Abstract
Objective
High-molecular-weight hydroxyethyl starch (HES) compromises blood coagulation more than does low-molecular-weight HES. We compared the effects of low- and high-molecular-weight HES for the treatment of vasospasm and investigated the dose relationship with each other.
Methods
Retrospectively, in a series of consecutive 102 patients with subarachnoid hemorrhage (SAH), 35 patients developed clinical symptoms of vasospasm of these fourteen patients were treated with low-molecular-weight HES for volume expansion while the other 21 received high-molecular-weight HES as continuous intravenous infusion. Prothrombin time (PT), partial thromboplastin time (PTT), fibrinogen level, and platelet count were all measured prior to initiation, during treatment and after termination of therapy for symptomatic vasospasm. The total dose of HES ranged from 5 L to 14 L and median infusion duration was 10 days.
Results
A more pronounced PTT prolongation was observed in high-molecular-weight HES group compared with low-molecular-weight HES group. No other coagulation parameters were altered. Dosage (=duration) shows a positive correlation with PTT. Clinically, significant bleeding episodes were noted in four patients who received high-molecular-weight HES.
Conclusion
Coagulopathy was developed in direct proportion to molecular weight of starch and dosages. We propose the extreme caution in the administration of HES solution for the vasospasm treatment.
Keywords: Coagulopathy, Hydroxyethyl starch (HES), Partial thromboplastin time (PTT), Subarachnoid hemorrhage, Vasospasm
INTRODUCTION
Cerebral vasospasm typically starts 3-5 days after the initial SAH, achieves maximal vessel narrowing at 5-14 days, and thereafter resolves gradually10). The exact mechanisms by which SAH induces arterial vasospasm continues to be a subject of considerable research and debate. This mechanism appears to be a multifactorial process that involves the generation of free radicals, lipid peroxidation and activation of protein kinase C as well as phospholipase C and A2 with resultant accumulation of diacylglycerol and the release of endothelin-12).
More than half of SAH patients develop cerebral vasospasm and approximately one-third develop symptomatic vasospasm which is associated with neurologic signs and symptoms of ischemia20). Volume expansion is one of the cornerstones in the prevention and treatment of postsubarachnoid hemorrhage vasospasm18). It has been shown to decrease the size of ischemic infarct and improve hemodynamic parameters4,18). Recently, synthetic colloids are commonly used as a volume expander. Among these solutions, hydroxyethyl starch (HES) solution has become widely used with limited side effects. Nevertheless, a coagulopathy can occur during HES administration, consisting of a prolongation of the partial thromboplastin time (PTT) and a decrease in factor VIII1).
We compared low- with high-molecular-weight HES that were used in the treatment of vasospasm and investigated the relationship between HES dosage and PTT prolongation to help in preventing further occurrences of bleeding pathologies.
MATERIALS AND METHODS
Between January, 2005 and December, 2006, 102 patients admitted to our hospital with a recent (<4 days of symptoms) SAH that required surgical clipping of an intracranial aneurysm underwent postoperative care in the neurosurgical intensive care unit.
Treatment of vasospasm
All patients received nimodipine for 21 days or until discharge. Hemodilution and hypervolemia were employed preventively in all patients using a crystalloid solution. Hunt and Hess grades were also documented on admission. Hematocrit was maintained between 30 and 35%. Systolic blood pressure was maintained above 180 mmHg. Prothrombin time (PT), partial thromboplastin time (PTT), fibrinogen level, and platelet count were all measured prior to the initiation during and termination of therapy for symptomatic vasospasm. There were no other differences in the treatment protocol and all patients were managed under the supervision of the senior author. Most data, including demographic data, clinical condition, radiographic findings, surgical details, onset and duration of vasospasm, use of medications (including hetastarch), complications, and outcome were collected retrospectively.
Administeration of HES
Of these 102 patients, 35 developed clinically significant vasospasm documented by a progressive postoperative neurological deficit that could not be explained on the basis of hydrocephalus, rebleeding, or other pathologies. The perioperative transcranial doppler examinations were obtained in all patients by experienced examiners using the standard technique. In those patients who developed clinical signs of vasospasm, hypervolemic therapy with a colloid solution and hypertensive therapy with phenylephrine were instituted. Either HES 70 (6% hetastarch, with an average molecular weight of 70 kd) or HES 670 (6% hetastarch, with an average molecular weight of 670 kd) were used as a colloid solution. Fourteen patients received HES 70 and 21 patients received HES 670. Both colloids were administered intravenously at a median rate of 40 cc/hour. The total dose of hetastarch ranged from 5 L to 14 L and median infusion duration was 10 days (ranged from 6 to 14 days). High molecular weight HES (HES 670) was the preferred volume expanding agent because volume expanding effect was excellent and penylephrine use reduced.
RESULTS
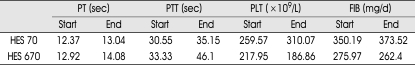
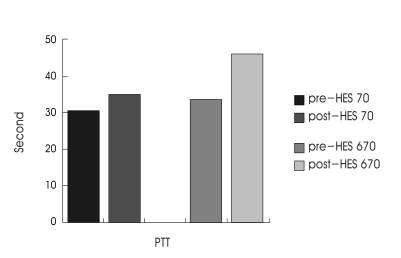
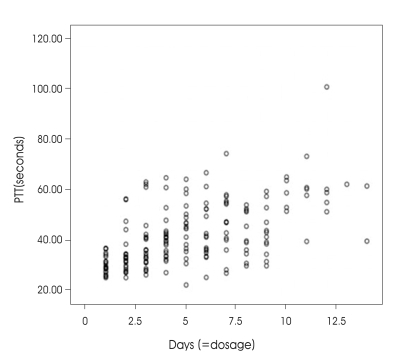
At the beginning of therapy, PT and PTT were in the normal range. The mean infusion rate for hetastarch was 0.67 cc/kg per hour. Table 1 demonstrates the hemorheologic parameters at the beginning and end of the HES treatment. Mean value of post-treatment PT was slightly elevated above pre-treatment levels in both groups, whereas platelet and fibrinogen levels revealed no significant change. PTT was found to be significantly prolongated. In HES 70 group, it ranged from 30.55 to 35.15 sec and in HES 670 group, from 33.33 to 46.1 sec (Table 2). The difference in PTT changes between two groups was significant (p<0.005 by Mann-Whitney test, Fig. 1). Dosage (=duration) shows a positive correlation with PTT (Fig. 2).
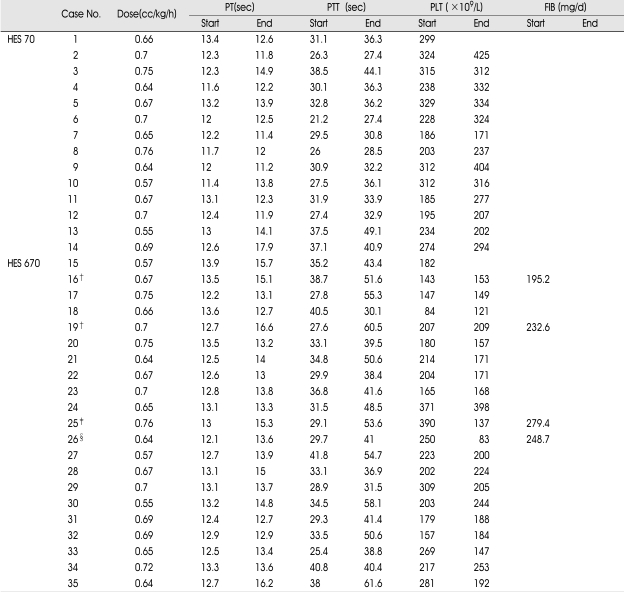
Table 1.
Hemorheological parameters at the beginning and end of treatment in patients receiving HES
Abbreviations : PT : prothrombin time, PTT : partial thromboplastin time, PLT : platelet count, FIB : fibrinogen level. *Not tested, †Subgaleal hematoma developed patients, ‡Delayed epidural hematoma developed patient, §Delayed epidural hematoma with microalveolar hemorrhage developed patient
Table 2.
Mean value of Hemorheological parameters at the beginning and end of treatment in patients receiving HES
Fig. 1.
Bar graph illustrating mean changes of partial thromboplastin time (PTT) in patients receiving either hydroxyethyl starch (HES) 70 or HES 670. The difference in PTT changes between HES groups is statistically significant (p < 0.005).
Fig. 2.
Graph displaying the distributions of partial thromboplastin time and dosage (=duration) in patients receiving hydroxyethyl starch (Pearson correlation=0.564, p < 0.005).
The elevation of PTT was evidenced clinically in high-molecular-weight hetastarch group but not in low-molecular-weight hetastarch group. Two patients (Case 16, 19), while receiving hetastarch, required re-exploration 4-5 days after surgery for subgaleal arterial bleeding from the operative site. Other two patients (case 25, 26), receiving hetastarch, developed delayed postoperative epidural hematoma that required evacuation. In case 26, additional microalveolar hemorrhage developed during hetastarch infusion. In all of these patients, outcome was believed to be negatively influenced by the aforementioned complications.
DISCUSSION
Hydroxylethyl starch (HES) is often used for plasma-expanding agent. Since bleeding complications have been reported repeatedly, a strict dose limitation of maximum of 1500 ml 6% solution per day is recommended1,18).
The use of plasma-expanding agents for the treatment of vasospasm requires a different treatment regimen than fluid resuscitation. The literature supports the use of various plasma substitutes for optimum hemodynamic and hemorheological effects in the cerebral circulation9). Previous studies have reported a reduction in cerebral infarct size with the use of these agents8,12) and improvement in cerebral blood flow in ischemic as well as normal brain6,19). Although the etiology of this improvement was unclear, they suggested theories including hemodilution, decreased plasma viscosity, increased cardiac output, or mild elevation of clotting time7,12,20). The mild elevation of clotting time was ascribed to precipitation by hetastarch of factor VIII5,7,10,15). Studies in humans used doses lower than those necessary for the treatment of vasospasm in normal volunteers8,15). We thought that 500 cc/day of hetastarch was inadequate to maintain pulmonary capillary wedge pressure and hemodilution. More than 1 L/day was usually necessary, and this dose was maintained as long as there was clinical concern about symptomatic vasospasm. Even these higher doses were less than the previously recommended maximum dose of 20 ml of hetastarch per kilogram of body weight for 24 hours.
In a recent series of 85 patients with cerebral vasospasm, Trumble et al.18) demonstrated that the infusion of hetastarch solution, instead of plasma protein fraction, increased the incidence of clinical bleeding and significantly prolonged PTT from 23.9 to 33.1 sec (p<0.001). Sanfellippo and Suberviola13) reported a patient with cerebral vasospasm who developed gingival bleeding after 10 days of hetastarch infusion at a rate of 1000 ml/day. The present study demonstrated that PTT was found to be significantly elevated in both groups. High-molecular-weight hetastarch significantly affected PTT prolongation than low-molecular-weight Hetastarch and also dosage (=duration) showed in direct proportion to PTT prolongation. The prolonged PTT may be associated with a disorder of the intrinsic clotting system caused mainly through a change in factor VIII/von Willebrand factor. von Willebrand factor supports the adhesion of the platelets to the injured blood vessel. A decrease in factor VIII/von Willebrand factor rarely leads to spontaneous bleeding but may considerably prolong after-bleeding from even small injuries17). In our study cases, we thought subgaleal bleeding and epidural hematoma developed from operation site at hemovac removing time (mainly postoperative 2-3 days) were from these cases.
The high molecular weight hetastarch used in this study has a high molecular weight (670 kd) and high degree of substitution (0.75) compared with low molecular weight hetastarch [molecular weight (70 kd), degree of substitution (0.5-0.55)] that would be difficult to be degraded and may induce an unexpected side effect3,11). Hetastarch is a very heterogeneous product, raising concern that its half-life may be extended further by its higher molecular weight and high degree of substitution. Most hetastarch is cleared by the kidney after enzymatic degradation by amylase17). Of particular importance is the fact that high molecular weight hetastarch is difficult to be degraded and cause accumulation in vivo. This affects factor VIII/von Willebrand factor which can lead to an acquired von Willebrand syndrome3,14,16,17). On the basis of this hetastarch-associated coagulopathy and recent experimental data shows that pentastarch causes lesser hemostatic abnormality than hetastarch16) and plasma protein fraction (5% PPF, which is albumin) may well be the most effective agent to increase cerebral blood flow and prevent infarction15). We discontinued the use of hetastarch in the neurosurgical intensive care unit and now use PPF exclusively.
CONCLUSION
The advantages of hetastarch use have been weighed against the disadvantages. The present study serves to point out one potentially serious disadvantage of this agent, especially, the coagulopathy. Such coagulopathy developed in direct proportion to molecular weight of starch and dosages.
This study suggests that daily coagulation screening shoud be monitored in all patients given hetastarch infusions, especially high-molecular-weight hetastarch. Additionally, an evaluation for potential intracranial hemorrhage must be followed in patients who receive hetastarch infusions especially there is a decline in mental status.
References
- 1.Baldassarre S, Vincent JL. Coagulopathy induced by Hydroxyethyl starch. Anesth Analg. 1997;84:451–453. doi: 10.1097/00000539-199702000-00040. [DOI] [PubMed] [Google Scholar]
- 2.Hall ED. Efficacy and mechanisms of action of the cytoprotective lipid peroxidation inhibitor tirilazad mesylate in subarachnoid haemorrhage. Eur J Anaesthesiol. 1996;13:279–289. doi: 10.1046/j.1365-2346.1996.00980.x. [DOI] [PubMed] [Google Scholar]
- 3.Jamnicki M, Bombeli T, Seifert B, Zollinger A, Camenzind V, Pasch T, et al. Low-and Medium molecular-weight Hydroxyethyl starches. Anesthesiology. 2000;93:1231–1237. doi: 10.1097/00000542-200011000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Kang SD. Prevention and Medical Management of Vasospasm. J Korean Neurosurg Soc. 1999;28:1226–1231. [Google Scholar]
- 5.Kuitunen A, Hynynen M, Salmenpera M. Hydroxyethyl starch as a prime for cardiopulmonary bypass : effects of two different solutions on haemostasis. Acta Anaesthesiol Scand. 1993;37:652–658. doi: 10.1111/j.1399-6576.1993.tb03783.x. [DOI] [PubMed] [Google Scholar]
- 6.Korosue K, Ishida K, Matsuoka H. Clinical hemodynamic and hemorheological effects of isovolemic hemodilution in acute cerebral infarction. Neurosurgery. 1988;23:148–153. doi: 10.1227/00006123-198808000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Korttila K, Grohn P, Gordin A. Effect of hydroxyethyl starch and dextran on plasma volume and blood hemostasis and coagulation. J Clin Pharmacol. 1984;24:273–282. doi: 10.1002/j.1552-4604.1984.tb01833.x. [DOI] [PubMed] [Google Scholar]
- 8.Kroemer H, Haass A, Muller K. Haemodilution therapy in ischaemic stroke : plasma concentrations and plasma viscosity during long-term infusion of dextran 40 or hydroxyethyl starch 200/0.5. Eur J Clin Pharmacol. 1987;31:705–710. doi: 10.1007/BF00541299. [DOI] [PubMed] [Google Scholar]
- 9.Levy ML, Giannotta SL. Cardiac performance indices during hypervolemic therapy for cerebral vasospasm. J Neurosurg. 1991;75:27–31. doi: 10.3171/jns.1991.75.1.0027. [DOI] [PubMed] [Google Scholar]
- 10.Mayberg MR, Batjer HH, Dacey R. Guidelines for the management of aneurysmal subarachnoid hemorrhage : a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 1994;25:2315–2328. doi: 10.1161/01.str.25.11.2315. [DOI] [PubMed] [Google Scholar]
- 11.Nearman HS, Herman ML. Toxic effects of colloids in the intensive care unit. Crit Care Clin. 1991;7:713–723. [PubMed] [Google Scholar]
- 12.Perez-Trepichio AD, Furlan AJ, Little JR. Hydroxyethyl starch 200/0.5 reduces infarct volume after embolic stroke in rats. Stroke. 1992;23:1782–1790. doi: 10.1161/01.str.23.12.1782. [DOI] [PubMed] [Google Scholar]
- 13.Sanfelippo MJ, Suberviola PD. Development of a von Willebrand-like syndrome after prolonged use of hydroxyethyl starch. Am J Clin Pathol. 1987;43:391–393. doi: 10.1093/ajcp/88.5.653. [DOI] [PubMed] [Google Scholar]
- 14.Stoll M, Treib J, Schenk JF, Windisch F, Haass A, Wenzel E, et al. No coagulation disorders under high-dose volum therapy with low-molecular-weight hydroxyethyl starch. Haemostasis. 1997;27:251–258. doi: 10.1159/000217464. [DOI] [PubMed] [Google Scholar]
- 15.Stump DC, Strauss RG, Henriksen RA. Effects of hydroxyethyl starch on blood coagulation, particularly factor VIII. Transfusion. 2002:27–36. doi: 10.1046/j.1537-2995.1985.25485273815.x. [DOI] [PubMed] [Google Scholar]
- 16.Stump DC, Strauss RG, Henriksen RA, Patersen RE, Saunders R. A randomized, blinded trial comparing the hemostatic effects of pentastach versus hetastarch. Transfusion. 1985;25:349–354. doi: 10.1046/j.1537-2995.2002.00003.x. [DOI] [PubMed] [Google Scholar]
- 17.Treib J, Haass A, Pindur G. Coagulation disorders caused by Hydroxyethyl starch. Thromb Haemost. 1997;78:974–983. [PubMed] [Google Scholar]
- 18.Trumble ER, Muizelaar JP, Myseros JS. Coagulopathy with the use of hetastarch in the treatment of vasospasm. J Neurosurg. 1995;82:44–47. doi: 10.3171/jns.1995.82.1.0044. [DOI] [PubMed] [Google Scholar]
- 19.Vorstup S, Andersen A, Juhler M. Hemodilution increases cerebral blood flow in acute ischemic stroke. Stroke. 1989;20:884–889. doi: 10.1161/01.str.20.7.884. [DOI] [PubMed] [Google Scholar]
- 20.Weir B. Protection of the brain after aneurysmal rupture. Can J Neurol Sci. 1995;22:177–186. doi: 10.1017/s0317167100039810. [DOI] [PubMed] [Google Scholar]