection for a newly diagnosed VSc at the authors' institution. In the operative field, the exact anatomical location and course of the FN were identified and depicted on the operative record in each patient. Among these, 163 patients in whom the operative record was available were entered into the study. The average age of the patients was 46.1 years (mean±SD, 46.1±12.3 years). One hundred and eight patients were female and 55 patients were male. The average length of the follow-up was 61.6 months (61.6±32.1 months).
Preoperative radiographic study
All of the patients underwent comprehensive radiographic examination : T1-weighted magnetic resonance imaging (MRI) with and without a contrast medium, T2-weighted MRI, and high-resolution temporal bone computerized tomography. The average tumor size was 3.7 cm (3.7±1.1 cm) which was measured in the largest diameter on MRI.
Surgical procedure and identification of the FN
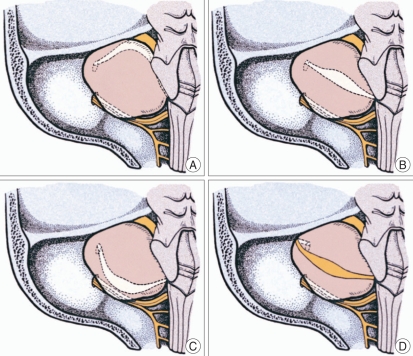
All of the operations were carried out by the senior author via the retrosigmoid approach. Resection of the tumor was generally accomplished in three steps : intracapsular debulking and dissection of the extrameatal portion of the tumor; removal of the intrameatal tumor after drilling out the posterior lip of the internal acoustic canal (IAC); and dissection and removal of the remaining extrameatal tumor. Throughout the microsurgical procedure, the FN function had been monitored using the electromyography (EMG) for the orbicularis oculi and oris muscles (NIM-2, Xomed Treace, Jacksonville, FL, USA). The exact location and course of the FN displaced along the tumor surface were identified by the microscopic inspection with the aid of the EMG monitoring, and were classified into 4 groups : 1) the FN displaced along the ventral and superior surface of the tumor (VS); 2) the ventral and central (VC); 3) the ventral and inferior (VI); and 4) the dorsal (Do) (Fig. 1).
Fig. 1.
Classification of the location and course of the facial nerve on the surface of vestibular schwannoma : the FN displaced along the ventral and superior surface of the tumor (A); the ventral and central (B); the ventral and inferior (C); and the dorsal (D).
Extent of tumor resection
Extent of tumor resection was graded by the operative records and postoperative MRI : total (absence of residual tumor); near total (residual tumor consisting of only a small and thin capsular peel adherent to the FN or brainstem); and subtotal resection (a substantial residual more than that of near total resection).
Outcome Measurement
The FN function was assessed using House-Brackmann grade4) (H-B grade) at 1 day and 1 year postsurgery, and was classified as excellent (H-B grade I/II), intermediate (H-B grade III/IV), or poor (H-B grade V/VI).
RESULTS
The location and course of the FN
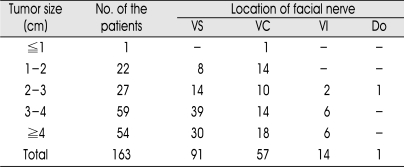
The location and course of the FN along the tumor was identified as the followings : VS in 91 patients (55.8%); VC in 57 (35.0%); VI in 14 (8.6%); and Do in 1 (0.6%) (Table 1). The location and course of the FN according to the tumor size is shown in Table 1. In the subgroup with tumors less than 2 cm in diameter (n=23), the FN displacement was VC in 15 patients (65.2%) and VS in 8 (35.8%), whereas, in the patients with tumors larger than 2cm (n=140), it was VS in 83 patients (59.3%), VC in 42 (30%), VI in 14 (10%), and Do in 1 (0.7%). Each example for the FN displacement is shown in Fig. 2, 3, 4.
Table 1.
The location and course of the facial nerve along the surface of vestibular schwannoma according to the tumor size
VS : vetral and superior surface, VC : ventral and central surface, VI : ventral and inferior surface, Do : Dorsal surface
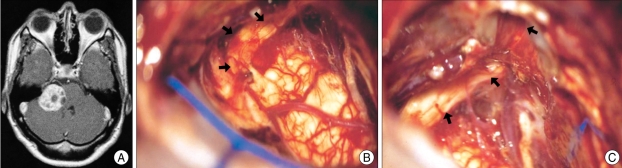
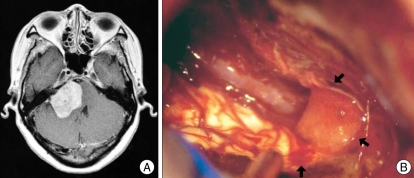
Fig. 2.
An example of ventral and superior displacement of the facial nerve. A : Preoperative magnetic resonance image. B and C : Microphotographs taken after removal of the tumor show that the facial nerve is flattened and displaced ventrally and superiorly.
Fig. 3.
An example of ventral and central displacement of the facial nerve. A : Preoperative magnetic resonance image. B and C : Microphotographs taken after removal of the tumor show that the facial nerve traverses the ventral surface of the tumor straight into the internal acoustic canal.
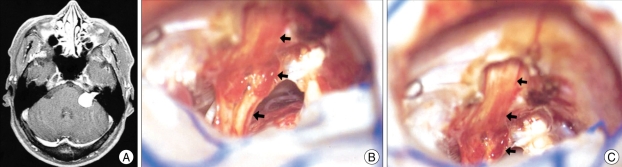
Fig. 4.
An example of ventral and inferior displacement of the facial nerve. A : Preoperative magnetic resonance image. B : A microphotograph taken after removal of the tumor shows that the facial nerve is flattened and displaced ventrally and inferiorly.
Extent of tumor resection
Total resection of the tumor was achieved in 148 patients (90.8%). Fifteen patients underwent near total (7 patients, 4.3%) or subtotal resection (8 patients, 4.9%), in whom some portion of the tumor considerably adherent to the FN or brainstem was left behind.
Preservation of the FN
The FN was preserved anatomically in 152 patients (93.3%). On 11 patients in whom the anatomical integrity of the nerve failed to be preserved, reanimation was performed using direct end-to-end anastomosis (9 patients), or interpositional grafting using the sural nerve (2 patients). The functional outcome of the FN at one day postsurgery was excellent in 69 patients (42.3%), intermediate in 76 patients (46.6%), and poor in 18 patients (11.1%). After one year of follow-up, it has recovered as excellent in 111 patients (71.6%), intermediate in 34 patients (21.9%), and poor in 10 patients (6.5%).
DISCUSSION
Surgical outcomes of VSc have steadily improved over the last century, and the primary goal of the surgical treatment today is to remove the tumor completely while preserving the cranial nerve functions. Refinement in microsurgical technique and intraoperative monitoring tools, and improvement in imaging studies bestowed more chances on neurosurgeons to achieve this goal. However, it is still difficult to accomplish a successful surgical outcome in large tumors mainly because of the progressive thinning and stretching of the adjacent cranial nerves, especially the facial and cochlear nerves, and subsequent their serious adhesion to the tumors1,12). Virtually there are no ways to visualize or predict the anatomy of the displaced cranial nerves preoperatively using contemporary diagnostic techniques, especially in large VSc. Thus, during the microsurgical resection, it is critical to identify the nerves with their anatomical relationship to the tumor as early as possible. In this context, informations on the anatomy of the displaced cranial nerves presented by the experienced VSc surgeons can offer a practical value. To date, there are a limited number of surgical series which describe the exact location and course of those cranial nerves displaced in VSc with their practical reference value8,9,11). In the present study, the authors report variations on the location and course of the displaced FN and their relative incidence based on their surgical experience of 163 VSc.
Operative nuances
Microsurgical excision of VSc has been performed in 3 steps in general as described elsewhere. Intracapsular debulking of the extrameatal portion of the tumor starts from the dorsal surface after verifying no nerve fibers present on it with careful inspection and EMG responses, as dorsal displacement of the facial and cochlear nerves is found uncommon1,11). Then debulking and dissection of the tumor alternate with modulating the directions of the maneuvers to maximize mobilization of the tumor. Generally, dissection of the tumor from the brainstem and the nerves proceeds from medial to lateral and from inferior to superior directions which enables the surgeon to notice the landmarks for the nerves earlier such as the lower cranial nerves, choroid plexus protruding from the foramen of Luschka, and the flocculus, and subsequently identify the proximal part of the nerves, almost invariably the eighth nerve first. By gently pulling the tumor from medial to lateral direction, more lateral part of the nerves can be visualized frequently on the ventral and inferior surface with the cochlear nerve, and on the ventral and superior with the FN, and further dissected. As dissection follows the nerves toward the porus, it becomes very difficult owing to the most severe fanning and adhesion of the nerves on the tumor surface at this point, especially in large tumors, and a small amount of the tumor is left in place at this step. The next step consists of opening of the posterior lip of the IAC and removal of the intrameatal tumor. Entrance into the canal by drilling should be undertaken after appreciation of the detailed bony anatomy on high-resolution computerized tomography. Removal of the intrameatal tumor is also carried out by means of debulking followed by dissection. Constant facial EMG monitoring are performed from this stage to the last dissection. Finally, dissection and removal of the remaining tumor near the entrance of the IAC is the critical step to preserve the nerve function. To ensure that dissection become practicable and less traumatic, remaining tumor is to be reduced as much as possible before dissection. Bimanual dissection of the nerves with continuous saline irrigation can improve the visualization and separation of the nerves from the tumor.
The location and course of the FN
It is a widely accepted operative strategy in VSc to identify the FN proximally at the brainstem or distally at the IAC, where its displacement and adhesion are the least8-10,14). On the brainstem side, the FN can usually be identified at the lateral end of the pontomedullary sulcus, just rostal to the glossopharyngeal nerve and just anterosuperior to the flocculus and choroid plexus protruding from the foramen of Luschka. To understand how the FN traverses its course along the tumor surface between the nerve exit zone and IAC is a key concept for a successful surgery for VSc. In the present series, variations on the orientation of the displaced FN along VSc was observed and several important remarks can be made based on the results : 1) ventral displacement of the FN was observed in all cases but one; 2) ventral and superior displacement was the most common; 3) ventral and superior, and ventral and central displacement constituted more than 90% of the cases; 4) more variations on the FN displacement was noted in tumors larger than 2 cm; and 5) incidence of variations was different between the small and large tumor : In tumors less than 2 cm in diameter, ventral and central displacement was observed in about two thirds, whereas, in larger tumors, ventral and superior displacement was around 60%. These observations compare favorably with the previous studies in some parts, but are inconsistent in another8,9,11). Sampath et al.11) reported with their large series that the most common location of the FN displacement was on the anterior middle third of the tumor capsule : the FN was found on the anterior and middle third in 41.4%; the anterior and superior third in 34.4%; the anterior and inferior third in 5.1%; the superior pole in 14.4%; and the inferior pole in 25 (2.6%). The posterior location was found in only 2.1%. When approximately correlating the anterior and superior third group plus the superior pole group of this classification to the ventral and superior group of the authors' classification, and, in the same way, the anterior and inferior third group plus the inferior pole group to the ventral and inferior group, these data can be compared more directly with those of the authors' series : 48.8% of the cases are correlated to the ventral and superior; 41.4% to the ventral and central; 7.7% to the ventral and inferior; and 2.1% to the dorsal group. Now these correlated data are quite consistent with those of the authors' own, which indicate that 1) ventral displacement of the FN was observed in most of the cases; 2) ventral and superior displacement was the most frequent; 3) ventral and superior, and ventral and central displacement constituted around 90%. However, variations on the FN displacement and their incidence was relatively uniform regardless of the tumor size in that study, if the size criteria was different from those of the authors' own. Dubiously, there were several cases with the FN passing through the tumor itself which have not been experienced ever by the authors. Although the FN displaced dorsally to VSc facing the surgeon has been reported up to 7 to 9%8), it was extremely rare in the present series.
CONCLUSION
It is of critical importance to understand the anatomical variations on the location and course of the FN to preserve the nerve during the microsurgical resection of VSc. The FN is displaced most frequently along the ventral and superior surface of the tumor, especially in large ones. It is suggested that, as the tumor size increases, the FN displacement tends to be variable, most frequently with ventral and superior location.
Acknowledgement
This paper was presented at 2006 Autumn Meeting of The Korean Neurosurgical Society.
References
- 1.Apuzzo MLJ. Brain surgery. ed 1. New York: Churchill-Livingstone; 1993. pp. 1743–1772. [Google Scholar]
- 2.Chakrabarti I, Appuzo ML, Giannota SL. Acoustic tumors : operation versus radiation-making sense of opposing viewpoints. Part I. Acoustic neuroma : Decision making with all the tools. Clin Neurosurg. 2003;50:293–312. [PubMed] [Google Scholar]
- 3.Harner SG, Laws ER. Clinical Findings in Patients with Acoustic Neuromas. Mayo Clin Proc. 1983;58:721–728. [PubMed] [Google Scholar]
- 4.House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1995;113:179–180. doi: 10.1177/019459988509300202. [DOI] [PubMed] [Google Scholar]
- 5.Kondziolka D, Lunsford LD, Flickinger JC. Acoustic tumors : operation versus radiation-making sense of opposing viewpoints. Part II. Acoustic neuromas : sorting out management options. Clin Neurosurg. 2003;50:313–328. [PubMed] [Google Scholar]
- 6.Machinis TG, Fountas KN, Dimpoulos V, Robinson JS. History of acousticneurinoma surgery. Neurosurg Focus. 2005;18:e9. doi: 10.3171/foc.2005.18.4.10. [DOI] [PubMed] [Google Scholar]
- 7.Myrseth E, Moller P, Pedersen PH, Vassobotn FS, Wentzel-Larsen T, Lund-Johansen M. Vestibular schwannomas : clinical results and quality of life after microsurgery or gamma knife radiosurgery. Neurosurgery. 2005;56:927–935. doi: 10.1055/s-2005-916493. [DOI] [PubMed] [Google Scholar]
- 8.Rand R. Microneurosurgery. ed 3. St. Louis: Mosby; 1985. pp. 335–365. [Google Scholar]
- 9.Rhoton AL, Jr, Tedeschi H. Microsurgical anatomy of acoustic neuroma. Otolaryngol Clin North Am. 1992;25:257–294. [PubMed] [Google Scholar]
- 10.Samii M, Matthies C. Management of 1000 vestibualr schwannomas (acoustic neuromas) : surgical management and results with an emphasis on complications and how to avoid them. Neurosurgery. 1997;40:11–23. doi: 10.1097/00006123-199701000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Sampath P, Rini D, Long DM. Microanatomical variations in the cerebellopontine angle associated with vestibular schwannomas (acoustic neuromas) : a retrospective study of 1006 consecutive cases. J Neurosurg. 2000;92:70–78. doi: 10.3171/jns.2000.92.1.0070. [DOI] [PubMed] [Google Scholar]
- 12.Sluyter S, Gramans K, Tulleken CA, Van Veelen CW. Analysis od the results obtained in 120 patients with large acoustic neuromas surgically treated via translabyrinthine-transtentorial approach. J Neurosurg. 2001;94:61–66. doi: 10.3171/jns.2001.94.1.0061. [DOI] [PubMed] [Google Scholar]
- 13.Toshinori H, Shigeru F, Shun K, Yoshihisa K, Masayuki Y, Joji K. Stereotactic radiosurgery for vestibular schwannomas : Analysis of 317 patients followed more than 5 years. Neurosurgery. 2005;57:257–265. doi: 10.1227/01.neu.0000166542.00512.84. [DOI] [PubMed] [Google Scholar]
- 14.Yasargil MG. Microneurosurgery. ed 2. New york: Thieme Medical; 1996. pp. 100–123. [Google Scholar]
- 15.Youmans JR. Neurosurgical surgery. ed 5. Philadelphia: WB Saunders; 2004. pp. 1150–1168. [Google Scholar]