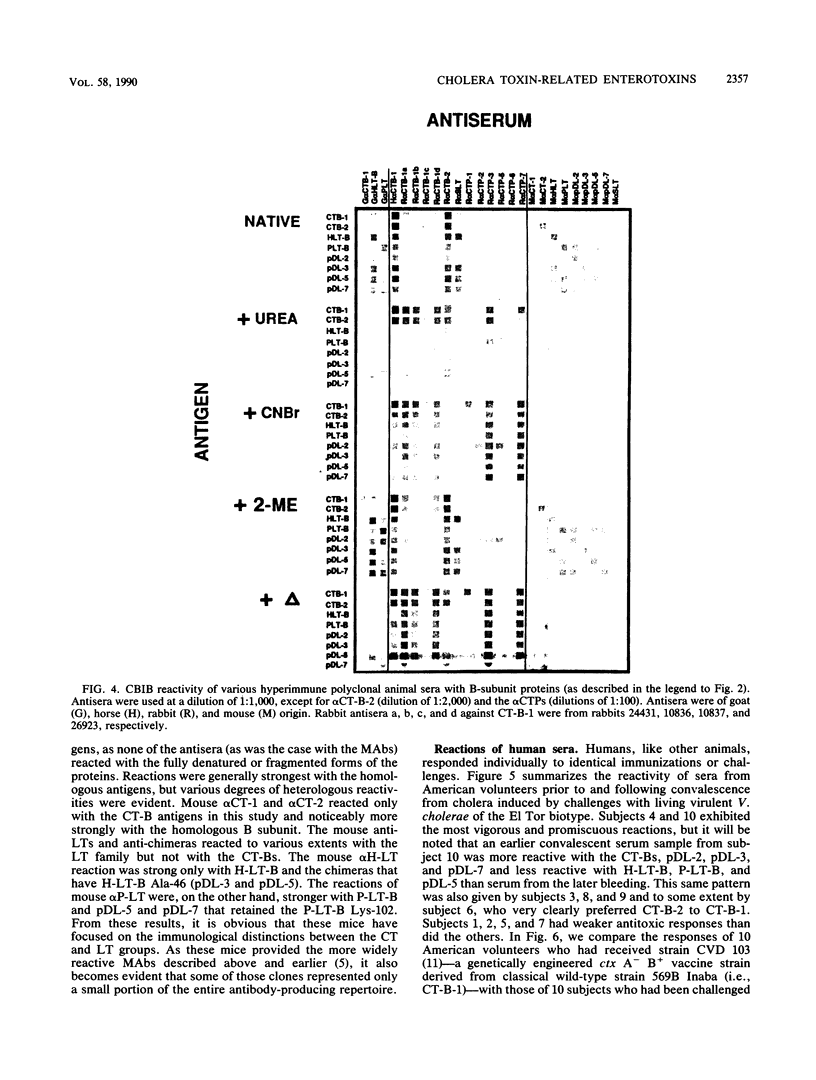
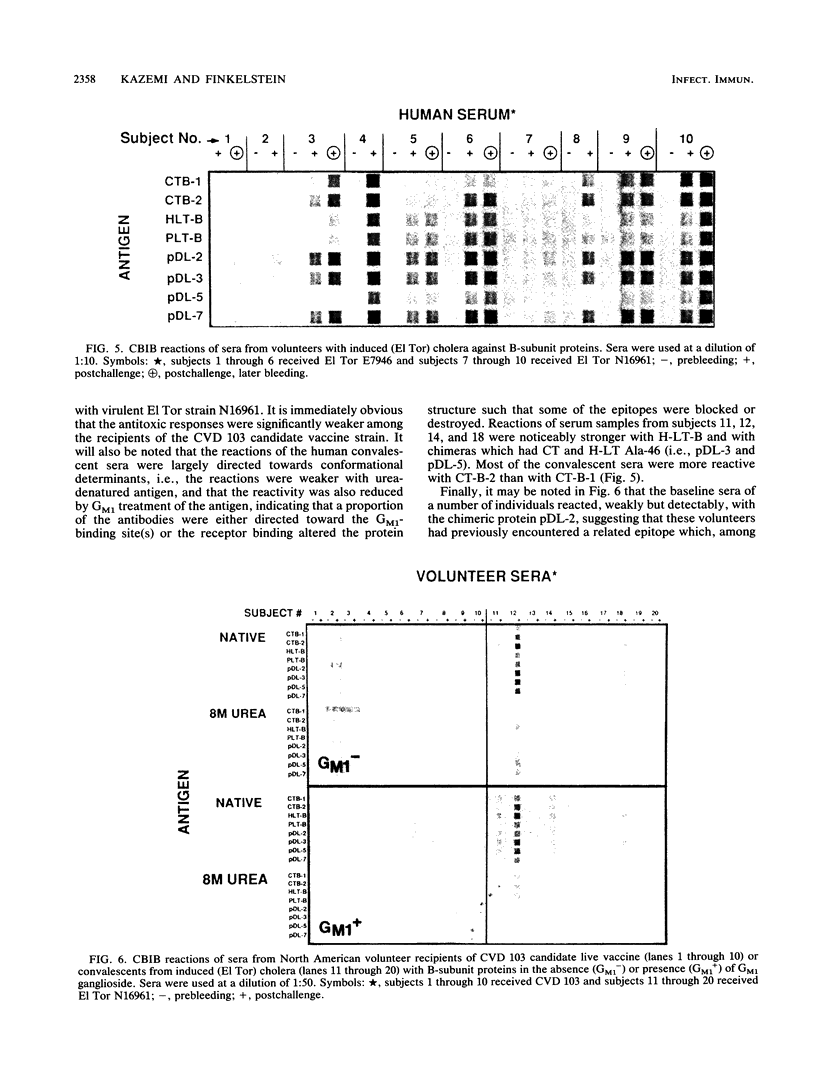
Abstract
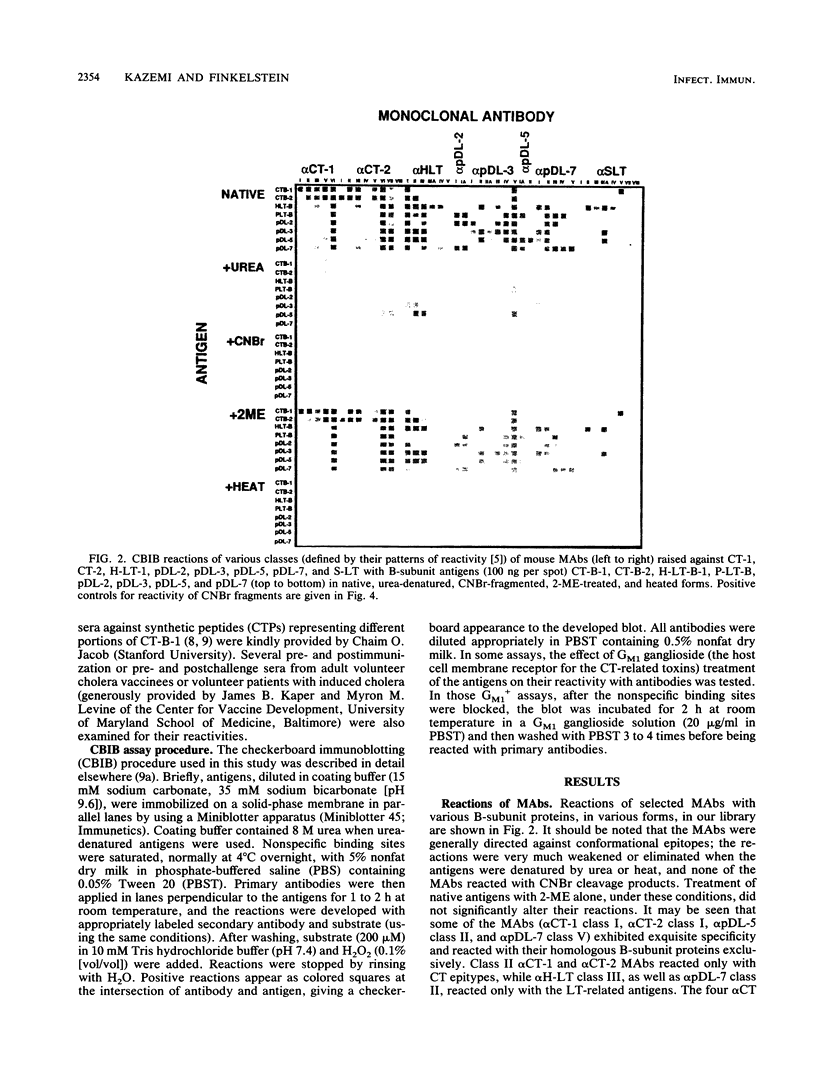
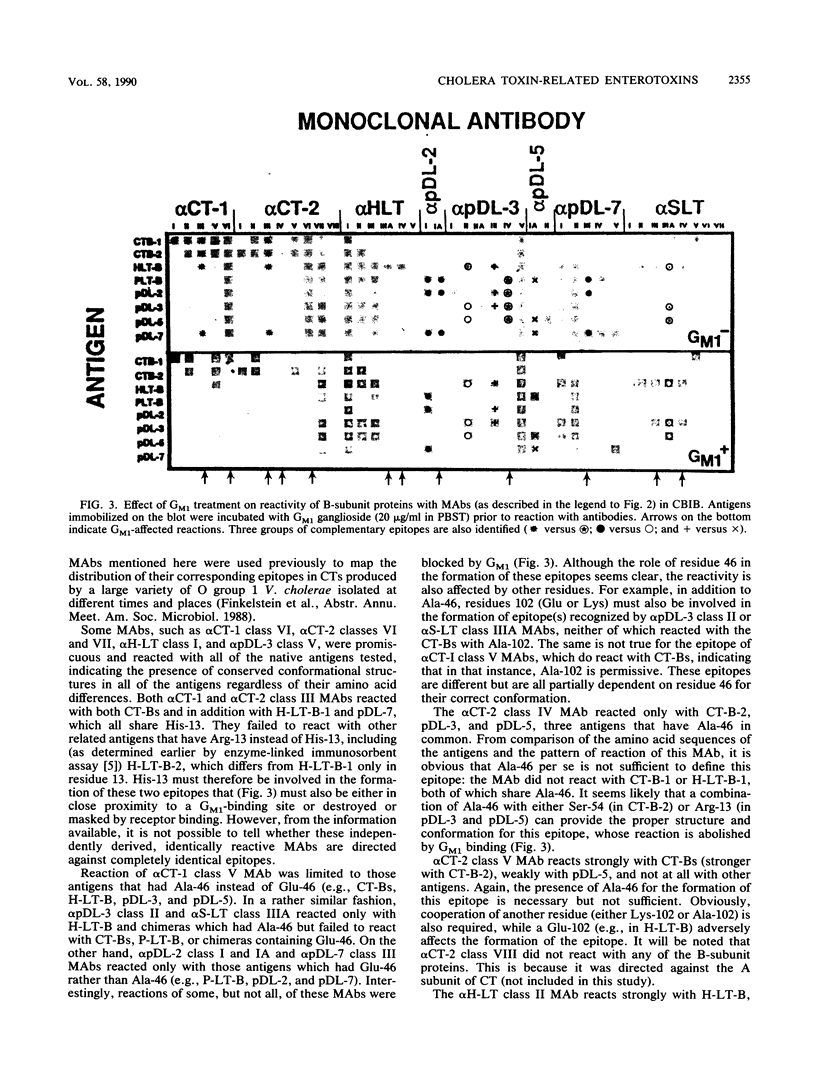
Checkerboard immunoblotting, a versatile new technique for examining multiple antigen and antibody interactions simultaneously, was applied in studies of epitopes in the cholera enterotoxin (CT)-related heat-labile enterotoxin (LT) family. The purified antigens used included the following: the B-subunit proteins from two CTs (CT-B-1 and CT-B-2), from classical and El Tor biotype strains of Vibrio cholerae, respectively; human LT-B-1 (H-LT-B-1) and porcine LT-B (P-LT-B) derived from LTs produced by Escherichia coli strains of human (H) and porcine (P) origins, respectively; and genetically engineered chimeric P-LT-Bs with amino acid substitutions from H-LT-B-1. The antigens were used in native, partially denatured, and CNBr-fragmented forms. The antisera included a variety of mouse monoclonal antibodies against these proteins as well as polyclonal hyperimmune sera and sera from adult American volunteer vaccinees or convalescents from induced cholera. Rabbit antisera against synthetic peptides of the CT-B-1 subunit were also used. In some instances, the effect of GM1 ganglioside on antibody binding was evaluated. The reactivity of the monoclonal antibodies was directed primarily against conformational epitopes: some were specific for homologous antigen; some were promiscuously reactive; and some recognized particular related proteins. Individual amino acids (most notably amino acid 46) exerted a dominant effect on epitope formation--in some instances, in a complementary fashion. Epitope expression was also affected by distant amino acid residues (polar effects). Some reactions were blocked by GM1 treatment of the immobilized antigen, indicating that the epitope was involved in or affected by GM1 binding. Polyclonal antibody responses varied within and among animal species. Human serum antitoxic responses were higher in convalescents from induced cholera than in recipients of a genetically engineered live vaccine, and the convalescent sera (from El Tor biotype cholera patients) generally preferred CT-B-2 to CT-B-1. The results demonstrate the potential significance of the differences among these immunologically related enterotoxins and may help provide direction to further vaccine development.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clemens J. D., Harris J. R., Sack D. A., Chakraborty J., Ahmed F., Stanton B. F., Khan M. U., Kay B. A., Huda N., Khan M. R. Field trial of oral cholera vaccines in Bangladesh: results of one year of follow-up. J Infect Dis. 1988 Jul;158(1):60–69. doi: 10.1093/infdis/158.1.60. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., Burks M. F., Zupan A., Dallas W. S., Jacob C. O., Ludwig D. S. Epitopes of the cholera family of enterotoxins. Rev Infect Dis. 1987 May-Jun;9(3):544–561. doi: 10.1093/clinids/9.3.544. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., LoSpalluto J. J. Pathogenesis of experimental cholera. Preparation and isolation of choleragen and choleragenoid. J Exp Med. 1969 Jul 1;130(1):185–202. doi: 10.1084/jem.130.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A. Monospecific equine antiserum against cholera exo-enterotoxin. Infect Immun. 1970 Dec;2(6):691–697. doi: 10.1128/iai.2.6.691-697.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Vasil M. L., Holmes R. K. Studies on toxinogenesis in Vibrio cholerae. I. Isolation of mutants with altered toxinogenicity. J Infect Dis. 1974 Feb;129(2):117–123. doi: 10.1093/infdis/129.2.117. [DOI] [PubMed] [Google Scholar]
- Jacob C. O., Arnon R., Finkelstein R. A. Immunity to heat-labile enterotoxins of porcine and human Escherichia coli strains achieved with synthetic cholera toxin peptides. Infect Immun. 1986 May;52(2):562–567. doi: 10.1128/iai.52.2.562-567.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob C. O., Sela M., Arnon R. Antibodies against synthetic peptides of the B subunit of cholera toxin: crossreaction and neutralization of the toxin. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7611–7615. doi: 10.1073/pnas.80.24.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazemi M., Finkelstein R. A. Checkerboard immunoblotting (CBIB): an efficient, rapid, and sensitive method of assaying multiple antigen/antibody cross-reactivities. J Immunol Methods. 1990 Mar 27;128(1):143–146. doi: 10.1016/0022-1759(90)90473-9. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Black R. E., Clements M. L. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev. 1983 Dec;47(4):510–550. doi: 10.1128/mr.47.4.510-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Herrington D., Ketley J., Losonsky G., Tacket C. O., Tall B., Cryz S. Safety, immunogenicity, and efficacy of recombinant live oral cholera vaccines, CVD 103 and CVD 103-HgR. Lancet. 1988 Aug 27;2(8609):467–470. doi: 10.1016/s0140-6736(88)90120-1. [DOI] [PubMed] [Google Scholar]
- Ludwig D. S., Finkelstein R. A., Karu A. E., Dallas W. S., Ashby E. R., Schoolnik G. K. Anti-idiotypic antibodies as probes of protein active sites: application to cholera toxin subunit B. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3673–3677. doi: 10.1073/pnas.84.11.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchlewicz B. A., Finkelstein R. A. Immunological differences among the cholera/coli family of enterotoxins. Diagn Microbiol Infect Dis. 1983 Jun;1(2):129–138. doi: 10.1016/0732-8893(83)90042-1. [DOI] [PubMed] [Google Scholar]
- Migasena S., Pitisuttitham P., Prayurahong B., Suntharasamai P., Supanaranond W., Desakorn V., Vongsthongsri U., Tall B., Ketley J., Losonsky G. Preliminary assessment of the safety and immunogenicity of live oral cholera vaccine strain CVD 103-HgR in healthy Thai adults. Infect Immun. 1989 Nov;57(11):3261–3264. doi: 10.1128/iai.57.11.3261-3264.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E. T., Clements J. D., Finkelstein R. A. Vibrio cholerae adherence and colonization in experimental cholera: electron microscopic studies. Infect Immun. 1976 Aug;14(2):527–547. doi: 10.1128/iai.14.2.527-547.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takao T., Watanabe H., Shimonishi Y. Facile identification of protein sequences by mass spectrometry. B subunit of Vibrio cholerae classical biotype Inaba 569B toxin. Eur J Biochem. 1985 Feb 1;146(3):503–508. doi: 10.1111/j.1432-1033.1985.tb08680.x. [DOI] [PubMed] [Google Scholar]
- Woodward W. E., Gilman R. H., Hornick R. B., Libonati J. P., Cash R. A. Efficacy of a live oral cholera vaccine in human volunteers. Dev Biol Stand. 1976;33:108–112. [PubMed] [Google Scholar]