Abstract
Objective
This multi-center clinical study was designed to determine the long-term results of patients who received a one-level posterior lumbar interbody fusion with expandable cage (Tyche® cage) for degenerative spinal diseases during the same period in each hospital.
Methods
Fifty-seven patients with low back pain who had a one-level posterior lumbar interbody fusion using a newly designed expandable cage were enrolled in this study at five centers from June 2003 to December 2004 and followed up for 24 months. Pain improvement was checked with a Visual Analogue Scale (VAS) and their disability was evaluated with the Oswestry Disability Index. Radiographs were obtained before and after surgery. At the final follow-up, dynamic stability, quality of bone fusion, interveretebral disc height, and lumbar lordosis were assessed. In some cases, a lumbar computed tomography scan was also obtained.
Results
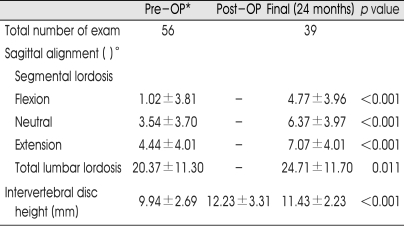
The mean VAS score of back pain was improved from 6.44 points preoperatively to 0.44 at the final visit and the score of sciatica was reduced from 4.84 to 0.26. Also, the Oswestry Disability Index was improved from 32.62 points preoperatively to 18.25 at the final visit. The fusion rate was 92.5%. Intervertebral disc height, recorded as 9.94±2.69 mm before surgery was increased to 12.23±3.31 mm at postoperative 1 month and was stabilized at 11.43±2.23 mm on final visit. The segmental angle of lordosis was changed significantly from 3.54±3.70° before surgery to 6.37±3.97° by 24 months postoperative, and total lumbar lordosis was 20.37±11.30° preoperatively and 24.71±11.70° at 24 months postoperative.
Conclusion
There have been no special complications regarding the expandable cage during the follow-up period and the results of this study demonstrates a high fusion rate and clinical success.
Keywords: Expandable cage, Degeneration, Interbody fusion, Lumbar spine
INTRODUCTION
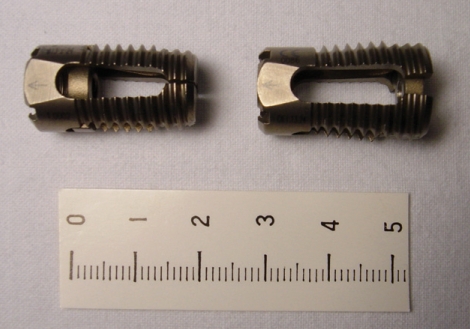
Back pain in the degenerative lumbar spine is caused by the mechanical compression of the cauda equina or abnormal micromotion due to degenerative changes in the motion segment8). Compression of the narrowing area can be treated with decompressive surgery; however, this procedure can also lead to another cause of back pain due to destruction of the anatomical structure or abnormal micromotion after surgery. The ideal treatment for replacing the degenerative segment with the new segment is realistically impossible. Thus, bone fusion is used to manage the area with the damaged segment3,5,16,23,27,29). Although bone fusion through posterolateral bone graft and pedicle screw fixation was become a common practice, complications such as pseudoarthrosis, graft bone resorption, instrument failure, and sagittal imbalance in the lumbar spine are possible1,4). The posterior lumbar interbody fusion (PLIF) technique with cages impacted with local bone has been developed to overcome these problems. The benefits of the PLIF are securing the fixation of vertebral body, maintaining the normal intervertebral space, and supporting the anterior column in charge of 80% of weight-bearing out of the vertebral column, thus providing satisfactory bone fusion while maintaining biomechanical stability20,23,27-29). However, not only the importance of the firm bone fusion after surgery has been found, but also the clinical or biomechanical importance of normal sagittal lumbar profile rearrangement has been recently emphasized2,4,11,13,15,18,21,22). New expandable cage products are being produced to maintain partial lordotic curve. Attia2) followed up 48 patients with degenerative lumbar spinal disorder for more than one year after the insertion of an expandable cage, and reported results compared with non-expandable cages. In his report, the expandable cages were effective in maintaining biomechanical stability and lumbar spinal lordosis. And, these were easily inserted in an accurate manner into the intervertebral space and resulted in firm traction, offering the benefits of normal sagittal lumbar profile. The Tyche® lumbar expandable cage (Kyungwon Medical Co Ltd., Seoul, Korea) (Fig. 1) is one of several products recently introduced for posterior lumbar interbody fusion. The present multi-center study was designed to determine the long-term results of patients who have undergone PLIF with the Tyche® lumbar expandable cage for a same period in each hospital.
Fig. 1.
The lateral view of the Tyche® lumbar expandable cage (Kyungwon Medical Co Ltd., Seoul, Korea before (left) and after (right) expansion. It consists of titanium and anterior part of the cage and can be expanded about 4 degrees with the cap inserted into the cage anteriorly.
MATERIALS AND METHODS
Subjects
Five centers were enrolled for this analysis. The investigators in each hospital evaluated their patients with identical management, follow-up, and examination forms. Once this was complete, the authors retrospectively reviewed the clinical results.
From June 2003 to December 2004, the multi-center clinical study was conducted on 57 patients who had at least 24 months follow-up data since undergoing surgery.
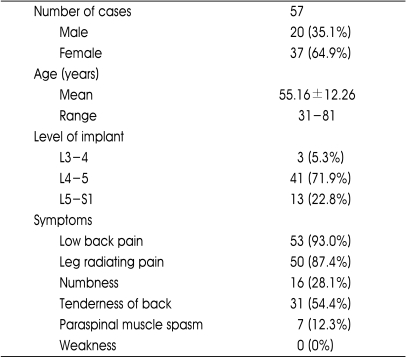
The age of patients ranged from 31 to 81 years with the mean of 55.16±12.26 years. There were 20 males (35.1%) and 37 females (64.9%). The mean hospital stay was 7.25 days. Main symptoms included low back pain in 53 cases (93.0%), radiating leg pain in 50 (87.4%), numbness in 16 (28.1%), lumbar tenderness in 31 (54.4%) but there were no weaknesses. The summary of patient demographic data is listed in Table 1.
Table 1.
The summary of demographic data of patients
Through preoperative and post operative one-on-one evaluation at 1, 6, 12 and 24 months on their hospital visit or a telephone survey, the severity of low back pain was evaluated by a Visual Analogue Scale (VAS) or Numeric Rating Scale (NRS) and the disability which interfered with carrying out everyday activities was examined by an Oswestry Disability Index (ODI) score36).
Radiologic Findings
Using the simple lumbar lateral X-ray and flexion-extension view obtained before surgery and at postoperative 1, 6, 12 and 24 months, stability was assessed. Unscheduled lumbar computed tomographies (CT) with fine slices through the fusion construct were obtained if they were required. Fusion was defined in a manner similar to other published studies7,19). Lateral flextion-extension radiographs and in some cases lumbar CT scans were checked to confirm the following findings : 1) an absence of radiolucent lines covering more than 50% of either implant, 2) translation of 3 mm or less and a range of motion of less than 5 degrees, 3) absence of halo, and 4) formation of anterior sentinel bone, or formation of the contiguous bony bridge between the upper and the lower vertebral bodies. The successful fusion was considered only if all 4 conditions were met. Using postoperative simple lumbar spine AP and lateral views, the changes in intervertebral disc height, segmental lordosis and total lumbar lordosis were recorded. The intervertebral height was calculated at the lateral X-ray by dividing the sum of the anterior, middle, and posterior intervertebral disc heights by three14). According to the method described by Cobb34), total lumbar lordosis was measured from the bottom of T12 to the bottom of L5. The degree of segmental lordosis at the site of surgery was measured from the lower endplate of the upper segment to the upper endplate of the lower segment (Fig. 2, 3).
Fig. 2.
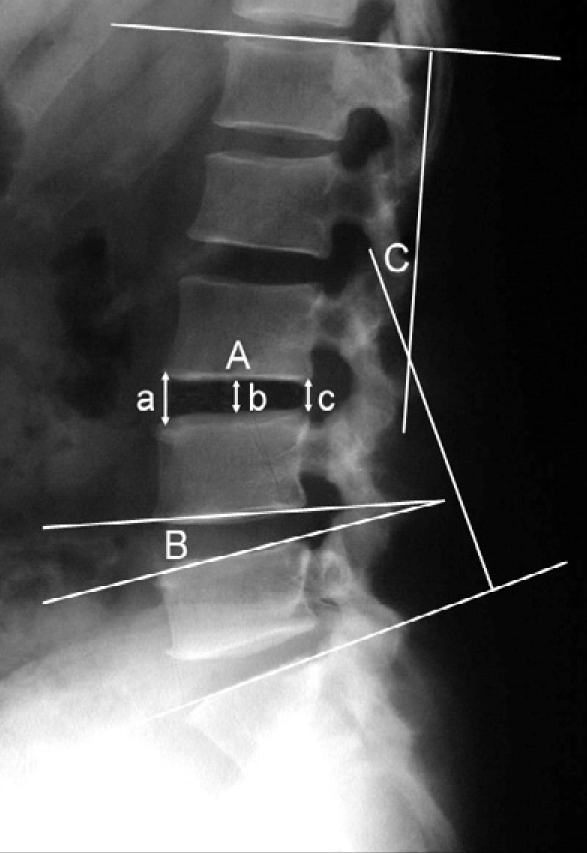
Lumbar spine X-ray, lateral view. The intervertebral height (A) is calculated by dividing the sum of anterior, middle, and posterior intervertebral disc heights by 3 (a+b+c/3). The angle between the superior and inferior endplates is the degree of segmental lordosis (B) at the site of surgery and total lordosis (C) is measured according to the Cobb method in which the angle appearing in each segment is based on the L1 superior endplate and the S1 superior endplate for lumbar lordosis.
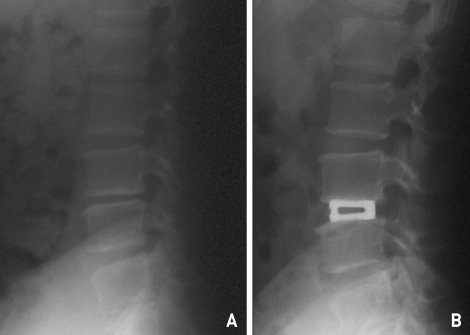
Fig. 3.
Lumbar spine X-ray, lateral view of pre-operative (A) and post operative (B). The inter-vertebral disc height is collapsed at pre-operative and rescued by expandable cage insertion to the level.
Surgical Indications and Techniques
The Tyche® lumbar expandable cage is a cylindrical shape, consisted of titanium. The front of the cage widens by about 4 degrees in the disc space to produce a normal lordotic angle. The principle is that when a cage is inserted into the disc space and the nut inside the cage is tightened, this nut moves to the front of the cage, which then widens the cage (Fig. 1). An additional benefit is that this widens the side slit of the cage, which increases the contacting surface between the graft bone and the endplate and facilitates the synostosis. There are three diameter sizes for the cage : 11, 13 and 15 mm, and the front of the cage can widen to 13, 15 and 17 mm, respectively. On the external surface of the cage there are ridges which play the role of a strong bone anchor.
Surgery was performed using this cage on the patients who mainly had low back pain aggravated by dynamic motion rather than radiating pain of the lower legs or neurogenic intermittent claudication. The operated lumbar disc showed definite low signal intensity suggestive of disc degeneration on preoperative T2 weighted magnetic resonance image (MRI) with definite decrease in the intervertebral disc height, and the low back pain did not show improvement even after a minimum of six months of conservative management.
The preparation, positioning, and initial exposure are similar to that for patients undergoing surgery for disc herniation or stenosis. Total or subtotal laminectomy and complete decompression of nerve roots are then performed. After medial facetectomy and foraminotomy are complete, the nerve roots on both sides should be freely movable to retract the dural sleeve.
Carefully retracting the nerve roots, a blade is then used to incise the annulus widely, and the soft disc material is removed with a pituitary rongeur bilaterally.
After deciding the size of the cage, a shaver is inserted to confirm the depth of cage insertion space. By rotating the shaver, removal of the remained disc can be done to help stabilize the cage's settlement.
A manual drill is inserted about 3 cm from the posterior margin of the vertebral body while being rotated the drill. After matching the size to the manual drill, the hollow cage is placed in the empty space where the annulus fibrosus was removed so that the posterior margin of the cage will be situated at 2-3 mm anterior to the posterior vertebral body. When the installed cap of the cage is inserted while rotating anteriorly, the anterior part of the cage is expanded about 4 degrees so that mechanical segmental lordosis is created. The procedure is now repeated on the other side. The cages are then filled with bone pieces harvested during laminectomy. Then, an end cap is installed to prevent the protrusion of the grafted bone fixed to the bone pieces. The incision site is sutured after confirming the root decompression.
Statistics
All statistical analysis was done using SPSS (version 8.0, SPSS Inc., Chicago, IL). The changes in preoperative and postoperative radiological findings were analyzed using the paired t-test. The Mann-Whitney test was used. Statistical significance was determined when p values were less than 0.05.
RESULTS
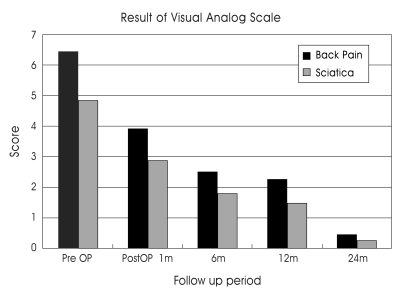
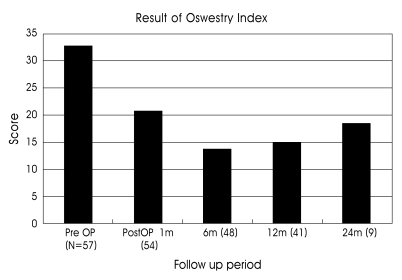
The mean score on the Visual Analogue Scale (VAS) of back pain was improved from 6.67 points during the preoperative period to 3.91 points at one month postoperative and decreased to 2.51, 2.25, and 0.44 at postoperative 6, 12, and 24 months, respectively. The VAS of sciatica was reduced from 4.84 at preoperative to 2.86 post, and it was reduced to 1.81, 1.48, and 0.26 at 6, 12, and 24 months later, respectively (Fig. 4). The ODI score was reduced from 32.6 points at the preoperative period to 20.7 points at one month postoperatively, and to 13.7, 15.1 and 18.3 at 6, 12, and 24 months later, respectively (Fig. 5).
Fig. 4.
The visual analog scale results in back pain and leg pain at post operative follow-up periods. Both the back pain and sciatica are reduced post operatively.
Fig. 5.
The results of the Oswestry Disability Index score. The 'N' means the number of patients followed up. The score was decreased to the post operative six-month period, but the day after, it was increased. This may be due to the reduced number of patients being followed up.
The angulation on flexion-extension film at the final visits was confirmed in all cases. Preoperatively, the segmental lordosis was 1.02±3.81° on flexion and 4.44±4.01° on extension. Post-operatively, it was 4.77±3.96° and 7.07±4.01°, respectively. The range of motion was less then 5 degrees. There were no pseudarthrosis or spondylolisthesis.
Plain X-rays were checked at post-operative 24 months with 39 patients. A total of 36 patients had obvious trabecular bridging on the plain x-ray. The total fusion rate was 92.3%. To confirm the fusion, post-operative CT scans were taken in 5 cases who showed the fusion state on the plan X-rays demonstrated bony material inside the cage (Fig. 6).
Fig. 6.
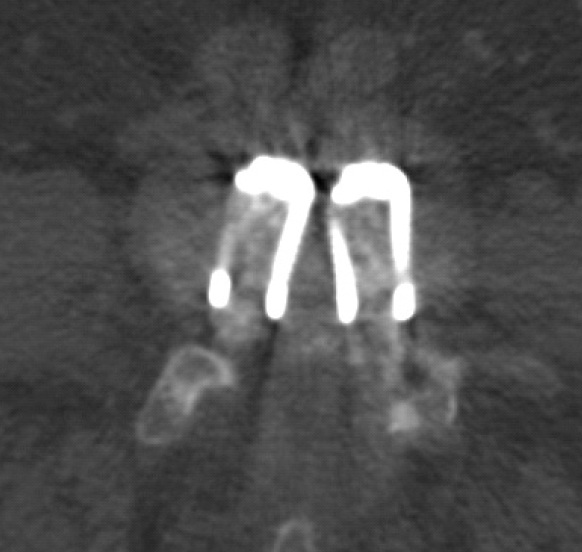
The post operative four-month follow-up computed tomography scan image of the level of cage insertion. The bony material inside the cage is seen.
The mean intervertebral disc height was 9.94±2.69 mm before surgery, and it was increased to 12.23±3.31 mm at postoperative 1 month and was stabilized to 11.43± 2.23 mm on the final visit. It was slightly decreased on the final visit compared with postoperative 1 month, but this difference was not statistically significant (p=0.103).
The segmental angle of lordosis at the neutral position was changed from 3.54±3.70° before surgery to 6.37±3.97° by postoperative 24 months, and total lumbar lordosis increased at postoperative 24 months to 24.71±11.70° from 20.37±11.30° before surgery (p=0.011). The results on the change of the intervertebral disc height, segmental lordosis, and total lordosis are also listed in Table 2.
Table 2.
Radiological change after surgery
*OP : operation
There were no other clinical complications or cases of re-operations.
DISCUSSION
Lumbar interbody fusion provides several theoretical advantages over other fusion techniques25,29,30). Biomechanically, the graft is placed at the weight-bearing center of the spine where 80% of the axial load occurs19,32). Furthermore PLIF can create a higher fusion rate by placing the graft under the compression with an extensive blood supply from the adjacent vertebral endplates19). The disc height and the sagittal balance can be restored just as well27). The pedicle screw fixation needs wide muscle retraction to expose the transverse process and it can induce mechanical damage or nerve injury at the time of screw insertion, but stand alone PLIF needs less muscle retraction and complications and post-operative pain are reduced10). Finally, the amount of bone required for the graft is significantly reduced28). Problems, however, do exist. Collapse, slippage, and graft migration have been reported in 3 to 10% of cases in large series1,16,25). Therefore, the interbody cages have been designed specifically to solve the structural and biological functions of the graft. Posterior interbody bone fusion does not require the full exposure down to the transverse process; it also offers biomechanical stability by fusing directly to the vertebral body, can prevent degenerative changes of the facet joint by helping to recover the narrowed disc height induced and can reduce the recurrence of disc protrusion since a significant amount of disc is removed at the time of interbody bone fusion6,16,20,25).
The expandable cages are available with different lordotic angles and allow better correction of the sagittal balance. To avoid migration, it is threaded. They can be inserted posteriorly, and have specific advantages and characteristics. Via a posterior approach, the spinal canal can be easily explored, fixation can be achieved during the same operative procedure, and the use of locally derived bone obviates the need to harvest iliac bone. Biomechanically, during all modes of loading except axial rotation, PLIF showed slightly better outcome than ALIF. But, dural and nerve root manipulations represent a particular risk of this procedure13,15,17).
Clinical outcome
In general, the clinical outcome after PLIF can vary widely depending on the selection criteria. We used VAS and ODI scores. The significant reduction of VAS and ODI was achieved at 12 months after surgery, and this represents a clinical success. Also, the reduction of VAS lasted to 24 months. The increase of the ODI score at 12 and 24 months follow-up might be seen due to the follow-up loss of the functionally increased patients and more follow-up of patients with more pain and disability.
Fusion outcome
Generally, the bone fusion rate of other interbody fusion method is more than 90%5,16,19,23-26,31). In this study, the fusion rate, which was confirmed by plain x-ray and CT scan, was 92.3%. And, the flexion-extension film showed stability in all patients.
Kumar et al.27) coined the phrase "functional arthrodesis" with the term indicating stability with less than two degrees of motion as seen on flexion and extension radiographs and bridging bone anterior or posterior to the femoral allograft although the fusion was less than complete. We had accepted fusion success with the meeting of all four criteria : low subsidence, stability on flexion-extension, absence of halo, and formation of anterior sentinel bone, or formation of the contiguous bony bridge. We accounted for the functional fusion in the majority of cases but were concerned more with the trabecular bone formation on the intervertebral space. We thought it could provide us more accurate results.
As a side note, Fraser27) stated that criteria-based radiographic determination of fusion status remained an imperfect modality, and operative exploration remained the gold standard. This means that it is difficult to specifically ascertain the fusion rate in radiologic findings and a simple comparison for fusion rate is impossible without any criteria. Therefore, in this study, we use the criteria offered by Ray30) in an Investigational Device Exemption study of titanium fusion cages.
Disc height, subsidence, and lordosis
The intervertebral disc height was reduced by approximately 1.74±1.82 mm at the last visit. The data obtained in our series showed a low subsidence rate and the cage seemed to maintain the intervertebral disc height as well in the long-term follow-up. The segmental angle of lordosis was also improved from 3.54° before surgery to 6.37° by 24 months postoperative. Expandable cages can be expanded by 2-2.5 mm, and 4°, after being inserted into the intervertebral space so that a firm fixation is possible. This requires little removal of unnecessary cancellous bone for the insertion of a cage larger than the intervertebral height, and results in little destruction of the facet joint. Furthermore, the contact surface between the cage and the end plate is widened by being flat rather than circular so that the possibility of sinking after bone fusion is minimized. Theoretically, these wedge-type cages could be quite firmly located in the intervertebral space, that they could correct 'flat back' which is related with back pain, and not result in irregular translational and shear force to the adjacent lumbar segment2,11,12). The physiological curve of the spine is related to the distribution of optimal weights loaded onto the spine so that the loss of physiological curve in the lumbar spine is related with the occurrence of backpain1,13,15,17,18,36). Wambolt and Spencer4,9,18,22,33) reported that lordosis in the physiological lumbar curve is formed from L3 to S1 in most cases and that the destruction of lordosis results from the decrease in intervertebral disc height and interspinous ligament damage due to degenerative changes. Also, according to the present study, normal lumbar lordosis by securing the normal segmental angle using expandable cages at the narrowed interbody of the lower lumbar spine can be expected. Total lumbar lordosis showed slight difference by postoperative 24 months with the value being 24.71° compared to the value before surgery at 20.37°. We suggest that the segmental angle recovery of the lower lumbar spine is not the only factor in deciding the total lumbar lordosis recovery but also it is important in the development of the facet joint degeneration, ligament hypertrophy, and back muscle atrophy. But, the significance of these factors was not evident in this study.
Procedural complication
Complications associated with PLIF can be serious, especially the neurological deficits often related to excessive retraction of the nerve roots or the dural sac. According to the various reports, these complications occur in 4 to 10% of patients16,23,25,30). Kuslich et al.23) reported that the complication of cage migration was observed in 3%. In addition, there have been increasing numbers of cage migration reported when stand-alone cages were used. The rate of cage migration in patients with no posterior instrumentation was significantly higher compared with the rate in those with posterior instrumentation (16.7% vs. 0%)32).
In this study, there were no significant procedure-related complications. The low rate of cage protrusion may be related to the threaded cage appearance and expandable nature. However, the cage insertion procedure is similar to other PLIF methods that the complication rate of neurologic deficit from nerve root or dural sac retraction should be similar to other reports.
CONCLUSION
This was a multi-center study to investigate the safety and efficacy of the expandable cage in the degenerative lumbar spinal disorders. There have been no special complications regarding the expandable cage over follow-up periods and the study results demonstrate a high functional stability and clinical success, but more longer follow-up study is mandatory to verify the superiority and to justify the general use of the expandable cage for degenerative lumbar spinal disorders.
References
- 1.Agazzi S, Reverdin A, May D. Posterior lumbar interbody fusion with cages : an independent review of 71 cases. J Neurosurg. 1999;91(Spine 2):186–192. doi: 10.3171/spi.1999.91.2.0186. [DOI] [PubMed] [Google Scholar]
- 2.Attia D. Posterior lumbar interbody fusion using an original screwed titanium device : LIFEC expandable cages. A retrospective study of 48 patients with an minimal follow-up of 1 year. In: Gunzburg R, Szpalski M, editors. Lumbar spinal stenosis. Lippincott Williams and Wilkins; 2000. pp. 263–273. [Google Scholar]
- 3.Barnes B, Rodts GE, McLaughlin MR, Haid RW., Jr Threaded cortical bone dowels for lumbar interbody fusion : over 1-year mean follow up in 28 patients. J Neurosurg. 2001;95(Spine 1):1–4. doi: 10.3171/spi.2001.95.1.0001. [DOI] [PubMed] [Google Scholar]
- 4.Bernhardt M, Bridwell KH. Segmental analysis of the sagittal plane alignment of the normal thoracic and lumbar spines and thoracolumbar junction. Spine. 1989;14:717–721. doi: 10.1097/00007632-198907000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Brantigan JW, Steffee AD. A carbon fiber implant to aid interbody lumbar fusion. Two-year clinical results in the first 26 patients. Spine. 1993;18:2106–2117. doi: 10.1097/00007632-199310001-00030. [DOI] [PubMed] [Google Scholar]
- 6.Brodke DS, Dick JC, Kunz DN, McCabe R, Zdeblick TA. Posterior lumbar interbody fusion. A biomechanical comparison, including a new threaded cage. Spine. 1997;22:26–31. doi: 10.1097/00007632-199701010-00005. [DOI] [PubMed] [Google Scholar]
- 7.Burkus JK, Dorchak JD, Sanders DL. Radiographic assessment of interbody fusion using recombinant human bone morphogenetic protein type 2. Spine. 2003;28:372–377. doi: 10.1097/01.BRS.0000048469.45035.B9. [DOI] [PubMed] [Google Scholar]
- 8.Cailliet R. Lumbar discogenic disease. Why the elderly are more vulnerable. Geriatrics. 1975;30:73–76. [PubMed] [Google Scholar]
- 9.Chernukha KV, Daffner RH, Reigel DH. Lumbar lordosis measurement. A new method versus Cobb technique. Spine. 1998;23:74–80. doi: 10.1097/00007632-199801010-00016. [DOI] [PubMed] [Google Scholar]
- 10.Davne SH, Myers DL. Complications of lumbar spinal fusion with transpedicular instrumentation. Spine. 1992;17:S184–S189. doi: 10.1097/00007632-199206001-00021. [DOI] [PubMed] [Google Scholar]
- 11.Diedrich O, Perlick L, Schmitt O, Kraft CN. Radiographic spinal profile changes induced by cage design after posterior lumbar interbody fusion. Preliminary report of a study with wedged implants. Spine. 2001;26:E274–E280. doi: 10.1097/00007632-200106150-00019. [DOI] [PubMed] [Google Scholar]
- 12.Dietl RHJ, Krammer M, Kettler A, Wilke HJ, Claes L. Pullout test with three lumbar interbody fusion cages. Spine. 2002;27:1029–1036. doi: 10.1097/00007632-200205150-00005. [DOI] [PubMed] [Google Scholar]
- 13.Fernand R, Fox DE. Evaluation of lumbar lordosis. A prospective and retrospective study. Spine. 1985;10:799–803. doi: 10.1097/00007632-198511000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Frobin W, Brinckmann P, Biggemann M. Objective measurement of the height of lumbar intervertebral disc from lateral roentgen views of the spine. Z Orthop Ihre Grenzgeb. 1997;135:394–402. doi: 10.1055/s-2008-1039407. [DOI] [PubMed] [Google Scholar]
- 15.Hansson T, Bigos S, Beecher P, Wortley M. The lumbar lordosis in acute and chronic low-back pain. Spine. 1985;10:154–155. doi: 10.1097/00007632-198503000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Hutter CG. Posterior intervertebral body fusion. A 25-year study. Clin Orthop Relat Res. 1983;179:86–96. [PubMed] [Google Scholar]
- 17.Itoi E. Roentgenographic analysis of posture in spinal osteoporosis. Spine. 1991;16:750–756. doi: 10.1097/00007632-199107000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Jackson RP, McManus AC. Radiographic analysis of sagittal plane alignment and balance in standing volunteers and patients with low back pain matched for age, sex, and size. Spine. 1994;19:1611–1618. doi: 10.1097/00007632-199407001-00010. [DOI] [PubMed] [Google Scholar]
- 19.Jeon JH, Kim SM, Jung DJ, Moon SM, Hwang HS, Choi SK. PLIF using cages at the instability level and additional transpedicular instrumental fusion in multilevel degenerative lumbar disease. J Korean Neurosurg Soc. 2004;35:372–378. [Google Scholar]
- 20.Jost B, Cripton PA, Lund T, Oxland TR, Lippuner K, Jaeqer P, et al. Compressive strength of interbody cages in the lumbar spine : the effect of cage shape, posterior instrumentation and bone density. Eur Spine J. 1998;7:132–141. doi: 10.1007/s005860050043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klemme WR, Owens BD, Dhawan A, Zeidman S, Pollv DW., Jr Lumbar sagittal contour after posterior interbody fusion : threaded devices alone versus vertical cages plus posterior instrumentation. Spine. 2001;26:534–537. doi: 10.1097/00007632-200103010-00017. [DOI] [PubMed] [Google Scholar]
- 22.Korovessis PG, Stamataskis MV, Baikousis AG. Reciprocal angulation of vertebral bodies in the sagittal plane in an asymptomatic Greek population. Spine. 1998;23:700–705. doi: 10.1097/00007632-199803150-00010. [DOI] [PubMed] [Google Scholar]
- 23.Kuslich SD, Danielson G, Dowdle JD, Sherman J, Fredrickson B, Yuan H, et al. Four-year follow-up results of lumbar spine arthrodesis using the Bagby and Kuslich lumbar fusion cage. Spine. 2000;25:2656–2662. doi: 10.1097/00007632-200010150-00018. [DOI] [PubMed] [Google Scholar]
- 24.Lehmann TR, Spratt KF, Tozzi JE, Weinstein JN, Reinarz SJ, el-Khoury GY, et al. Long-term follow-up of lower lumbar fusion patients. Spine. 1987;12:97–104. doi: 10.1097/00007632-198703000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Lin PM, Cautilli RA, Joyce MF. Posterior lumbar interbody fusion. Clin Orthop Relat Res. 1983;180:154–168. [PubMed] [Google Scholar]
- 26.Ma GW. Posterior lumbar interbody fusion with specialized instruments. Clin Orthop Relat Res. 1985;193:57–63. [PubMed] [Google Scholar]
- 27.McAfee PC, Maryland T. Interbody fusion cages in reconstructive operations on the spine. J Bone Joint Surg AM. 1999;81:859–880. doi: 10.2106/00004623-199906000-00014. [DOI] [PubMed] [Google Scholar]
- 28.Mizuno J, Nakaqawa H. Threaded fusion cage for lumbar spondylolisthesis. Neurol Med Chir (Tokyo) 1998;38:155–160. doi: 10.2176/nmc.38.155. [DOI] [PubMed] [Google Scholar]
- 29.Onesti ST, Ashkenazi E. The ray threaded fusion cage for posterior lumbar interbody fusion. Neurosurgery. 1998;42:200–205. doi: 10.1097/00006123-199801000-00046. [DOI] [PubMed] [Google Scholar]
- 30.Ray CD. Threaded titanium cages for lumbar interbody fusions. Spine. 1997;22:667–679. doi: 10.1097/00007632-199703150-00019. [DOI] [PubMed] [Google Scholar]
- 31.Rish BL. A comparative evaluation of posterior lumbar interbody fusion for disc disease. Spine. 1985;10:855–857. doi: 10.1097/00007632-198511000-00016. [DOI] [PubMed] [Google Scholar]
- 32.Schlegel KF, Pon A. The biomechanics of posterior lumbar interbody fusion (PLIF) in spondylolisthesis. Clin Orthop Relat Res. 1985;193:115–119. [PubMed] [Google Scholar]
- 33.Tencer AF, Hampton D, Eddy S. Biomechanical properties of threaded fusion inserts for lumbar interbody spinal fusion. Spine. 1995;20:2408–2414. doi: 10.1097/00007632-199511001-00007. [DOI] [PubMed] [Google Scholar]
- 34.Tribus CB, Belanger TA, Zdeblick TA. The effect of operative position and short-segment fusion on maintenance of sagittal alignment of the lumbar spine. Spine. 1999;24:58–61. doi: 10.1097/00007632-199901010-00014. [DOI] [PubMed] [Google Scholar]
- 35.Wetzel FT, LaRocca H. The failed posterior lumbar interbody fusion. Spine. 1991;16:839–845. doi: 10.1097/00007632-199107000-00027. [DOI] [PubMed] [Google Scholar]
- 36.West JL, 3rd, Bradford DS, Ogilvie JW. Results of spinal arthrodesis with pedicle screw-plate fixation. J Bone Joint Surg AM. 1991;73:1179–1183. [PubMed] [Google Scholar]