Abstract
We have investigated whether exposure to Gram-negative bacterial endotoxin in early neonatal life can alter neuroendocrine and immune regulation in adult animals. Exposure of neonatal rats to a low dose of endotoxin resulted in long-term changes in hypothalamic–pituitary–adrenal (HPA) axis activity, with elevated mean plasma corticosterone concentrations that resulted from increased corticosterone pulse frequency and pulse amplitude. In addition to this marked effect on the development of the HPA axis, neonatal endotoxin exposure had long-lasting effects on immune regulation, including increased sensitivity of lymphocytes to stress-induced suppression of proliferation and a remarkable protection from adjuvant-induced arthritis. These findings demonstrate a potent and long-term effect of neonatal exposure to inflammatory stimuli that can program major changes in the development of both neuroendocrine and immunological regulatory mechanisms.
Developmental plasticity in physiological systems is an important mechanism by which organisms can adapt their physiological responses to better meet environmental demands. Although such alterations may prove beneficial for immediate survival, they may also result in a permanent alteration in physiological responses to environmental challenges and alter predisposition to pathology later in life (1–3).
The hypothalamic–pituitary–adrenal (HPA) axis is a neuroendocrine system that is subject to programming by early life events (4–7). In addition to its purely endocrine role, the HPA axis has bidirectional communication with the immune system, and during development signals from one system impinge on the functional development of the other (8–10). Exposure to pathogens early in life is a common occurrence and, because of the fragile immunological status of the neonate, represents a particularly salient challenge requiring not only immune, but also endocrine, responses for survival. Endotoxin or Gram-negative lipopolysaccharide (LPS) elicits both acute-phase and inflammatory responses that are suppressed by glucocorticoids and may be lethal in the absence of an adequate steroid response (11, 12). We previously observed that animals exposed to endotoxin (ENDO) during the first week of life are more stress responsive as adults, exhibiting increased adrenocorticotropic hormone (ACTH) and corticosterone responses to restraint stress compared with saline-treated controls (SALINE) (13). ENDO animals also have decreased glucocorticoid receptor binding in the hypothalamus, frontal cortex, and hippocampus in adult life. This decrease in glucocorticoid receptor binding is thought to contribute to decreased steroid negative-feedback efficacy when terminating corticosterone stress responses, and it has been observed that dexamethasone is less effective in suppressing stress responses in ENDO animals (13). On the basis of these data, it was suggested that ENDO animals might be exposed to greater glucocorticoid levels throughout their lifespan and that this may alter predisposition to pathologies that have a significant HPA regulatory component, such as metabolic and inflammatory disease states.
Under some conditions rat strains with relatively low levels of basal HPA activity and poor HPA responses to stressful stimuli are susceptible to inflammatory disease, and this susceptibility can be reversed by administration of exogenous glucocorticoids (14–16). Conversely, rats exhibiting greater stress responsivity and HPA basal activity are generally not as susceptible to inflammation, unless they are subjected to adrenalectomy or administered glucocorticoid antagonists (17). Early life environments may contribute further to this variation, and it has been shown that maternally deprived or neonatally handled animals, which have altered stress responsiveness as adults, are differentially susceptible to developing experimental allergic encephalomyelitis (18, 19). While predisposition to inflammatory disease is multifaceted, it has been suggested that immune tolerance is critical for determining disease predisposition, whereas HPA activity determines disease severity (20). What is not clear from the existing literature is whether alterations in the long-term predisposition to inflammation associated with maternal deprivation or other early-life environmental factors are a consequence of increased HPA activity in response to proinflammatory signals, or whether neuroendocrine–immune interactions are altered. Therefore, we examined whether animals exposed to neonatal handling (HANDLED), a paradigm shown to decrease HPA stress responses later in life (4–6), or neonatal ENDO treatment, which we show to increase HPA stress responses (13), differed in their susceptibility to chronic inflammation associated with adjuvant-induced arthritis (AA). In the present study we have, therefore, determined whether ENDO animals show elevated glucocorticoid levels under both basal and mild stress conditions, and whether this neonatal programming alters long-term susceptibility to inflammation.
Materials and Methods
Animals.
Sprague–Dawley female rats were obtained from Harlan UK at 200–250 g and maintained under standard animal housing conditions, including a 14-h light (lights on 0500), 10-h dark illumination cycle. All animals were bred in house, and litters were culled to 10 pups on the day of birth (day 0). All litters were weaned at 20–23 days of age and housed 4 or 5 animals per cage until 10–14 weeks of age, when the long-term effects of early-life events were assessed. Care and use of the rats were in accordance with protocols approved by the Home Office (Animals Scientific Procedures, Act 1986 U.K.).
Procedures.
Early-life manipulations.
Neonatal handling procedures involved removing the dam from the nest and placing her in a holding cage and then removing the pups and placing them together in another small cage. After 15–20 min the pups were returned to the nest in the home cage, and then the dam was returned. This handling procedure was performed daily from days 1 to 21 of life until the animals were weaned.
Neonatal endotoxin exposure involved injecting rat pups i.p. with either 0.05 mg/kg Salmonella enteritidis endotoxin (L-6761, lot 55F4013, Sigma) in sterile saline with an injection volume not exceeding 0.1 ml or an equivalent volume of sterile saline vehicle on days 3 and 5 postpartum. Maternal behavior was videotaped for 8 h after injection. Litters were then left undisturbed until weaning (21–23 days postpartum). At least five litters are represented in each experimental condition, and at least 8 animals are used for each experimental group.
Basal corticosterone determination.
Basal secretion patterns of corticosterone in adult ENDO and SALINE rats were assessed over 24 h (21). Briefly, this procedure involved surgically implanting jugular cannulae, and sampling was performed 5 days postoperatively. Small blood samples (10–20 μl) were taken every 10 min over 24 h starting at 0800 (lights on 0500) with an automated sampling system. The following morning stress responses were determined by exposing the animals to a mild noise stress (100 dB, 10 min white noise), and both endocrine and behavioral responses were recorded. Behaviors measured included active exploration and rearing in the home cage for 10 min before, during, and after noise stress exposure. At 1100 each animal was injected with a bolus of LPS (i.v., 25 μg, Escherichia coli O26:B6 from Sigma), and sampling was continued for a further 2 h.
Pulsar analysis.
The patterns of basal corticosterone secretion were analyzed for 6 h into each of the light and dark cycles and over the 24 h of blood sampling. The number of corticosterone pulses and mean pulse amplitudes were determined by using pulsar computer software as previously described (21, 22).
Corticosterone radioimmunoassay.
Total plasma corticosterone concentrations were measured directly in heparinized plasma by using a citrate buffer at pH 3.0 to denature the binding globulin (1 μl of plasma fraction diluted to 100 μl with buffer), antiserum kindly supplied by G. Makara (Institute of Experimental Medicine, Budapest) and 125I-labeled corticosterone (ICN Biomedicals, Irvine, CA) with a specific activity of 2–3 mCi/μg (1 mCi = 37 MBq).
AA.
When the animals were 10–12 weeks of age they were given an intradermal injection of a suspension of ground heat-killed Mycobacterium butyricum in paraffin oil (0.1 ml, 10 mg/ml) into the tail base to induce mycobacterial arthritis, or they were injected with paraffin oil vehicle. Severity of the arthritis was determined in all animals by measuring paw volumes 14 days after arthritis induction. The paw volume of each hind paw from all animals was measured by plethysmometry (Ugo Basile, Varese, Italy). Hind paws were submerged to the level of the lateral malleolus, and the mean of three recordings taken for each paw was recorded as the paw volume (23).
In situ hybridization.
Coronal sections (12 μm) were cut through the area of the paraventricular nucleus (PVN), mounted on gelatin-coated slides, and stored at −80°C. In situ hybridization was performed as previously described by Ma and Lightman (24). For determination of corticotropin-releasing hormone (CRH) mRNA, the probe used was a 48-mer oligonucleotide complementary to part of the exonic portion the CRH mRNA coding sequence, and for arginine-vasopressin (AVP) determinations another 48-mer oligonucleotide probe, complementary to the exonic portion of the coding sequence for AVP mRNA, was used. Both probes were end labeled with α-[35S]thio-dATP by using terminal deoxynucleotidyltransferase (Boehringer Mannheim). The specific activity of the probe was determined as 1.0 × 105 counts per slide. All sections were hybridized in the same hybridization reaction. The autoradiographic images of the probe bound to PVN AVP or CRH mRNA, together with 14C-labeled standards (to compensate for the nonlinear response of the film to radioactivity), were measured by using a computer-assisted image analysis system (Image 1–22, developed by W. Rasband, National Institutes of Health, Bethesda, MD) and run on a Macintosh 11Ci. The results are presented as integrated optical densities and expressed as arbitrary units.
Lymphocyte proliferation.
Spleens were dissected under aseptic conditions and placed in supplemented RPMI medium 1640 (GIBCO) containing 10 mM Hepes, 2 mM glutamine, and 50 μg of gentamicin per ml. Spleens were then ground between frosted ends of microscope slides to obtain single-cell suspensions in supplemented medium containing 10% FCS. Cells were counted by using a hemocytometer and diluted to a concentration of 5 × 106 cells per ml. Cells were plated in triplicate at a volume of 100 μl with 100 μl of medium supplemented with LPS (E. coli O26:B6) in triplicate across three final concentrations (0, 5.0, and 10 μg/ml). The cells were left to incubate for 48 h at 37°C under a 5% CO2/95% air atmosphere. At 44 h of incubation, plates were pulsed with 1 μCi of [3H]thymidine in 50 μl of medium per well and allowed to incubate for 4 more h. Cells were harvested onto glass filters by using a 96-well Tomtec cell harvester, and radioactivity on the filters was quantified on a Microbeta counter (Wallac, Milton Keynes, U.K.). Data are expressed as means of corrected cpm for [3H]thymidine incorporation.
Statistical analysis.
All values are expressed as mean ± SEM. All data were analyzed by using analysis of variance (ANOVA) (P < 0.05) and, when deemed appropriate, patterns of mean differences were determined by using Newman–Keuls multiple comparisons or planned contrasts (α = 0.05).
Results and Discussion
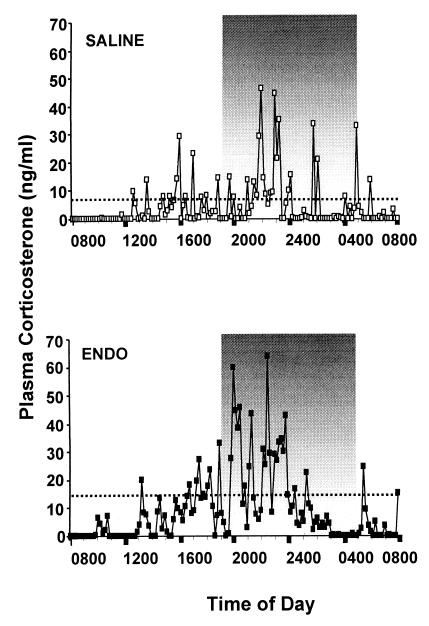
Both ENDO and SALINE animals displayed a pulsatile pattern of corticosterone secretion with a circadian variation in pulse amplitude (see Fig. 1 and Table 1). However, compared with SALINE animals, basal levels of corticosterone were increased in both the light and dark phases of the diurnal cycle in ENDO animals, because of both an increased number of pulses and an increased pulse amplitude. These data demonstrate that neonatal programming through exposure to inflammatory stimuli can exert long-term effects on the mechanisms generating basal pulsatile HPA activity in adult animals and that this programming does not simply increase tonic activity.
Figure 1.
Examples of plasma corticosterone levels sampled every 10 min for 24 h in adult rats that had been neonatally injected with either sterile saline (Upper) or endotoxin (Lower). The diurnal pattern of steroid secretion is observable, with peak levels during the dark phase of the light cycle (1900–0500) and a nadir early in the light phase. Dotted lines indicate the mean corticosterone levels over 24 h, and it was observed that on average ENDO animals exhibited greater overall levels of corticosterone. Analysis of the ultradian secretion patterns revealed that ENDO animals exhibited more pulses of corticosterone secretion and greater pulse amplitudes compared with SALINE animals (see Table 1).
Table 1.
pulsar analysis of basal corticosterone
| Treatment | Plasma corticosterone, ng/ml | No. of pulses | Pulse, amplitude, ng/ml |
|---|---|---|---|
| 24-h cycle | |||
| ENDO | 24.5 ± 4.2* | 15.1 ± 1.7** | 54.4 ± 6.2** |
| SALINE | 7.5 ± 1.5 | 6.8 ± 1.0 | 32.9 ± 3.1 |
| First 6 h of light phase (0500–1100) | |||
| ENDO | 17.9 ± 5.7* | 3.3 ± 0.4** | 41.0 ± 4.4** |
| SALINE | 5.7 ± 2.3 | 0.8 ± 0.3 | 15.3 ± 4.4 |
| First 6 h of dark phase (1900–0100) | |||
| ENDO | 35.4 ± 5.0* | 5.8 ± 0.4** | 64.1 ± 6.1** |
| SALINE | 11.3 ± 2.0 | 2.0 ± 0.3 | 29.6 ± 5.6 |
Basal corticosterone was analyzed over 24 h and during the first 6 h of the light phase (nadir, 0500–1100) and the first 6 h of the dark phase (acrophase, 1900–0100) for ENDOS (n = 14) and SALINE (n = 12) animals. Data are expressed as means ± SEM for corticosterone levels, total number of pulses, and pulse amplitude of data points contributing to pulses. ENDO animals exhibit greater mean levels of corticosterone, mean number of pulses, and mean pulse amplitude over 24 h and in each of the light and dark phases of the light cycle. *, P < 0.01; **, P < 0.001.
In view of previous data indicating that the dam's interactions with her litter can program HPA development (7), we assessed the amount of time the dam spent off the nest and the frequency of leaving the nest over the 8 h after endotoxin or saline injection. We did not observe any differences (P > 0.05) in maternal behavior between the two treatment conditions, with dams of ENDO and SALINE litters showing the same number of periods away from the nest (ENDO 14.77 ± 1.9 vs. SALINE 12.00 ± 1.7) and time spent off the nest (ENDO 56.94 ± 11.8 min vs. SALINE 57.19 ± 13.8 min), suggesting that the programming of the HPA axis after endotoxin treatment is unlikely to be due to reduced maternal contact.
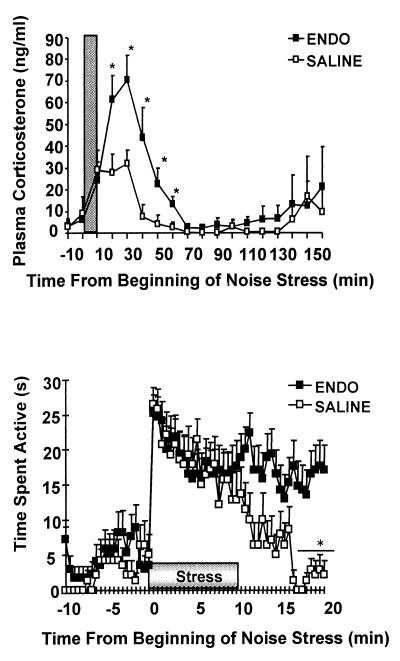
In addition to increased basal levels of circulating steroid, we investigated whether ENDO animals showed altered behavioral and corticosterone responses to noise stress. Noise stress is a mild psychological challenge that can be applied remotely without removing animals from their home cage and is sufficiently sensitive to be able to detect differences between hyper- and hypocorticosteronemic states, such as in the Lewis and Fischer strains of rat (14). We observed that plasma corticosterone levels were increased in both groups of animals after noise exposure, but to a greater extent in ENDO animals (see Fig. 2). Behavioral observations confirmed that ENDO animals and SALINE controls did not differ in their activity levels before stress exposure, and that both groups increased their activity with the onset of stress. However, whereas activity levels returned to baseline within 10 min after stress exposure in SALINE controls, ENDO animals maintained elevated activity levels beyond 10 min after stress.
Figure 2.
(Upper) Mean (+SEM) plasma corticosterone levels in response to 10 min of noise stress. ENDO animals exhibit greater corticosterone stress responses and take significantly longer to return to basal levels compared with SALINE controls. *, P < 0.05 vs. SALINE. (Lower) The animals' behavioral responses to noise stress while in their home cages were also observed. Mean (+SEM) activity levels increased in both ENDO and SALINE animals in response to the noise stress. SALINE animals returned to baseline levels of activity within 10 min after stress exposure, whereas ENDO animals continued to exhibit increased activity. *, P < 0.05 vs. ENDO.
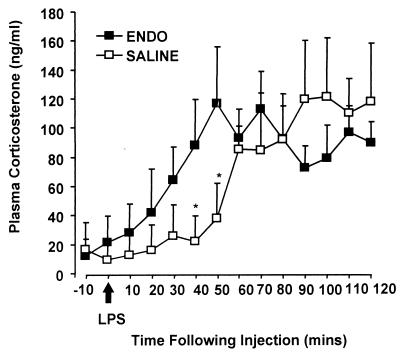
ENDO and SALINE animals were also given an acute inflammatory challenge with an i.v. bolus injection of LPS (E. coli, Sigma lot 63H4010). Both groups of animals responded to LPS with a marked increase in plasma corticosterone, although the onset of the response was more rapid in the ENDO animals compared with the SALINE animals (see Fig. 3). These data show that exposure to an inflammatory stimulus during the first week of life increases both endocrine and behavioral stress reactivity in adult animals. The long-term effects of neonatal inflammatory challenge on the central regulation of the HPA axis, therefore, expose an animal to greater basal and stress-induced steroid signals throughout its lifespan.
Figure 3.
Mean (+SEM) plasma corticosterone levels after an i.v. bolus injection of 25 μg of LPS (E. coli O26:B6, Sigma) in adult ENDO and SALINE rats. Peak levels of corticosterone in response to the stimulus did not differ between the two groups of rats; however, ENDO animals reached peak levels 20–30 min before the SALINE animals. *, P < 0.05 vs. ENDO. These data indicate possible differences in the dynamics of the inflammatory response to acute LPS or differences in the ability of the HPA axis to respond to acute inflammatory signals.
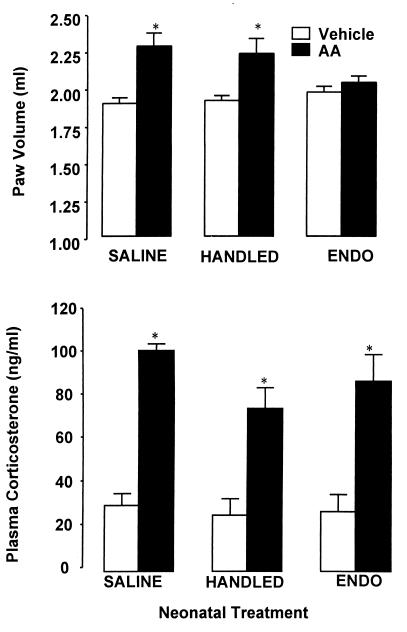
Although HPA responses to acute inflammatory challenge were not significantly greater, they did develop more rapidly, indicating that the dynamics of the acute-phase response and/or subsequent endocrine regulation are altered in ENDO animals. The compelling question, therefore, was whether these long-term alterations in HPA function and regulation have any implications with respect to predisposition to inflammatory disease. Both HANDLED and SALINE animals had increased paw volumes in response to adjuvant (P < 0.05), whereas the ENDO animals did not exhibit significant signs of arthritis and were indistinguishable from vehicle-treated controls (see Fig. 4). In contrast, however, resting levels of corticosterone were increased in all animals with AA (P < 0.05) and did not appear to be related to paw inflammation.
Figure 4.
(Upper) Mean paw volume (+SEM) 14 days after intradermal injection of Mycobacterium suspended in oil (AA) or injection of oil vehicle in adult rats that had been neonatally handled (HANDLED) or neonatally injected with endotoxin (ENDO) or sterile saline (SALINE). AA was indicated by paw inflammation in AA SALINE and HANDLED animals; however, inflammation was not observed in the ENDO animals. *, P < 0.05 vs. Vehicle controls. (Lower) Mean plasma corticosterone levels (+SEM) on day 14 after AA or vehicle treatment illustrates the HPA response to the chronic inflammation, and this was observed in all animals into which Mycobacterium was injected, including ENDO animals, where no inflammation was observed. *, P < 0.01 vs. Vehicle controls.
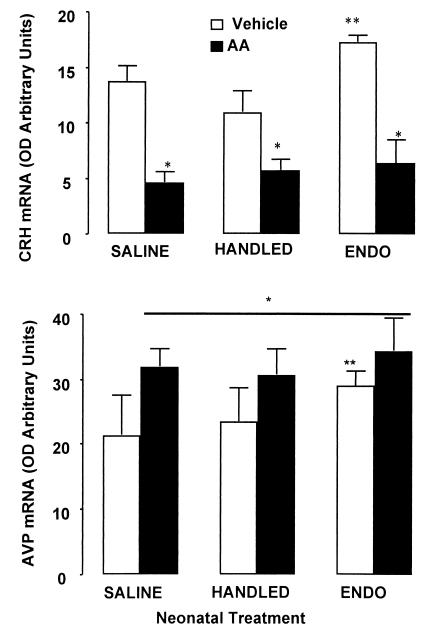
CRH and AVP produced by the hypothalamic PVN drive the HPA axis by stimulating the pituitary release of adrenocorticotropic hormone (ACTH) and ultimately the adrenocortical release of corticosterone. HPA drive by CRH and AVP has also been shown to be altered by early-life events wherein both mRNA and peptide levels within the PVN and median eminence, respectively, are decreased by neonatal handling, yet increased by endotoxin treatment (Fig. 5) (4, 13). Although CRH and AVP mRNA generally both increase in response to acute stress, PVN CRH mRNA levels decrease and AVP mRNA levels increase in response to chronic inflammation associated with AA (23–26). In our current studies, we observed similar increments in PVN AVP mRNA and decrements in CRH mRNA in SALINE, ENDO, and HANDLED animals (Fig. 5). These data and the lack of any significant difference in the corticosterone responses for these three groups of animals were surprising in light of the differences in the development of arthritis in the ENDO animals. Thus, although the neuroendocrine responses to chronic inflammatory challenge were similar in the animals that had undergone different neonatal treatments, the pathological outcomes differed markedly.
Figure 5.
Mean (+SEM) CRH mRNA (Upper) and AVP mRNA (Lower) in the PVN of adult SALINE, ENDO, and HANDLED rats 14 days after AA or vehicle injection. mRNA was measured as optical density arbitrary units relative to standard values from autoradiograph images. In situ hybridizations were performed with oligonucleotide probes corresponding to rat CRH or AVP mRNA coding sequences. CRH mRNA was reduced in all neonatal treatment conditions in animals injected with adjuvant; *, P < 0.05. Control levels of CRH mRNA were greater in the ENDO animals relative to HANDLED animals; **, P < 0.05. An increase in AVP mRNA expression was observed in all AA experimental groups by day 14 after injection compared with Vehicle controls; *, P < 0.05. Overall, AVP mRNA levels were greater in ENDO animals compared with the other neonatal treatment conditions; **, P < 0.05.
Our data indicate that HPA activation is observed in ENDO animals after AA, but it is possible that the immunoregulatory impact of HPA factors might differ in this population of animals. Alternatively, it may suggest that effects of neonatal exposure to endotoxin on endocrine function have nothing to do with altered HPA responsivity in these animals. Indeed, the different experimental groups displayed equivalent endocrine responses, but differed in paw inflammation. Exposure to an acute inflammatory stimulus during development may exert effects directly on inflammatory processes that alter immune responses later in life. The idea that immune regulation is altered in ENDO animals is further supported by observations that in vitro lymphocyte proliferative responses to LPS stimulation are differentially suppressed after acute exposure to restraint stress (P < 0.05). Proliferative responses of splenocytes collected from SALINE animals were relatively insensitive to modulation after acute stress exposure, whereas there was a marked suppression of proliferation of splenocytes collected from ENDO animals immediately after restraint stress (see Fig. 6). Although the factors that mediate stress-induced suppression of lymphocyte proliferation within the spleen are not clear, these data indicate that splenocytes from ENDO animals are more sensitive to stress than are those from SALINE controls. Thus, although nonstressed ENDO animals can respond to inflammatory stimuli to an extent similar to that of control animals, they may differ with respect to stress-related immunoregulation. Indeed, recent data suggest that the cellular composition of immune organs is altered after acute stress exposure, and it may be the case that spleens in ENDO animals are more susceptible to stress-related shifts in cell populations and subsequent stress effects on proliferative responses (27).
Figure 6.
Mean (+SEM) splenocyte proliferation in response to various concentrations of LPS (E. coli O26:B6) by cells collected from adult ENDO and SALINE rats immediately after exposure to 30 min of restraint stress or in nonstressed controls. Lymphocyte proliferation was determined by measuring incorporation of [3H]thymidine in 5 × 105 cells incubated (37°C, 5% CO2) with LPS for 44 h and a further incubation for 4 h after the addition of 1 μCi of [3H]thymidine per well. Cells were harvested on a 96-well harvester and cpm was determined. Restraint stress significantly reduced splenocyte proliferation in ENDO animals; however, this effect was not observed in stressed SALINE animals (*, P < 0.05). These data suggest that ENDO animals can mount a proliferative response to LPS equivalent to controls, but their lymphocytes are more sensitive to “stress factors.”
We have demonstrated that exposure to endotoxin during the first week of life not only exerts long-term effects on endocrine and central nervous system development but also dramatically alters predisposition to inflammatory disease. It has been demonstrated previously, however, that exposure of adult animals to LPS can alter hypothalamic peptide regulation and subsequent HPA responsiveness that can last a number of weeks (28). Little is known about how acute inflammatory events exert their effects and can alter predisposition to chronic inflammation over time. We suggest that one possible mechanism could be long-term functional alterations of immune inflammatory responses. Alterations in inflammatory responsiveness could merely be associated with altered HPA development and not a consequence of it, or alternatively they could reflect differences in efficacy of endocrine suppression. On the basis of our data, it does appear, however, that activation of endocrine and immune systems during neonatal development can program or “reset” functional development of both the endocrine and immune systems. In this respect it is noteworthy that exposure to steroids during immunization schedules in early life can alter the development of immune tolerance, and that animals raised in pathogen-free environments have increased susceptibility to inflammatory disease (20, 29–31). The environment in which a mammal develops is often the environment in which it must survive throughout life, and developmental plasticity must surely be of adaptive advantage. We suggest that “immune environments” during development not only can alter inflammatory and neuroendocrine responses throughout life but also may alter predisposition to stress-related pathologies associated with HPA activation.
Acknowledgments
We express our appreciation to Mrs. Susan Wood, Mrs. Yvonne Kershaw, and Mr. Derek Renshaw for their excellent technical assistance. This work was supported by grants from the Wellcome Trust (N.S., S.L.L., and C.D.I.) and the United Bristol Health Care National Health Service Trust (N.S. and S.L.L.).
Abbreviations
- AA
adjuvant-induced arthritis
- AVP
arginine-vasopressin
- CRH
corticotropin-releasing hormone
- ENDO
endotoxin-treated
- HPA
hypothalamic–pituitary–adrenal
- LPS
lipopolysaccharide
- PVN
paraventricular nucleus
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.090571897.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.090571897
References
- 1.Arborelius L, Owens M J, Plotsky P M, Nemeroff C B. J Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- 2.McEwen B S, Stellar E. Arch Intern Med. 1993;153:2093–2101. [PubMed] [Google Scholar]
- 3.Munck A, Naray-Fejes-Toth A. Mol Cell Endocrinol. 1992;90:C1–C4. doi: 10.1016/0303-7207(92)90091-j. [DOI] [PubMed] [Google Scholar]
- 4.Meaney M J, Bhatnagar S, Diorio J, Larocque S, Francis D, O'Donnell D, Shanks N, Sharma S, Smythe J, Viau V. Cell Mol Neurobiol. 1993;13:321–347. doi: 10.1007/BF00711576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meaney M J, Tannenbaum B, Francis D, Bhatnagar S, Shanks N, Viau V, O'Donnell D, Plotsky P M. Sem Neurosci. 1994;6:247–259. [Google Scholar]
- 6.Levine S. Science. 1967;156:258–260. doi: 10.1126/science.156.3772.258. [DOI] [PubMed] [Google Scholar]
- 7.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky P, Meaney M J. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 8.O'Grady M P, Hall N R S. In: Psychoneuroimmunology. 2nd Ed. Ader R, Felten D L, Cohen N, editors. San Diego: Academic; 1991. pp. 561–572. [Google Scholar]
- 9.Pierpaoli W, Kopp H G, Müller J, Keller M. Cell Immunol. 1977;29:16–27. doi: 10.1016/0008-8749(77)90271-4. [DOI] [PubMed] [Google Scholar]
- 10.Stein M, Miller A H. Adv Exp Med Biol. 1993;335:1–5. doi: 10.1007/978-1-4615-2980-4_1. [DOI] [PubMed] [Google Scholar]
- 11.Ramchandra R N, Sehon A, Berczi I. Brain Behav Immun. 1992;6:157–169. doi: 10.1016/0889-1591(92)90015-g. [DOI] [PubMed] [Google Scholar]
- 12.Witek-Janusek L. Am J Physiol. 1988;18:E525–E530. doi: 10.1152/ajpendo.1988.255.4.E525. [DOI] [PubMed] [Google Scholar]
- 13.Shanks N, Larocque S, Meaney M J. J Neurosci. 1995;15:376–384. doi: 10.1523/JNEUROSCI.15-01-00376.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Windle R J, Wood S A, Lightman S L, Ingram C D. Endocrinology. 1998;139:4044–4052. doi: 10.1210/endo.139.10.6238. [DOI] [PubMed] [Google Scholar]
- 15.Mason D. Immunol Today. 1991;12:57–60. doi: 10.1016/0167-5699(91)90158-P. [DOI] [PubMed] [Google Scholar]
- 16.Sternberg E M, Hill J M, Chrousoso G P, Kamilaris T, Listwak S J, Gold P W, Wilder R L. Proc Natl Acad Sci USA. 1989;86:2374–2378. doi: 10.1073/pnas.86.7.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sternberg E M, Wilder R L, Gold P W, Chrousos G P. Ann NY Acad Sci. 1990;594:289–292. doi: 10.1111/j.1749-6632.1990.tb40488.x. [DOI] [PubMed] [Google Scholar]
- 18.Laban O, Dimitrijevic M, von Hoersten S, Markovic B M, Jankovic B D. Brain Res. 1995;676:133–140. doi: 10.1016/0006-8993(95)00106-z. [DOI] [PubMed] [Google Scholar]
- 19.Laban O, Markovic B M, Dimitrijevic M, Jankovic B D. Brain Behav Immun. 1995;9:9–19. doi: 10.1006/brbi.1995.1002. [DOI] [PubMed] [Google Scholar]
- 20.van de Lagerijt A G, van Lent P L, Hermus A R, Sweep C G, Cools A R, van den Berg W B. Clin Exp Immunol. 1994;97:33–38. doi: 10.1111/j.1365-2249.1994.tb06575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Windle R J, Wood S, Shanks N, Lightman S L, Ingram C D. Endocrinology. 1998;139:443–450. doi: 10.1210/endo.139.2.5721. [DOI] [PubMed] [Google Scholar]
- 22.Merriam G R, Wachter K W. Am J Physiol. 1982;243:E310–E3118. doi: 10.1152/ajpendo.1982.243.4.E310. [DOI] [PubMed] [Google Scholar]
- 23.Chowdrey H S, Larsen P J, Harbuz M S, Jessop D S, Aguilera G, Eckland D J, Lightman S L. Br J Pharmacol. 1995;116:2417–2424. doi: 10.1111/j.1476-5381.1995.tb15089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma X M, Lightman S L. J Physiol (London) 1998;510:605–614. doi: 10.1111/j.1469-7793.1998.605bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harbuz M S, Rees R G, Eckland D, Jessop D S, Brewerton D, Lightman S L. Endocrinology. 1992;130:1394–1400. doi: 10.1210/endo.130.3.1537299. [DOI] [PubMed] [Google Scholar]
- 26.Harbuz M S, Lightman S L. J Endocrinol. 1992;134:327–339. doi: 10.1677/joe.0.1340327. [DOI] [PubMed] [Google Scholar]
- 27.Miller A, Spencer R, Hassett J, Kim C, Rhee R, Ciurea D, Dhabhar F, McEwen B, Stein M. Endocrinology. 1994;135:1934–1944. doi: 10.1210/endo.135.5.7956914. [DOI] [PubMed] [Google Scholar]
- 28.Tilders F J H, Schmidt E D. Ann NY Acad Sci. 1998;840:65–73. doi: 10.1111/j.1749-6632.1998.tb09550.x. [DOI] [PubMed] [Google Scholar]
- 29.Dahlquist G, Kallen B. Pediatr Res. 1997;42:489–491. doi: 10.1203/00006450-199710000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Madar J, Chesnovkova V M. Folia Biol. 1992;38:103–112. [PubMed] [Google Scholar]
- 31.Ohsugi T, Kurosawa T. Lab Anim Sci. 1994;44:386–388. [PubMed] [Google Scholar]