Abstract
Objective
Spinal cord hemangioblastoma is an uncommon vascular neoplasm with a benign nature and is associated with von Hippel-Lindau (VHL) disease in 20-30% of patients. Total removal of these tumors without significant neurological deficit remains a great challenge. The purpose of this study was to investigate the efficacy of VHL mutation analysis and to evaluate surgical outcome of patients with spinal cord hemangioblastomas.
Methods
This study included nine patients treated for spinal cord hemangioblastomas at our institute between December 1994 and March 2006. There were four male and five female patients. Mean age was 37.8 years. The mean follow-up period was 22.4 months. Magnetic resonance imaging (MRI) of the complete neuraxis was done in all cases and VHL mutation analysis was performed in three cases for a definite diagnosis.
Results
Six patients had intramedullary tumor, and the remaining patients had intradural extramedullary lesions. Five patients were associated with VHL disease. The von Hippel-Lindau mutation analysis was done in three patients and two of them showed VHL gene abnormality. Tumors were located in the cervical cord in five cases and in the thoracic cord in four cases. All patients underwent surgical intervention, and total removal was achieved in six cases. All patients showed improvement or, at least, clinically stationary state. Surgical complications did not develop in any cases.
Conclusion
Spinal hemangioblastoma in this series has been safely and effectively removed via a posterior approach. Postoperatively, clinical outcome was excellent in the majority of cases. The VHL mutation analysis was useful in patients with family history and in those with multiple hemangioblastomas.
Keywords: Hemangioblastoma, Von Hippel-Lindau disease, Mutation analysis
INTRODUCTION
Hemangioblastomas are vascular tumors that can be found throughout the central nervous system including spinal cord that account for approximately 2% of primary spinal cord tumors9,18,35). They are benign neoplasm (World Health Organization Grade I), but can cause significant morbidity and mortality through mass effect according to the intraspinal lesion20,27).
Von Hippel-Lindau (VHL) disease denotes an important subset of patients who present with hemangioblastomas in the central nervous system (CNS). Hemangioblastomas of the spinal cord occur more commonly as sporadic isolated lesions (70-80% of cases) rather than as multiple lesions in the cerebellum and retina as part of the VHL disease1,7,15,18-20,35). The basis of familial inheritance of VHL disease is a germline mutation in the VHL tumor suppressor gene, which is located in the short arm of chromosome 3p2510). A method of VHL gene mutation analysis is accessible and is used for genetic testing within families with VHL disease31). The actual rate of sensitivity and specificity for applied VHL molecular genetic analysis is reported to be up to 99% by use of automated sequencing, Southern blotting, and fluorescence in situ hybridization6,12).
Magnetic resonance (MR) imaging is a useful examination for diagnosis of hemangioblastomas of the spinal cord22). On T1-weighted MR imaging with contrast enhancement, the tumors appear as bright enhancing lesions mainly on the dorsal surface of the spinal cord. On T2-weighted image, associated edema and syrinx is shown13,25). Many of these tumors originate in the region of the dorsal roots or the spinal cord at the dorsal root entry zone (DREZ)13).
In the present study, we reviewed nine patients who were treated for spinal cord hemangioblastoma in our institute between December 1994 and March 2006, and analyzed retrospectively clinical features including neuroradiological findings, surgical treatments and clinical outcomes. We also investigated the efficacy of VHL mutation analysis.
MATERIALS AND METHODS
Between December 1994 and March 2006, nine patients were treated for spinal cord hemangioblastomas including five patients with VHL disease. Four men and five women were included in the study, ranging from 17 to 66 years in age (mean age, 37.8 years). The sex, age at the time of onset, initial symptom, duration, location of the tumor, association with VHL disease and the presence of syrinx are summarized in Table 1. Clinical diagnosis for VHL disease was made according to the criteria that Melmon and Rosen17) described. If a family history of the CNS or retinal hemangioblastoma exists, only one hemangioblastoma or visceral lesion (renal tumors, pancreatic cysts or tumors, pheochromocytoma, papillary cystadenomas of the epididymis) is required to make the diagnosis of VHL disease. For an isolated case without a clear family history, two or more CNS and/or retinal hemangioblastoma or one hemangioblastoma and a visceral manifestation are required17). The von Hippel-Lindau mutation analysis was performed in three patients. Mutations in the VHL gene was determined by direct sequencing of all coding exons and flanking intronic sequences31). Serial neurological examination was done and preoperative and postoperative MRI studies were performed in all patients. Preoperative spinal angiography was performed in four patients, and preoperative embolization in two (Fig. 1). Preoperative and intraoperative somatosensory-evoked potentials (SSEP) were performed in four cases.
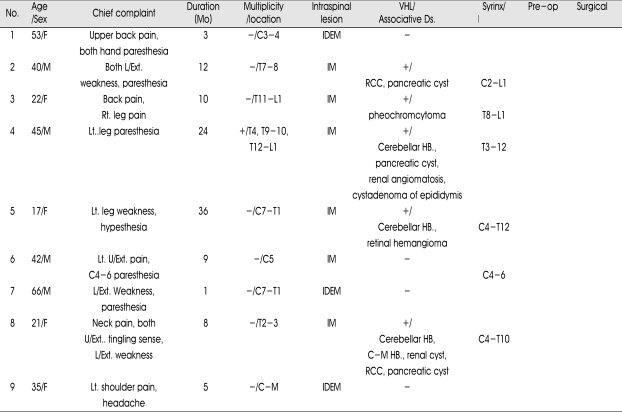
Table 1.
Clinical data for nine patients with hemangioblastomas of spinal cord
U/Ext. : upper extremity; L/Ext. : lower extremity; Rt. : right; Lt. : left; C : cervical; T : thoracic; L : lumbar; C-M : cervico-medullary; IDEM : intradural and extramedullary; IM : intramedullary; RCC : renal cell carcinoma; HB : hemangioblastoma; GTR : gross total removal; STR : subtotal removal
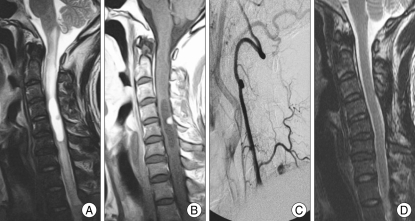
Fig. 1.
Preoperative magnetic resonance imaging (MRI) : T2-weighted sagittal image (A) showing a hyperintense lesion with spinal cord swelling from lower medulla to T2-3 level. Gadolinium-enhanced sagittal image (B) reveals well enhancing mass, approximately 1 cm diameter at the C5 spinal cord level. Preoperative right vertebral angiography (C) demonstrates hypervascular tumor staining fed by the ascending cervical artery of right thyrocervical trunk. Postoperative MRI : T2-weighted sagittal image (D) shows a marked diminution of the hyperintense lesion that existed previously in the preoperative MRI. The swelling of the cervical cord was nearly disappeared.
Indications for operation included the presence of lesion consistent with a spinal cord hemangioblastoma on MR imaging in a patient with objective and usually progressive neurological deficit. All patients underwent surgical removal through posterior approach via laminectomy.
Patients were followed from 4 to 39 months (mean follow-up period, 22.4 months) postoperatively. The neurological function of patients was graded according to the clinical scale described by McCormick, et al.16) The grade was evaluated before surgery, at the time point of discharge, and in the follow-up period for all patients.
RESULTS
The clinical features of the cord hemangioblastomas were similar to those of other spinal cord tumors. Initial symptoms in our series appeared as a combination of several sensory/motor manifestations. Sensory disturbance was encountered in seven patients (78%), pain in five patients (55%), and motor disturbances in four patients (45%). The duration between onset of initial symptom and diagnosis ranged from 1 month to 36 month (mean 12.9 month). Tumors were located in the cervical cord including cervico-medullary junction area in five patients and in thoracic area in four patients. In six cases, they were located intramedullary and in three cases intradural extramedullary. Based on a review of MR imaging, syrinx was accompanied in six patients (66%).
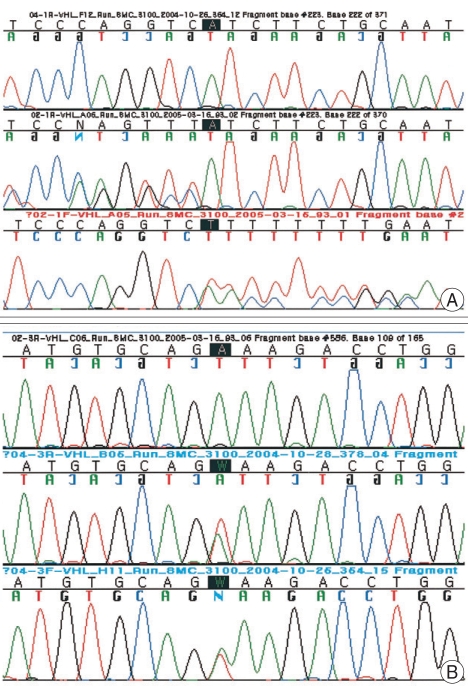
Five of nine patients (55%) met the criteria for the diagnosis of VHL disease. Two patients presented the family history of CNS or retinal hemangioblastoma. In three patients, multiple lesions of CNS hemangioblastomas were seen on MR imaging. In five patients, additional organ systems were involved including kidney (three cases), retina (two cases), pancreas (three cases), adrenal gland (one case) and epididymis (one case) (Table 1). VHL mutation analysis was performed in three patients and two of them showed gene abnormality. Two different mutations of the VHL gene, including 1 missense (c.586A>T;p.Lys196X) and frameshift insertion mutation (c.233_224insT;p.Ile75TyrfsX57), were detected in two patients (Fig. 2).
Fig. 2.
Sequencing results of von Hippel-Lindau gene mutations analysis identified in the present study. A : Insertion T in exon 1 of the VHL gene (c.223_224insT), resulting in a frame-shift mutation (p.Ile75Tyrfs×57). B : A heterozygous A to T transversion in exon 3 of the VHL gene (c.586A>T), resulting in a Lys196X nonsense mutation.
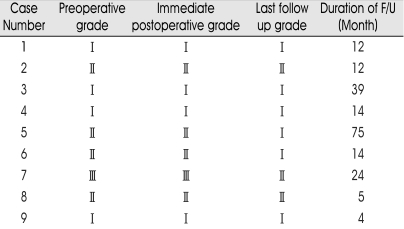
On the operation field, abnormally dilated and tortuous vessels were found on the surface of the tumors. In all cases, tumors had a capsule and there was a border demarcating clearly the tumor from the normal spinal cord. Syrinx and edema were seen in spinal cord close to the tumor. In three patients (case 2, 4 and 5) tumors were subtotally removed and for other patients tumors were totally removed. There was no perioperative mortality. Table 2 represents the preoperative, postoperative and last follow-up grade changes according to McCormick, et al.16) Most patients remained at their preoperative grade level or improved. There was no significant difference in surgical outcomes for patients with VHL disease compared to those with sporadic tumors. Intraoperative findings also did not differ between two groups. Two patients, who underwent subtotal removal (case 4, 5) showed stationary and improved outcome, respectively.
Table 2.
Clinical outcome of the patients in this series according to McCormick, et al.16)
Grade I : Neurologically normal, mild focal deficit not significantly affecting function of involved limb, mild spasticity or reflex abnormality, normal gait; Grade II : Presence of sensorimotor deficit affecting function of involved limb, mild/moderate gait difficulty, severe pain or dysesthetic syndrome impairing patient's quality of life, still functions and walks independently; Grade III : More severe neurological deficit, requires cane/brace for walking of significant bilateral upper-extremity impairment, may or may not function independently
DISCUSSION
Hemangioblastomas can occur throughout CNS system that originate primarily in the cerebellum (83-95%), spinal cord (3.2-13%), and medulla oblongata (2.1%)2). In about 40% of patients with VHL disease, a hemangioblastoma is the manifestation of the disease24,29). Spinal hemangiobalstoma can occur in 13-59% of patients with VHL disease depending on the families selected for study. These tumors occur more commonly as sporadic isolated lesions (70-80% of cases) rather than as multiple lesions in the cerebellum and retina as part of the dominantly inherited familial cancer syndrome, von Hippel-Lindau disease (16-25% of cases)6,26). In our series, five of nine patients (55%) with spinal cord hemangioblastomas were affected by VHL disease. The age of the patients at the time of diagnosis was not different from the data from other reports, ranging from 17 to 66 years, and hemangioblastoma in VHL disease was manifested in early adulthood, ranging from 17 to 40 years5,14,15,19,23).
Recently, the importance of early detection of VHL disease has been emphasized20). The majority of mutations in the VHL disease patients are represented by point mutations including missense, nonsense mutations, splicing, microinsertions or microdeletions10,12,31). The VHL gene resides on the short arm of chromosome 3 and encodes a ubiquitously expressed 4.7-kilobase (kb) messenger RNA (mRNA) that encodes 3 alternately spliced exons14,31,33). In our study, five patients were clinically associated with VHL disease. Two of them showed gene abnormality in VHL gene mutation analysis. Two different mutations of the VHL gene were detected in each patient : insertion T on exon 1 of the VHL gene (c.223_224insT), resulting in a frameshift mutation (p.Ile75TyrfsX57) and a heterozygous A to T transversion in exon 3 of the VHL gene (c.586A>T), resulting in a Lys196X nonsense mutation (Fig. 2). We confirmed these in the genetic mutation web site (http://molgen-www.uia.ac.be/CMTMutations) and these mutations were novel not described previously. None of the novel variants were observed in 100 control chromosomes by direct sequencing of the corresponding exons. We are investigating the resultant VHL proteins (pVHL) formed by these mutant genes and their significance on the tumorigenesis.
The most common neurological symptoms at presentation were sensory disturbance and pain as those in the other spinal cord tumor. A difference in neurological symptom was not observed between patients harboring sporadic hemangioblastomas and those harboring hemangioblastomas as a manifestation of VHL disease. Regards to the location of the tumor, intramedullary hemangioblastomas were reported as rare cases in previous review4,18,20,26,28,30,32,34-36), but in our study intramedullary location (66%) was the most common.
Magnetic resonance imaging (MRI) is an examination of choice for spinal hemangioblastomas, and is helpful in preoperative planning and the differential diagnosis of spinal cord neoplasms and vascular lesions. On contrast-enhanced images, hemangioblastomas usually show a typically bright enhancing mass, clearly delineated from the surrounding spinal cord tissue. On T1-weighted images, hemangioblastomas produce signals that are isointense or slightly hyperintense, and on T2-weighted images the signals are hyperintense and the signals of the associated edema and cyst are hypointense22,25,34).
Although radiosurgery has been used to treat multiple hemangioblastoma, particularly in the cerebellum21), complete microsurgical removal is the treatment of choice for spinal cord hemangioblastoms26,35). It has been shown that preoperative spinal angiography and embolization may be used as an adjunct to surgery35). Although the number of our cases is small, spinal angiography and embolization helped determine the location and the nature of the tumor and anatomy of the feeding artery and delineate vascular supply. Surgical removal via posterior approach has been advocated in the majority of cases to achieve complete removal because a posterior laminectomy provides adequate exposure of the tumor11,25,35). Spinal cord hemangioblastomas are almost always associated with a syrinx or significant edema13,25). In our study, 66% of the patients exhibited the tumor associated edema and syrinx. Cases associated edema and syrinx are more space-occupying than those only with solid part of the tumor. Consequently, the mass effect producing neurological symptoms derives from the cyst rather than the tumor itself. On removal of heamangiobalstomas in association with syrinx, the syrinx is spontaneously opened and always stops growing and usually regresses in size. Thus, additional opening of the syrinx, or surgical removal of the syrinx is not necessary20,26,32). In our series, a posterior approach via laminectomy was performed in all cases, and total removal was achieved in six cases. On postoperative MR imaging, it was found that the syrinx was regressed spontaneously in six cases. No patient's condition worsened after surgery, and complications did not develop in any cases in our series.
CONCLUSION
Spinal hemangioblastoma in this series could be safely and effectively removed via a posterior approach. Postoperatively, edema and syrinx resolved spontaneously and clinical outcome was excellent in the majority of cases. For early diagnosis and family consultation, the VHL mutation analysis was useful in patients with family history and in those with multiple hemangioblastomas. Cautious neurological observation and timely selective removal are necessary for spinal cord hemangioblastoma.
Acknowledgement
The authors are deeply grateful for Jong-Won Kim, M.D., Ph.D. at the Samsung Medical Center, Sungkyunkwan University, School of Medicine, for his great contribution to this manuscript with the VHL gene analysis.
References
- 1.Browne TR, Adams RD, Roberson GH. Hemangioblastoma of the spinal cord. Review and report of five cases. Arch Neurol. 1976;33:435–441. doi: 10.1001/archneur.1976.00500060041009. [DOI] [PubMed] [Google Scholar]
- 2.Catapano D, Muscarella LA, Guarnieri V, Zelante L, D'Angelo VA, D'Agruma L. Hemangioblastomas of central nervous system : molecular genetic analysis and clinical management. Neurosurgery. 2005;56:1215–1122. doi: 10.1227/01.neu.0000159646.15026.d6. discussion 1221. [DOI] [PubMed] [Google Scholar]
- 3.Constans JP, Meder F, Maiuri F, Donzelli R, Spaziante R, de Divitiis E. Posterior fossa hemangioblastomas. Surg Neurol. 1986;25:269–275. doi: 10.1016/0090-3019(86)90238-7. [DOI] [PubMed] [Google Scholar]
- 4.Cristante L, Herrmann HD. Surgical management of intramedullary hemangioblastoma of the spinal cord. Acta Neurochir (Wien) 1999;141:333–339. doi: 10.1007/s007010050308. discussion 339-340. [DOI] [PubMed] [Google Scholar]
- 5.Filling-Katz MR, Choyke PL, Oldfield E, Charnas L, Patronas NJ, Glenn GM, et al. Central nervous system involvement in von Hippel-Lindau disease. Neurology. 1991;41:41–46. doi: 10.1212/wnl.41.1.41. [DOI] [PubMed] [Google Scholar]
- 6.Gallou C, Chauveau D, Richard S, Joly D, Giraud S, Olschwang S, et al. Genotype-phenotype correlation in von Hippel-Lindau families with renal lesions. Hum Mutat. 2004;24:215–224. doi: 10.1002/humu.20082. [DOI] [PubMed] [Google Scholar]
- 7.Glenn GM, Linehan WM, Hosoe S, Latif F, Yao M, Choyke P, et al. Screening for von Hippel-Lindau disease by DNA polymorphism analysis. JAMA. 1992;267:1226–1231. [PubMed] [Google Scholar]
- 8.Humphrey JS, Klausner RD, Linehan WM. von Hippel-Lindau syndrome : hereditary cancer arising from inherited mutations of the VHL tumor suppressor gene. Cancer Treat Res. 1996;88:13–39. doi: 10.1007/978-1-4615-6343-3_2. [DOI] [PubMed] [Google Scholar]
- 9.Hurth M. Intraspinal hemangioblastomas. Neurochirurgie. 1975;21(Suppl 1):1–136. [PubMed] [Google Scholar]
- 10.Latif F, Tory K, Gnarra J, Yao M, Duh FM, Orcutt ML, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260:1317–1320. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- 11.Lee DK, Choe WJ, Kim DY, Lee CH, Chung CK, Kim HJ. Clinical analysis of spinal cord hemangioblastoma. J Korean Neurosurg Soc. 2001;30:1291–1299. [Google Scholar]
- 12.Linehan WM, Lerman MI, Zbar B. Identification of the von Hippel-Lindau (VHL) gene. Its role in renal cancer. JAMA. 1995;273:564–570. [PubMed] [Google Scholar]
- 13.Lonser RR, Weil RJ, Wanebo JE, DeVroom HL, Oldfield EH. Surgical management of spinal cord hemangioblastomas in patients with von Hippel-Lindau disease. J Neurosurg. 2003;98:106–116. doi: 10.3171/jns.2003.98.1.0106. [DOI] [PubMed] [Google Scholar]
- 14.Maddock IR, Moran A, Maher ER, Teare MD, Norman A, Payne SJ, et al. A genetic register for von Hippel-Lindau disease. J Med Genet. 1996;33:120–127. doi: 10.1136/jmg.33.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maher ER, Yates JR, Harries R, Benjamin C, Harris R, Moore AT, et al. Clinical features and natural history of von Hippel-Lindau disease. Q J Med. 1990;77:1151–1163. doi: 10.1093/qjmed/77.2.1151. [DOI] [PubMed] [Google Scholar]
- 16.McCormick PC, Torres R, Post KD, Stein BM. Intramedullary ependymoma of the spinal cord. J Neurosurg. 1990;72:523–532. doi: 10.3171/jns.1990.72.4.0523. [DOI] [PubMed] [Google Scholar]
- 17.Melmon KL, Rosen SW. Lindau's Disease. Review of the literature and study of a large kindred. Am J Med. 1964;36:595–617. doi: 10.1016/0002-9343(64)90107-x. [DOI] [PubMed] [Google Scholar]
- 18.Murota T, Symon L. Surgical management of hemangioblastoma of the spinal cord : a report of 18 cases. Neurosurgery. 1989;25:699–707. doi: 10.1097/00006123-198911000-00003. discussion 708. [DOI] [PubMed] [Google Scholar]
- 19.Neumann HP, Eggert HR, Scheremet R, Schumacher M, Mohadjer M, Wakhloo AK, et al. Central nervous system lesions in von Hippel-Lindau syndrome. J Neurol Neurosurg Psychiatry. 1992;55:898–901. doi: 10.1136/jnnp.55.10.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neumann HP, Eggert HR, Weigel K, Friedburg H, Wiestler OD, Schollmeyer P. Hemangioblastomas of the central nervous system. A 10-year study with special reference to von Hippel-Lindau syndrome. J Neurosurg. 1989;70:24–30. doi: 10.3171/jns.1989.70.1.0024. [DOI] [PubMed] [Google Scholar]
- 21.Pan L, Wang EM, Wang BJ, Zhou LF, Zhang N, Cai PW, et al. Gamma knife radiosurgery for hemangioblastomas. Stereotact Funct Neurosurg. 1998;70(Suppl 1):179–186. doi: 10.1159/000056420. [DOI] [PubMed] [Google Scholar]
- 22.Rebner M, Gebarski SS. Magnetic resonance imaging of spinal-cord hemangioblastoma. AJNR Am J Neuroradiol. 1985;6:287–289. [PMC free article] [PubMed] [Google Scholar]
- 23.Richard S, Campello C, Taillandier L, Parker F, Resche F. Haemangioblastoma of the central nervous system in von Hippel-Lindau disease. French VHL study group. J Intern Med. 1998;243:547–553. doi: 10.1046/j.1365-2796.1998.00337.x. [DOI] [PubMed] [Google Scholar]
- 24.Richard S, David P, Marsot-Dupuch K, Giraud S, Beroud C, Resche F. Central nervous system hemangioblastomas, endolymphatic sac tumors, and von Hippel-Lindau disease. Neurosurg Rev. 2000;23:1–22. doi: 10.1007/s101430050024. discussion 23-24. [DOI] [PubMed] [Google Scholar]
- 25.Roonprapunt C, Silvera VM, Setton A, Freed D, Epstein FJ, Jallo GI. Surgical management of isolated hemangioblastomas of the spinal cord. Neurosurgery. 2001;49:321–327. doi: 10.1097/00006123-200108000-00012. discussion 327-328. [DOI] [PubMed] [Google Scholar]
- 26.Samii M, Klekamp J. Surgical results of 100 intramedullary tumors in relation to accompanying syringomyelia. Neurosurgery. 1994;35:865–873. doi: 10.1227/00006123-199411000-00010. discussion 873. [DOI] [PubMed] [Google Scholar]
- 27.Silver ML, Hennigar G. Cerebellar hemangioma (hemangioblastoma) : a clinicopathological review of 40 cases. J Neurosurg. 1952;9:484–494. doi: 10.3171/jns.1952.9.5.0484. [DOI] [PubMed] [Google Scholar]
- 28.Solomon RA, Stein BM. Unusual spinal cord enlargement related to intramedullary hemangioblastoma. J Neurosurg. 1988;68:550–553. doi: 10.3171/jns.1988.68.4.0550. [DOI] [PubMed] [Google Scholar]
- 29.Sora S, Ueki K, Saito N, Kawahara N, Shitara N, Kirino T. Incidence of von Hippel-Lindau disease in hemangioblastoma patients : the University of Tokyo Hospital experience from 1954-1998. Acta Neurochir (Wien) 2001;143:893–896. doi: 10.1007/s007010170019. [DOI] [PubMed] [Google Scholar]
- 30.Spetzger U, Gertalanffy H, Huffmann B, Mayfrank L, Reul J, Gilsbach JM. Hemangioblastomas of the spinal cord and the brainstem : diagnostic and therapeutic features. Neurosurg Rev. 1996;19:147–151. doi: 10.1007/BF00512042. [DOI] [PubMed] [Google Scholar]
- 31.Stolle C, Glenn G, Zbar B, Humphrey JS, Choyke P, Walther M, et al. Improved detection of germline mutations in the von Hippel-Lindau disease tumor suppressor gene. Hum Mutat. 1998;12:417–423. doi: 10.1002/(SICI)1098-1004(1998)12:6<417::AID-HUMU8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 32.Trost HA, Seifert V, Stolke D. Advances in diagnosis and treatment of spinal hemangioblastomas. Neurosurg Rev. 1993;16:205–209. doi: 10.1007/BF00304329. [DOI] [PubMed] [Google Scholar]
- 33.Woodward ER, Buchberger A, Clifford SC, Hurst LD, Affara NA, Maher ER. Comparative sequence analysis of the VHL tumor suppressor gene. Genomics. 2000;65:253–265. doi: 10.1006/geno.2000.6144. [DOI] [PubMed] [Google Scholar]
- 34.Xu QW, Bao WM, Mao RL, Yang GY. Magnetic resonance imaging and microsurgical treatment of intramedullary hemangioblastoma of the spinal cord. Neurosurgery. 1994;35:671–675. doi: 10.1227/00006123-199410000-00013. discussion 675-676. [DOI] [PubMed] [Google Scholar]
- 35.Yasargil MG, Antic J, Laciga R, de Preux J, Fideler RW, Boone SC. The microsurgical removal of intramedullary spinal hemangioblastomas. Report of twelve cases and a review of the literature. Surg Neurol. 1976;3:141–148. [PubMed] [Google Scholar]
- 36.Yasui T, Hakuba A, Katsuyama J, Nishimura S. Microsurgical removal of intramedullary spinal cord tumors : report of 22 cases. Acta Neurochir Suppl (Wien) 1988;43:9–12. doi: 10.1007/978-3-7091-8978-8_2. [DOI] [PubMed] [Google Scholar]