Abstract
Objective
Arsenic trioxide (As2O3) has been used as an anticancer agent in traditional Chinese medicine for thousand years and berberine is an isoquinoline alkaloid present that has indicated significant antimicrobial activity. We have examined the combined anticancer effects of As2O3 and berberine against the human neuroblastoma (HNB) SH-SY5Y cells in vitro, and to elucidate underlying molecular mechanism.
Methods
HNB SH-SY5Y cells were treated with 2 µM As2O3 and 75 µg/ml berberine, and their survival, cell death mechanism as well as synergistic cytotoxic effects were estimated by using MTT assay, DAPI staining, agarose gel electrophoresis, flow cytometric analysis, and western blot analysis.
Results
The combined treatment of two drugs also markedly decreased cell viability. The cytotoxic effects of two drugs were revealed as apoptosis characterized by chromatin condensation, DNA fragmentation, and the loss of mitochondrial membrane potential. The apoptotic cytotoxicity was accompanied by activation of caspase-3 protease as well as decreased the expression of Bcl-2, Bid, and Bcl-x/L. In addition, the cells treated with combination of two drugs also showed significantly increased intracellular reactive oxygen species levels and lipid peroxidation compared to cells As2O3 or berberine only.
Conclusion
Combined treatment of As2O3 with berberine induced activation of apoptotic signaling pathways in HNB SH-SY5Y cells. These results suggest that the possibility of the combined treatment of two chemotherapeutic agents with low concentration improving cytotoxic effect for cancer cells with minimal side effects.
Keywords: Human neuroblastoma SH-SY5Y cells, Arsenic trioxide, Berberine, Apoptosis
INTRODUCTION
Neuroblastoma is the most common extracranial neoplasm of childhood derived from precursor of immature cells of the sympathetic nervous system. Neuroblastomas show a very complex biological and tremendous clinical heterogenesity. The same remarkable cure rate that has been achieved in most other childhood malignancies has not yet occurred in this tumor. The important factors that determine the prognosis and therapeutic modality are patient age, stage, molecular defect and pathology. The majority of the neuroblastomas in children over 1 year age are aggressive metastatic tumors with poor clinical outcome despite intensive multimodal therapy including surgery, irradiation and chemotherapy.
Arsenic trioxide (As2O3) has been used as an anticancer agent in traditional Chinese medicine for thousand years. Recently, As2O3 was approved by the Food and Drug Administration for use in the treatment of relapsed/refractory acute promyelocytic leukemia, head and neck cancer, neuroblastoma, etc4,9,15). The mechanism of action is apoptosis induced by making reactive oxygen species (ROS), change of mitochondrial membrane potential (MMP), decreasing Bcl-2 protein expression, and caspases activation. Also, arsenic acid induces DNA damage and regression cell cycle by effect on G0 or G2/M phase3,24).
Berberine, a main component of Coptidis Rhizoma, is a type of alkaloid that was initially isolated from Chinese herbal medicine. Berberine has been used in treatments of various diseases, because of its antibacterial, anti-inflammatory, and anticancer effect1,2,8,16). In USA, it is also used as prostatic cancer drug, the brand name of PC-SPECS®. Berberine exhibits the ability to induce apoptosis in human cancer cells. Apoptosis is triggered by DNA fragmentation of S-phase cells with specific inhibition of DNA topoisomerase I and II in biochemical system by berberine15,26).
One of the primary issues for cancer chemotherapy is how to kill cancer cells selectively without damaging normal cells. In particular, combination treatment of two chemotherapeutic agents with low concentration has been reported to improve cytotoxic effect for cancer cells with minimal side effects2,10,17,19,22,27). In this study, we examined the apoptotic effects of combination treatment of berberine and As2O3 on HNB SH-SY5Y cell line.
MATERIALS AND METHODS
Chemical reagents
Minimum Essential Medium (MEM) and F12 medium were purchased from Gibco Life Technologies (Gaithersburg, MD, USA). Fetal bovine serum (FBS) was purchased from Hyclone. 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyl tetrazolium bromide (MTT), trypsin, streptomycin, penicillin, berberine, and As2O3 were obtained from Sigma Co. (St. Louis, MO, USA). 2'7'-Dichlorofluorescein diacetate (DCFH-DA), 4',6-diamidine-2-phenylindole (DAPI), Rhodamine123 were purchased from Molecular Probes (Eugene, OR, USA). Antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Peroxidase-linked secondary antibody was bought from Amersham Life Science (Piscataway, NJ). All other chemicals used were of the highest grade available commercially.
Cell culture
HNB SH-SY5Y cells (American Type Culture Collection, USA) were maintained in MEM and F12 supplemented with 10% (v/v) heat-inactivated FBS and 100 µg/ml penicillin/ streptomycin. Cells were maintained in an incubator with a humidified atmosphere of 95% air and 5% CO2 at 37℃. Experiments were carried out 48 h after cells were seeded onto plates or dishes.
Measurement of cell viability
Cell viability was quantified by MTT assay. Briefly, cells were plated in 48-well culture plates at the density of 5×104 cells/well and allowed to adhere at 37℃ for 24 h. The following day, various doses of berberine or As2O3 were added and the cells incubated for 48 h, after which cell growth and viability were measured using MTT. The absorbance was then measured at 595 nm using a Digiscan Microplate Reader (Assys Hitech, Kornenburg, Austria). Wells without cells were used as blanks and were subtracted as background from each sample. Results were expressed as a percentage of control.
Nuclear morphology
To assess apoptosis, the nuclei of SH-SY5Y cells were stained with DAPI. Cells were fixed in phosphate buffered saline (PBS) containing 3.7% paraformaldehyde for 15 min. After fixation, cells were washed twice with PBS and then treated with DAPI in PBS. After three washes, cells were observed under fluorescence microscope.
Analysis of DNA fragmentation by agarose gel electrophoresis
To prepare genomic DNA, SH-SY5Y cells (4×106 cells) were incubated with berberine and As2O3, or in combination for 48 h, then the cells were detached from 6 cm culture dishes and centrifuged at 100 g for 10 min. The cell pellet was then washed twice with ice-cold PBS and genomic DNA was isolated using a DNA isolation kit (Promega, Basel, Switzerland) according the manufacturer's instructions. The DNA samples were analysed on a 1.5% agarose gel containing ethidium bromide (1 µg/mL) in Tris-borate-EDTA (TBE) buffer and run for 90 min at 70 V. After electrophoresis, the DNA was visualized under ultraviolet (UV) light and photographed.
Determination of mitochondrial membrane potential
MMP was quantified using the ratio metric probe Rhodamine123. Briefly, cells in 6 cm tissue culture plates were treated with berberine or As2O3 alone or combined treatment for the indicated times. Twenty minutes before the cells were harvest, Rhodamine123 was added directly to the culture medium to final concentration of 30 nM. The cells were harvested by trypsinization, washed with 5 ml PBS at 37℃, pelleted by centrifugation, resuspended in 500 µL fluorescence-activated cell sorting (FACS) buffer, and analyzed immediately for Rhodamine123 fluorescence intensity by flow cytometry (FACSCalibur, BD Bioscience, San Jose, CA).
Western blot analysis
Cytosolic protein extracts were prepared as described by Kumagai et al.15) Briefly, cells were collected by centrifugation at 300 g for 5 min at 4℃ and washed with ice-cold PBS. The cell pellet was then resuspended in 500 µL of lysis buffer (20 mM Hepes- KOH, pH 7.5, 210 mM sucrose, 70 mM mannitol; 1.5 mM MgCl2, 10 mM KCl2, 10 µg/ml leupeptin, and 10 µM digitonin). After 10 minutes of incubation at 25℃, the sample was spun at 14,000 g for 15 min, and the supernatant containing cytosolic proteins were stored at -70℃ until analysis by 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The protein extracts were subjected to standard SDS-PAGE, transferred onto polyvinylidene difluoride membranes (Millipore), and probed with appropriate antibodies as described individually in the figure legends. The bound primary antibody was detected using appropriate horseradish peroxidase-conjugated secondary antibody, and protein was visualized using an enhanced chemiluminescence (ECL) detection kit. β-actin was used as an internal control to confirm that the amounts of protein load were equal.
Measurement of intracellular reactive oxygen species
To monitor the intracellular ROS, we utilized cell-permeable probes, DCFH-DA. Nonfluorescent DCFH-DA, hydrolyzed to DCFH inside of cells, yields highly fluorescent DCF in the presence of intracellular hydrogen peroxide and related peroxides. Therefore, the DCF fluorescence intensity is proportional to the amount of hydrogen peroxide formed intracellulary. At the end of treatment, cells were washed once with PBS, and then incubated in growth media containing 10 µM DCF-DA for 30 min at 37℃. Cells were washed once with PBS and suspended in cold PBS, and the fluorescence was monitored by flow cytometry. As a positive control, cells were separately treated with berberine, As2O3 alone or combined and processed for ROS detection.
Measurement of lipid peroxidation
Lipid peroxidation assay was based on that described by Ghafourifar et al21), with some modifications. Briefly, 50 µL of PBS resuspension of harvested cells (1-5×106 cells) was added to 50 µL of 3.2% SDS in PBS, and incubated for 10 min at room temperature. Following incubation, 150 µL of 20% acetic acid (pH 3.5) and 150 µL of 0.8% thiobarbituric acid (TBA) in 0.05 N NaOH were added and the mixture was then boiled for 1 h. The mixtures was cooled and extracted with 1 ml each of an n-butanol-pyridine mixture (1:3, v/v) to avoid turbidity. The upper layer (approximately 240 µL) of each sample was aspirated, and the absorbance was measured at 532 nm. Concentrations of 2-TBA were determined using the extinction coefficient of 1.56×105 M-1cm-1. The results were expressed as mol of malondialdehyde (MDA) per mg-protein.
Statistical analysis
The data given in this experiment were expressed as means±standard deviation (S.D.). Statistical comparison was carried out with three or more groups using one-way analysis of variance (ANOVA). A difference was considered to be significant at (p-value)<0.05.
RESULTS
Combined effects of As2O3 and berberine on cell viability
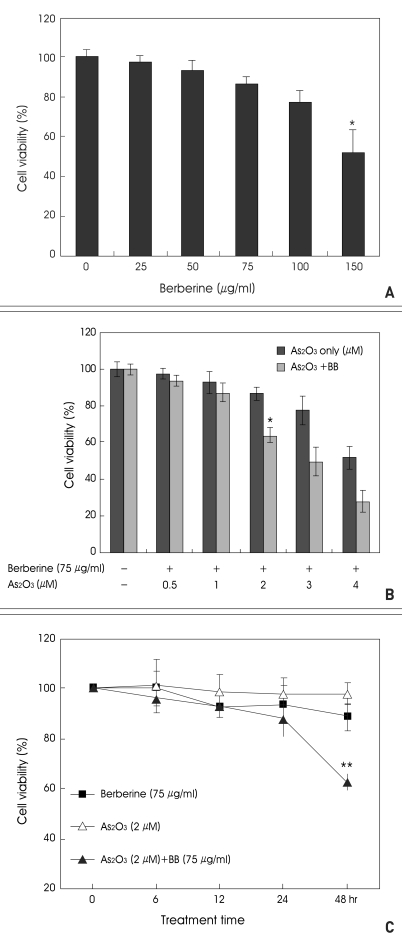
To investigate the combined anticancer effects of As2O3 and berberine, we first examined the cytotoxic effects of berberine in SH-SY5Y cells. After SH-SY5Y cells were treated with various doses of berberine for 48 h, cell viability was measured by MTT assay (Fig. 1A). These experiments for berberine were done to identify a concentration that produced modest growth inhibition to test subsequently its combination with As2O3. We noticed that a dose of berberine as low as 75 µg/ml caused little effects on cell viability. However, berberine significantly enhanced cytotoxicity (61%), when combined with 2 µM As2O3. Hence, 75 µg/ml berberine and 2 µM As2O3 were selected as the dose used for the combination effects in SH-SY5Y cells.
Fig. 1.
A : Cytotoxic effect of berberine on SH-SY5Y cells. Cells were treated with various concentrations of berberine for 48 h. Cell viability was determined by 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyl tetrazolium bromide (MTT) assay. Results represent the mean±S.D. of three independent experiments (*p<0.05). B : Combined treatment of berberine with As2O3 decreased the viability of SH-SY5Y cells in a dose-dependent manner. Cells were treated with various concentrations of As2O3 and berberine for 48 h. Then, cell viability was determined by MTT assay (*p<0.05). C : Combined treatment of berberine with As2O3 decreased the viability of SH-SY5Y cells in a time-dependent manner. Cells were treated with berberine and As2O3 for the indicated periods after then, cell viability was determined by MTT assay.
The viability of SH-SY5Y cells treated with berberine and As2O3 was inversely proportional to the dose of As2O3. In the concentration of As2O3 more than 2 µM showed prominent synergistic effect (Fig. 1B). Also, the combined treatment of two drugs showed a significant cytotoxicity in a time-dependent manner (Fig. 1C).
Apoptosis induced by combined treatment of As2O3 and berberine
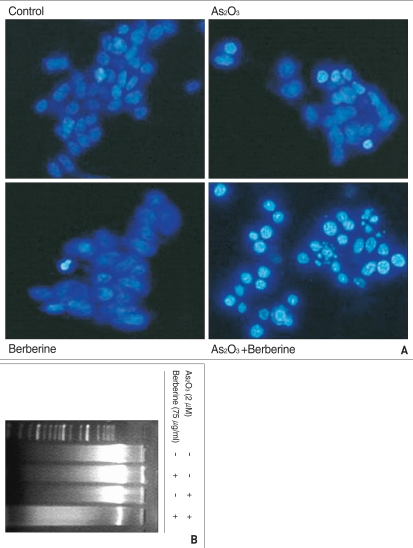
In order to define the mode of cell death induced by combined treatment of As2O3 with berberine, we examined nuclear morphology and DNA fragmentation. After SH-SY5Y cells were treated with 2 µM As2O3 and 75 µg/ml berberine, nuclear morphology was examined by DAPI staining. In contrast to control group and single treated groups (berberine or As2O3), combined treatment group of As2O3 with berberine showed chromatin condensation, DNA fragmentation under fluorescent microscopy (Fig. 2A). To reconfirm the DNA fragmentation, genomic DNA was extracted and electrophoresed on 1.5% agarose gel. Combined treatment with As2O3 and berberine induced apoptic DNA fragmentation, however, single treated groups with 75 µg/ml berberine or 2 µM As2O3 were not (Fig. 2B).
Fig. 2.
Combined treatment of berberine with As2O3. A : Combination treatment with As2O3 and berberine induced the chromatin condensation and nuclear fragment of SH-SY5Y cells. Cells were treated with berberine (75 µg/ml) and As2O3 (2 µM) and stained with 4',6-diamidine-2-phenylindole (DAPI). The nuclei of cells were observed under fluorescent miroscope. B : Combination treatment with As2O3 and berberine increased the fragmentation of genomic DNA in SH-SY5Y cells. Cell were treated with berberine (75 µg/ml) and As2O3 (2 µM for 48 h. Genomic DNA was extracted, separated on 1.5% agarose gel with size marker, and stained with ethidium bromide to visualized under ultraviolet light.
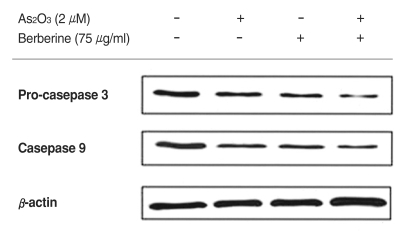
To address the particular role of caspase in berberine and As2O3 induced apoptosis, we investigated activities of caspase-3, -9. The western blot analysis of pro-caspase-3 and caspase-9 protein is illustrated in Fig. 3. As presented, combined treatment of berberine and As2O3 decreased expression of pro-caspase-3 and caspase-9 protein in SH-SY5Y cells markedly.
Fig. 3.
Combination treatment with berberine and As2O3 resulted in a decreased expression of pro-caspase 3 and caspase 9 protein in SH-SY5Y cells. Cells were treated with 75 µg/ml berberine and 2 µM of As2O3 for 48 h. The equal amount of protein from cell lysate was subjected on 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred onto polyvinylidene difluoride-nitrocellulose filters (PVDF) membranes, and immunoblotted with anti-procaspase 3, anti-caspase 9 and anti-β, actin antibodies, respectively. The immunoreactive signals were visualized by enhanced chemiluminescence (ECL) kit.
Combined treatment with As2O3 and berberine-induced apoptosis is associated with a reduction of mitochondrial membrane potential and alteration of Bcl-2 family proteins
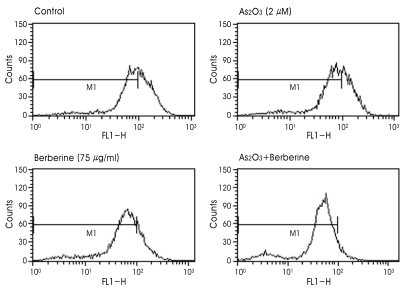
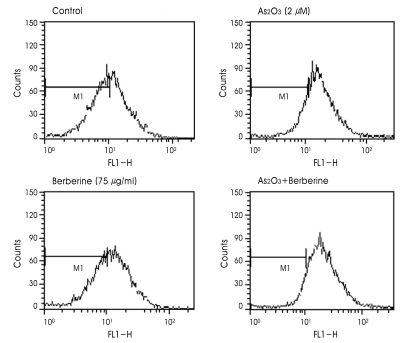
A decline of the MMP may be an early event in the process of cell death. The loss of the MMP is the marker for the activation of mitochondrial pathway, which is regulated by pro- and anti-apoptotic proteins of the Bcl-2 family. Since disruption of the MMP is a critical step in cells undergoing apoptosis, we evaluated whether combined treatment with As2O3 and berberine had any effect on the MMP by using Rhodamine123 as a marker of mitochondrial membrane integrity. Disruption of MMP causes a decrease in FL-1 fluorescence as analyzed by flow cytometry. As indicated in Fig. 4, there was no substantial change of the MMP after treatment with 2 µM As2O3 or 75 µg/ml berberine alone. However, when the cells were treated with As2O3 and berberine in combination, a markedly decreased Rhodamine123 fluorescence was observed, indicating disruption of the MMP in these cells.
Fig. 4.
Combined treatment of berberine and As2O3 induced the mitochondrial membrane potential transition (MMPT) in SH-SY5Y cells. Cells were treated with 75 µg/ml berberine and 2 µM As2O3 48 h. Then, cells were incubated with 50 nM Rhodamine123 for 30 min at 37℃C and used to analyze MMPT with flow cytometry. The horizontal axis indicates the relative fluorescence intensity and the vertical axis denotes the relative cell number.
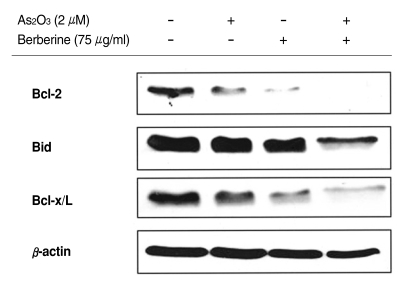
It has been recognized that the proteins of Bcl-2 family play crucial roles in regulation of apoptosis by functioning as inhibitors (Bcl-2, Bid or Bcl-x/L) of cell death. We therefore reasoned to determine the effect of berberine on the expression of these proteins. The results revealed that the combined treatment with As2O3 and berberine resulted in a marked reduction in the levels of protein Bcl-2, Bid and Bcl-x/L (Fig. 5).
Fig. 5.
Combined treatment of berberine and As2O3 decreased the expression of anti-apoptotic Bcl-2 proteins in SH-SY5Y cells. Cells were treated with 75 µg/ml berberine and 2 µM of As2O3 for 48 h. The equal amount of protein from cell lysate was subjected on 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred onto polyvinylidene difluoride-nitrocellulose filters (PVDF) membranes, and immunoblotted with anti-Bcl-2, anti-Bid, anti-Bcl-x/L, and anti-β, actin antibodies, respectively. The immunoreactive signals were visualized by enhanced chemiluminescence (ECL) kit.
Combined treatment with As2O3 and berberine in significantly increased intracellular ROS generation and lipid peroxidation
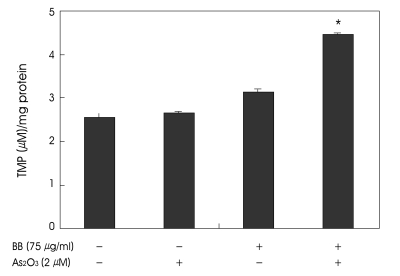
Because of the mitochondrial respiratory chain on the inner mitochondrial membrane is a major intracellular source of ROS, we next examined whether combined treatment with As2O3 and berberine induces ROS generation. We examined the level of ROS production in combination with berberine and As2O3 using DCF-DA, ROS-sensitive probe. In contrast to the control and As2O3 or berberine alone, treatment with 2 µM As2O3 and 75 µg/ml berberine markedly induced intense fluorescence in SH-SY5Y cells (Fig. 6). We next addressed the effect of combined treatment with As2O3 and berberine on the content of intracellular lipid peroxidation products (thiobarbituric acid reactive substance). Although cells treated with As2O3 or berberine alone showed no significantly differences in lipid peroxidation, when cells were combined with As2O3 and berberine for 48 h, the rate of lipid peroxidation significantly increased in 2 folds (Fig. 7).
Fig. 6.
Combination treatment with berberine and As2O3 increased the intracellular reactive oxygen species (ROS) in SH-SY5Y cells. Cells were treated with 75 µg/ml berberine and 2 µM As2O3 for 32 h. After harvesting, cells were stained with 2',7'-dichlorofluorescin diacetate (DCFH-DA) dye and subjected on flow cytometric analysis to estimate intracellular ROS levels.
Fig. 7.
Combination treatment with berberine and As2O3 increased the lipid peroxidation of SH-SY5Y cells. Cells were treated with berberine (75 µg/ml) and As2O3 (2 µM) for 48 h. Cell homogenates were mixed with thiobarbituric acid (TBA) in glass test tubes and incubated in a boiling water bath for 60 min. The level of lipid peroxidation was expressed in absorbance at 532 nm using tocopherol-mediated peroxidation (TMP) as an external standard, and expressed as mean±S.D. (n=3) (*p<0.05).
DISCUSSION
Berberine has been used to treat diabetes for more than 1,000 years in the long history of chinese medicine. Berberine has a wide range of pharmacologic actions such as antidiarrheric, antimicrobial, anticancer, anti-inflammatory, and antiarrhythmic activities7,26). Berberine was also shown to inhibit DNA and protein synthesis, cell cycle progress of arrest, and the in vitro growth of a number of human cancer cell lines, however, the molecular mechanisms underlying berberine-induced apoptosis are not yet well defined7).
Although As2O3 has potent activity against cell growth in a series of leukemia cell lines, little information is available regrading this compound's effect on cell growth in solid tumor cell lines14). The mechanisms of As2O3 cytotoxicity are not known completely, but preclinical studies have provided insight into the processes involved. The mechanisms include cellular differentiation, induction of apoptosis, and inhibition of angiogenesis4). As2O3-induced apoptosis has been described in three major apoptotic mechanisms; mitogen-activated protein (MAP) kinase, activation of caspase pathway, and production of ROS2-4,14,24).
Because As2O3 at higher concentrations induces many side effects (ventricular arrhythmia, skin reaction, peripheral neuropathy, electrolyte changes, leukocytosis, hepatic dysfunction, GI reactions, etc.) as well as association linking carcinogenesis with chronic arsenic exposure, low-dose combination therapy is required2,9,10,17,27,28). In our study, low dose combination of two chemotherapeutic agents (75 µg/ml berberine + 2 µM As2O3) decreased cell viability markedly, however each of low dose single agent (75 µg/ml berberine or 2 µM As2O3) could not decreased cell viability significantly. Combination of berberine and As2O3 at non-toxic level has synergistic effects of apoptosis on HNB SH-SY5Y cells in vitro, therefore, we can expect that clinically it may reduce the side effect of therapeutic agents8,23).
To determine apoptotic effects of combination chemotherapy (As2O3+berberine) in HNB SH-SY5Y cell line, we had investigated the cell morphology, ROS production, lipid peroxidation, mitochondrial membrane potential transition (MMPT), and expression of apoptosis induced protein. The treatment of SH-SY5Y cells with combination of berberine and As2O3 was sufficient to induce apoptosis and cleavage of several key proteins related to the mitochondrial death pathway, which was examined by western blot analysis6,7,13). These experiments demonstrated that the combined treatment of As2O3 and berberine significantly activates the mitochondrial apoptosis pathway which are regulated by the anti-apoptotic proteins of the Bcl-2 family8,23,25). In mitochondria-mediated pathway, cytochrome c and other apoptogenic proteins are released from the mitochondrial intermembrane space into the cytosol. Once released, cytochrome c binds to Apaf-1 and induces sequential activation of caspase-9 and caspase-3. Activation of caspase-8, result from death factors (e.g. Fas ligand, tumor necrosis factor-α) bind to their relevant cell-surface receptors, provides a link between receptor-mediated and mitochondria-mediated pathways of apoptosis. Caspase-8 activation induces cleavage of Bid to truncated Bid (tBid) that triggers Bax activation, resulting in change in mitochondrial permeability and thereby release of cytochrome c into the cytosol. Hwang et al.7) reported that cytotoxicity of berberine is due to the induction of apoptosis which activates procaspase and DNA fragmentation. Furthermore, the activation of caspases results to decrease the bcl-2, Bid, and Bcl-x/L. Therefore, the berberine-induced apoptosis could be almost completely inhibited by caspase inhibitors.
One of mechanisms of As2O3-induced apoptosis is through activation of caspases. As2O3 activates these proteases, which play an important role in the degradation phase of apoptosis, in NB4 cell lines4). A key event in the effector phase of apoptosis is the progressive permeabilization of the mitochondrial membrane secondary to the action of permeability transition pore complex. As2O3 can lead to changes of MMP and increased membrane permeability as a result of degradation phase of apoptosis4). The results of our study were also consistent with the findings of aforementioned. The combination treatment of As2O3 with berberine decreased expression of pro-caspase 3, caspase 9 protein, bcl-2, Bid, Bcl-x/L, and induced the MMPT in HNB SH-SY5Y cells.
ROS are free radicals that react with all biological macromolecules, modify the structure and function of proteins as well as cause oxidative damage to DNA12,14). Hydrogen peroxide (H2O2) is a common intermediate in the oxidative metabolic precess, which has been shown to induce DNA damage in human cells4,5,27,28). Despite varied antioxidant defenses occurring in cells, there is always a steady state concentration of ROS presents1,3,4). ROS damages cellular DNA through oxidative stress-induced destruction of pyrimidine and purine bases and single strand breaks. A critical component of the cellular response to oxidative stress is the glutathione redox system, a known modulator of arsenical induced cell killing1,4,20). As previously studied by other authors4,11), our study revealed that combined treatment with As2O3 and berberine increased the intracellular ROS than single treatment in HNB SH-SY5Y cells.
CONCLUSION
Combined treatment of As2O3 and berberine at non-toxic dose level induced activation of apoptotic signal transmission system, DNA fragmentation and caspase family protein, decreased expression of MMPT, and increased production of intracellular ROS in SH-SY5Y cells. These results suggest that the possibility of the combined treatment of two chemotherapeutic agents with low concentration can improve cytotoxic effect for cancer cells with minimal side effects.
Acknowledgement
This paper was supported by Wonkwang University in 2006.
References
- 1.Akihter MH, Sabir M, Bhide NK. Anti-inflammatory effect of berberine in rats injected locally with cholera toxin. Indian J Med Res. 1977;65:133–141. [PubMed] [Google Scholar]
- 2.Amin AH, Subbaiah TV, Abbasi KM. Berberine sulfate : antimicrobial activity, bioassay, and mode of action. Can J Microbiol. 1969;15:1067–1076. doi: 10.1139/m69-190. [DOI] [PubMed] [Google Scholar]
- 3.Baumgartner M, Sturlan S, Roth E, Wessner B, Bachleitner-Hofmann T. Enhancement of arsenic trioxide-mediated apoptosis using Docosahexaenoic acid in arsenic trioxide-resistant sold tumor cells. Int J Cancer. 2004;112:707–712. doi: 10.1002/ijc.20462. [DOI] [PubMed] [Google Scholar]
- 4.Evans AM, Tallman MS, Gartenhaus RB. The potential of arsenic trioxide in the treatment of malignant disease : past, present, and future. Leuk Res. 2004;28:891–900. doi: 10.1016/j.leukres.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Hail NJ, Lotan R. Apoptosis induction by the natural product cancer chemopreventive agent deguelin is mediated through the inhibition of mitochondrial bioenergetics. Apoptosis. 2004;9:437–447. doi: 10.1023/B:APPT.0000031449.57551.e1. [DOI] [PubMed] [Google Scholar]
- 6.Hougardy BM, van der Zee AG, van den Heuvel FA, Timmer T, de Vries EG, de Jong S, et al. Sensitivity to Fas-mediated apoptosis in high-risk HPV-positive human cervical cancer cells : Relationship with Fas, caspase-8, and Bid. Gynecol Oncol. 2005;97:353–364. doi: 10.1016/j.ygyno.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 7.Hwang JM, Kuo HC, Tseng TH, Liu JY, Chu CY. Berberine induces apoptosis through a mitochondria/caspases pathway in human hepatoma cells. Arch Toxicol. 2006;80:62–73. doi: 10.1007/s00204-005-0014-8. [DOI] [PubMed] [Google Scholar]
- 8.Iizuka N, Hazama N, Yoshimura S, Yoshino K, Tangoku S, Miyamoto A, et al. Anticachectic effects of the natural herb Coptidis rhizoma and Berberine on mice bearing colon 26/clone 20 adenocarcinoma. Int J Cancer. 2002;99:286–291. doi: 10.1002/ijc.10338. [DOI] [PubMed] [Google Scholar]
- 9.Jakubowicz-Gil J, Paduch R, Piersiak T, Glowniak K, Gawron A, Kanderfer-Szerszen M. The effect of quercetin on pro-apoptotic activity of cisplatin in HeLa cells. Biochem Pharmacol. 2005;69:1343–1350. doi: 10.1016/j.bcp.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Jantova S, Cipak L, Cernakova M, Kost'alova D. Effect of berberine on proliferation, cell cycle and apoptosis in HeLa and L1210 cells. J Pharm Pharmacol. 2003;55:1143–1149. doi: 10.1211/002235703322277186. [DOI] [PubMed] [Google Scholar]
- 11.Jantova S, Cipak L, Letasiova S. Berberine induces apoptosis through a mitochondrial/caspase pathway in human promonocytic U937 cells. Toxicol In Vitro. 2007;21:25–31. doi: 10.1016/j.tiv.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Kang YH, Lee E, Choi MK, Ku JL, Kim SH, Park YG, et al. Role of reactive oxygen species in the induction of apoptosis by α-tocopheryl succinate. Int J Cancer. 2004;112:385–392. doi: 10.1002/ijc.20424. [DOI] [PubMed] [Google Scholar]
- 13.Kerr JF, Wyllie AH, Currie AR. Apoptosis : a basic biological phenomenon with wide ranging implication in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kong B, Huang S, Wang W, Ma D, Qu X, Jiang J, et al. Arsenic trioxide induces apoptosis in cisplatin-sensitive and -resistant ovarian cancer cell lines. Int J Gynecol Cancer. 2005;15:872–877. doi: 10.1111/j.1525-1438.2005.00251.x. [DOI] [PubMed] [Google Scholar]
- 15.Kumagai T, Shih LY, Hughes SV, Desmond JC, O'Kelly J, Hewison M, et al. 19-Nor-1,25(OH)2D2 (a novel, noncalcemic vitamin D analogue), combined with arsenic trioxide, has potent antitumor activity against myeloid leukemia. Cancer Res. 2005;65:2488–2497. doi: 10.1158/0008-5472.CAN-04-2800. [DOI] [PubMed] [Google Scholar]
- 16.Kuo CL, Chi CW, Liu TY. The anti-inflammatory potential of berberine in vitro and in vivo. Cancer Lett. 2004;203:127–137. doi: 10.1016/j.canlet.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Li XK, Motwani M, Tong W, Bornmann W, Schwarts GK. Huanglian, a Chinese herbal extract, inhibits cell growth by suppressing the expression of cyclin B1 and inhibiting CDC2 kinase activity in human cancer cells. Mol Pharmacol. 2000;58:1287–1293. doi: 10.1124/mol.58.6.1287. [DOI] [PubMed] [Google Scholar]
- 18.Liu Q, Hilsenbeck S, Gazitt Y. Arsenic trioxide-induced apoptosis in myeloma cells : p53-dependent G1 or G2/M cell cycle arrest, activation of caspase-8 or caspase-9, and synergy with APO2/TRAIL. Blood. 2003;101:4078–4087. doi: 10.1182/blood-2002-10-3231. [DOI] [PubMed] [Google Scholar]
- 19.Maeda H, Hori S, Ohizumi H, Segawa T, Kakehi Y, Ogawa O, et al. Effective treatment of advanced solid tumors by the combination of arsenic trioxide and L-buthioninesulfoximine. Cell Death Differ. 2004;11:737–746. doi: 10.1038/sj.cdd.4401389. [DOI] [PubMed] [Google Scholar]
- 20.Maeda S, Suqiura T, Saikawa Y, Kubota T, Otani Y, Kumai K, et al. Docetaxel enhances the cytotoxicity of cisplatin to gastric cancer cells by modification of intracellular platinum metabolism. Cancer Sci. 2004;95:679–684. doi: 10.1111/j.1349-7006.2004.tb03329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nazarewicz RR, Zenebe WJ, Parihar A, Larson SK, Alidema E, Choi J, et al. Tamoxifen induces oxidative stress and mitochondrial apoptosis via stimulating mitochondrial nitric oxide synthase. Cancer Res. 2007;67:1282–1290. doi: 10.1158/0008-5472.CAN-06-3099. [DOI] [PubMed] [Google Scholar]
- 22.Notarbartolo M, Poma P, Perri D, Dusonchet L, Cervello M, D'Alessandro N. Antitumor effects of curcumin, alone or combination with cisplatin or doxorubicin, on human hepatic cancer cells. Analysis of their possible relationship to changes in NF-kB activation levels and in IAP gene expression. Cancer Lett. 2005;224:53–65. doi: 10.1016/j.canlet.2004.10.051. [DOI] [PubMed] [Google Scholar]
- 23.Sauer H, Wartenberg M, Hescheler J. Reactive oxygen species as intracellula messengers during cell growth and differentiation. Cell Physiol Biochem. 2001;11:173–186. doi: 10.1159/000047804. [DOI] [PubMed] [Google Scholar]
- 24.Scholz C, Wieder T, Starck L, Essmann F, Schulze-Osthoff K, Dorken B, et al. Arsenic trioxide triggers a regulated form of caspase-independent necrotic cell death via the mitochondrial death pathway. Oncogene. 2005;24:1904–1913. doi: 10.1038/sj.onc.1208233. [DOI] [PubMed] [Google Scholar]
- 25.Tada-Oikawa S, Hiraku Y, Kawanishi M, Kawanish S. Mechanism for generation of hydrogen peroxide and change of mitochondrial membrane potential during rotenone-induced apoptosis. Life Sci. 2003;73:3277–3288. doi: 10.1016/j.lfs.2003.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Takara K, Horibe S, Obata Y, Yoshikawa E, Ohnishi N, Yokoyama T. Effects of 19 herbal extracts on the sensitivity to paclitaxel or 5-fluorouracil in HeLa cells. Biol Pharm Bull. 2005;28:138–142. doi: 10.1248/bpb.28.138. [DOI] [PubMed] [Google Scholar]
- 27.Takeuchi H, Kondo Y, Fujiwara K, Kanzawa T, Aoki H, Mills GB, et al. Synergistic Augmentation of Rapamycin-Induced Autophagy in Malignant Glioma Cells by Phosphatidylinositol 3-Kinase/Protein Kinase B Inhibitors. Cancer Res. 2005;65:3336–3346. doi: 10.1158/0008-5472.CAN-04-3640. [DOI] [PubMed] [Google Scholar]
- 28.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]