Abstract
Objective
Gamma Knife Stereotactic Radiosurgery (GK SRS) has become an important treatment modality for vestibular schwannomas. We evaluated the tumor control rate, patterns of tumor volume change and preservation of hearing following low-dose radiation for vestibular schwannomas in a homogeneous cohort group in which the mean marginal dose was 12 Gy.
Methods
A total of 59 patients were enrolled in this study. All enrolled patients were followed-up for at least 5 years and the radiation dose was 11-13 Gy. Regular MRI, audiometry and clinical evaluations were done and tumor volumes were obtained from MRI using the OSIRIS program.
Results
The tumor control rate was 97%. We were able to classify the patterns of change in tumor volume into three categories. Transient increases in tumor volume were detected in 29% of the patients and the maximum transient increase in tumor volume was identified at 6 to 30 months after GK SRS. The transient increases in tumor volume ranged from 121% to 188%. Hearing was preserved in 4 of the 12 patients who had serviceable hearing prior to treatment. There were no other complications associated with GK SRS.
Conclusion
Low-dose GK SRS was an effective and safe mode of treatment for vestibular schwannomas in comparison to the previously used high-dose GK SRS. Transient increases in tumor volume can be identified during the follow-up period after low-dose GK SRS for vestibular schwannomas. Physicians should be aware that these increases are not always indicative of treatment failure and that close observation is required following treatments. Unfortunately, a satisfactory hearing preservation rate was not achieved by reducing the radiation dose. It is thought that hearing preservation is a more sophisticated problem and further research is required.
Keywords: Vestibular schwannomas, Gamma knife, Low dose, Long-term outcome
INTRODUCTION
Vestibular schwannomas account for 10% of newly diagnosed intracranial tumors18). Among several treatment options for vestibular schwannomas, such as the "wait-and-see" approach, microsurgical resection, and radiosurgery, Gamma Knife Stereotactic Radiosurgery (GK SRS) has become an important treatment modality because it is less invasive and has been associated with a good tumor control rate and low morbidity since its introduction in 196927). Although GK SRS has become widely recommended as an initial treatment for small-sized vestibular schwannomas, there is no consensus on the optimal marginal dose of GK SRS for vestibular schwannomas. From a historical point of view, radiation dose has been strongly associated with the functional preservation of the cranial nerve and tumor control rates. In the initial reports in the 1980s, the marginal radiation doses were over 20 Gy, which led to high incidences of facial palsy and trigeminal nerve dysfunction21). Thereafter, the marginal radiation dose was decreased to 16-18 Gy but it was still associated with high rates of facial nerve palsy and hearing loss7,8,11,14). Recently, much lower doses were attempted with documented reductions in cranial nerve dysfunction, including hearing loss. However, there was limited data as to whether such low doses of radiation were as effective as the high doses in achieving long-term disease control30). Thus, we evaluated the tumor control rate chronologically and assessed the pattern of changes in tumor volume and the preservation of hearing following GK SRS for vestibular schwannomas in which a mean dose of 12 Gy was prescribed to the tumor margin.
MATERIALS AND METHODS
A total of 118 patients with vestibular schwannomas were treated with GK SRS at the Gamma Knife Center at our Hospital, between December 1997 and September 2001. A total of 122 lesions were treated. Four of the patients had bilateral lesions. Eleven patients were excluded because the radiation dose administered did not meet the study criteria (11-13 Gy) and 11 other patients were excluded because they were diagnosed as having type 2 neurofibromatosis, which is known to be associated with a distinct biologic behavior. The remaining 96 patients were initial candidates of this study. Fifty-nine of these 96 patients were enrolled in this study. However, the other 37 patients were excluded from this study because they were not followed up for 5 years. Twenty-nine patients were treated with GK SRS as primary treatment and 30 patients were treated with GK SRS after undergoing an operation. Low-dose GK SRS was defined as a marginal dose of 11-13. Twenty-seven patients were male and the other 32 patients were female. Median age was 48 year old and mean initial tumor volume was 3.41cc (±2.76, range : 0.14 cc-11.10 cc). Mean follow up duration was 6.47 years (clinical follow up) and 6.07 years (MRI scan follow up).
The median marginal dose was 12 Gy (11-13 Gy) and the median prescription isodose line was 50% (45-52%). Twenty-one patients were treated with 13 Gy, two patients were treated with 11.5 Gy and nine patients were treated with 11 Gy at the tumor margin. The remaining 28 patients were treated with a marginal dose of 12 Gy. Magnetic Resonance Imaging (MRI) was performed before GK SRS and at 6, 12, 18, 24 months post-treatment and once annually thereafter. The pretreatment tumor volumes were calculated using the treatment planning software (Leksell Gamma Plan®). Post-treatment tumor volumes were measured by the OSIRIS program®, which consisted of general medical image manipulation and analysis software program that was developed at the University Hospital of Geneva. An increase or decrease of more than 20% of the tumor volume was defined as a significant change. Tumor control was considered to have failed when a tumor volume increase above 20% was detected in the final follow-up images or when surgical intervention was necessary.
The tumor volume change was expressed as the tumor volume percentage. The initial tumor volume was defined as 100%, and the tumor volume changes in the follow-up images were examined as percentage of the initial tumor volume.
Twelve of the enrolled patients had serviceable hearing before GK SRS, which was defined as a hearing level of Gardener-Robertson (G-R) Grade I and II9). Pure tone audiometry (PTA) and speech discrimination scores (SDS) were analyzed in the patients with serviceable hearing. G-R grade I means that hearing result is included in 0-30dB (PTA) and 70-100% (SDS). G-R grade II means that hearing result is included in 31-50 dB and 50-59%. Hearing considered as preserved when the hearing level was maintained at G-R grade I or II after GK SRS, and to have deteriorated when the hearing level was aggravated to G-R grade III, IV, V. All statistical analyses, including the Kaplan-Meier method and multivariate analysis, were performed by Statistical Package for the Social Sciences (SPSS) for Windows®, version 12 K (SPSS Inc, Chicago). Patients' clinical data were assessed using medical records and electronic databases.
RESULTS
Tumor control was achieved in 57 (97%) of the 59 patients enrolled in the study. The mean tumor volume decrease was 52% in the primary GK SRS group and 49% in post-operation group. One patient underwent a tumor removal operation due to aggravation of hemifacial spasm. His tumor volume decreased from 100% to 54% over a period of two years after GK SRS, but his symptoms of hemifacial spasm were aggravated. Thus, microvascular decompression and tumor removal were performed at the same time.
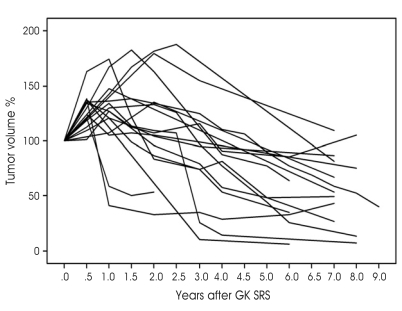
The pattern of change in tumor volume was classified into three categories: (1) decreased continuously; (2) stationary; and (3) transiently increased above 20% and then decreased (Fig. 1). A total of 17 patients were included in the third category. The maximum transient increase in tumor volume was identified at 6 to 30 months after GK SRS and the mean time to maximum tumor volume was 13.4 months. The transient increases in tumor volume ranged from 121% to 188%.
Fig. 1.
Transiently increased tumor volume. 29% of the patients are included in this category.
Statistical analysis was performed about the transient increases in tumor volume. Initial tumor volume was 4.5 cc in the transiently increased group and 2.97 cc in the not-transiently increased group. But, p-value was 0.052, indicative of no statistical significance. Radiation dose also showed no statistical significance (p=0.57)
Tumor control was failed in two patients. One patient was included in the primary GK SRS group and the other patient in the post-operation group. One patient, a 32-year-old-woman, underwent retromastoid suboccipital craniotomy and tumor removal. GK SRS was performed for the residual tumor at eight months after the initial surgery. At 54 months after GK SRS, MRI showed that the size of solid enhancing portion of the tumor had decreased; however, a new peritumoral cystic change was observed near the cerebellum. Due to complaints of severe headache, she underwent burr-hole trephination and navigation-guided Ommaya reservoir insertion. Her headache subsided after the operation. However, two weeks later, she visited the hospital due to severe headache and diplopia. MRI revealed that the small cyst that was located adjacent to the solid portion of the tumor shrank, but the size of the other cyst remained unchanged. So, cyst wall removal and Ommaya reservoir insertion by craniotomy were performed. The cyst has remained stable thus far.
The other patient, a 60-year-old man, underwent GK SRS for a cystic vestibular schwannoma. The initial tumor volume was 10.60 cc and the marginal dose at 50% was 11 Gy. After GK SRS, the tumor volume was decreased from 10.60 cc to 6.94 cc over 36 months. However, the tumor regrew to a volume of 23.50 cc (180.75%) 6 years after GK SRS, primarily due to tumor cyst enlargement. Retromastoid suboccipital craniotomy and tumor removal were then performed 6 years after GK SRS. A small residual tumor remained after the surgery, but follow-up examination showed that it had remained stable.
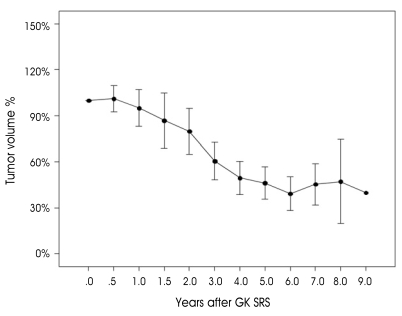
Fig. 2 shows the long-term changes in tumor volume among patients in the continuously decreased and transiently increased groups. A confidence interval of 95% is also shown. This graph reveals the transient increase in tumor volume and it is predicted that the tumor volume will be stable at five years after GK SRS.
Fig. 2.
Pattern of long-term changes in tumor volume. 95% confidence interval is shown.
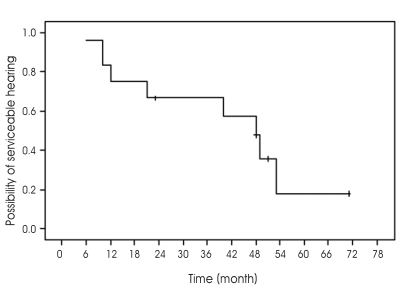
The hearing level of all the patients in the post-operation group was classified as G-R grade V. Twelve patients in primary GK SRS group had serviceable hearing before GK SRS. A serviceable level of hearing was preserved in 4 of the 12 patients who had serviceable hearing before GK SRS (Fig. 3). The mean audiometry follow-up duration was 4.07 years. However, the hearing level was only maintained in one of these patients; the hearing level of the other three patients was aggravated from G-R grade I to G-R grade II. The hearing levels of the eight patients with deteriorated hearing were aggravated from G-R grade I to G-R grade III in three patients, from G-R grade II to G-R grade III in four patients, and from G-R grade II to G-R grade IV in one patient. There were no statistically significant factors affecting hearing preservation.
Fig. 3.
Kaplan-Meier survival plot of hearing preservation in patients treated with Gamma Knife Stereotactic Radiosurgery.
We also analyzed the intraauditary canal (IAC) portion of the tumor. In the hearing preserved group, mean IAC tumor volume was 0.13 cc and initial tumor volume was 3.22 cc. In the hearing deteriorated group, mean IAC tumor volume was 0.22 cc and initial tumor volume was 5.12 cc. Proportion of the tumor located in the IAC was 14% in the hearing preserved group and 8% in the hearing deteriorated group. But, there was no statistically significant difference between the two groups (p=0.22, 0.39, 0.42, respectively), probably because of the small sample of patients.
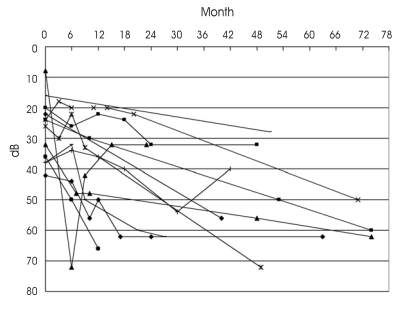
The pure-tone average change during the follow-up period is shown in Fig. 4. We found that the majority of hearing deterioration occurred within 2 years after GK SRS, but it may occur in later years.
Fig. 4.
Sequential plotting of pure tone average changes in patients treated with Gamma Knife Stereotactic Radiosurgery.
The other 17 patients in the primary GK SRS group had no serviceable hearing before GK SRS. The hearing level was of G-R grade III in seven patients, G-R grade IV in two patients and G-R grade V in the other eight patients. All patients with G-R III hearing maintained their level of hearing after GK SRS, but the hearing levels in two patients were aggravated from G-R VI to G-R grade V after GK SRS.
During the follow-up periods, the other side effects of GK SRS requiring treatment, such as aggravation of hydrocephalus, other cranial nerve palsy, occurrence of malignancy and brain edema that required treatment, were not observed.
DISCUSSION
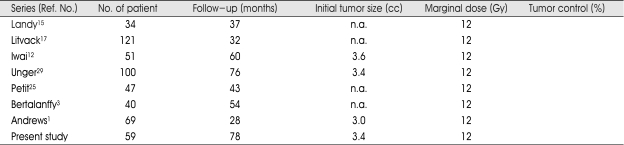
The tumor control rate of vestibular schwannomas by GK SRS has been reported to be between 86 and 98% when the mean or median radiation dose was 12 Gy (Table 1). The tumor control rate was 97% in the present study. In other studies concerning GK SRS for vestibular schwannomas using a marginal dose of more than 12 Gy, the tumor control rate was reported to be between 89 and 100%6-8,11,14). The results of several low-dose GK SRS studies have shown that there is no decrease in tumor control rate in comparison to the results of high-dose GK SRS19). Compared to other studies, more patients were enrolled in this study and the duration of the follow-up period was longer. Thus, it provides strong evidence to support the effectiveness of low-dose GK SRS for vestibular schwannomas.
Table 1.
Comparison of the results of GK SRS for vestibular schwannomas (n.a.: not available)
It was well known that the initial tumor volume is correlated with the tumor control rate after GK SRS. Transient increases in tumor volume have been reported after GK SRS in many studies19,22,26,31). It remains controversial whether the transient increase in tumor volume should be regarded as a treatment failure26). Noren20) noted that approximately 5% of tumors will exhibit such transient "swelling" and that at least 2 years should pass before determining a lack of response from radiosurgery. In other reports, 14-15% of patients demonstrated transient tumor enlargement5,26) .
Picture Archiving and Communication System (PACS) was adapted in our hospital, and all images were stored as Digital Imaging and Communications in Medicine (DICOM) files. By using OSIRIS programs, we measured the volume of the Regions of Interest (ROI) in DICOM files of the images. A volume can be described by a set of ROI's assigned to several images in a contiguous set of slices to calculate volume. It was more accurate than the previously used two-dimensional measurement. In our study, 17 of 59 patients (29%) showed a transient increase in tumor volume. In two other studies22,31), the tumor volume was measured using a sequential volume mapping technique on a computer and transient increases in tumor volume were observed in 45% and 62% of patients, respectively. Such results and this study support that transient increases in tumor volume are a more common finding after GK SRS for vestibular schwannomas.
In this study, initial tumor volume was considerably different between the transiently increased group and the not-transiently increased group, although P-value was 0.052, so there was no statistical significance. We believe that further study with more patients is required to verify the factors affecting transient increase in tumor volume.
Transient increases in tumor volume were observed for up to 30 months after GK SRS in this study. The maximum increase in tumor volume was 188%, which is consistent with the findings of other studies19,22). Thus, we recommend that tumor volume increases that remain under 200% for at least 2.5 years after GK SRS should not be regarded as a treatment failure. In this setting, close serial MRI follow-ups are recommended. Microsurgical resection of tumors, which increased after GK SRS, showed hyalinized thrombosis, thickening of the vascular wall, vascular obstruction, and granulomatous changes10,13). These changes could be reasons for transient tumor swelling after GK SRS.
It was suggested that enlargement of the cystic component of the tumor may occur regardless of the effectiveness of GK SRS and changes in the cystic components cause changes in the tumor volume and the unpredictable behavior of the tumor19). In this study, six patients had cystic vestibular schwannomas. The tumor was controlled successfully in four patients. Two patients in the tumor control failure group underwent microsurgical resection due to cyst enlargement. Unpredictable volume changes were found owing to a newly developed cystic component in one patient. The cystic components of the tumor were found to be enlarged in another patient. It has been suggested that cyst formation in vestibular schwannomas results from the microhemorrhage24). We recommend that tumor volume changes should be carefully interpreted, especially if there is a cystic component of the tumor.
In this study, we determined a pattern in the long-term changes in tumor volume GK SRS (Fig. 5). It seems that tumor volume changes become stationary by about five years after GK SRS, which means that at least five years of follow-up are necessary to evaluate the effect of GK SRS. Although a limitation of this study is the relatively small number of patients studied, the tumor volume change can be statistically predicted by this result.
Improvements in the GK SRS technique and dose reduction have reduced the complication rates of GK SRS. However, the preservation of hearing is still a major concern with GK SRS. In other studies, the cranial nerve preservation rates were dramatically increased after GK SRS, but the hearing preservation rate was still low4,6,12). Iwai et al.12) reported that useful hearing was preserved in only 10 (56%) of 18 patients after GK SRS, although a good tumor control rate (96%) and no development of facial palsy or trigeminal neuropathy were observed with a marginal dose of 12 Gy. Another study23), which was performed at our institution, showed that the hearing preservation rate was 52% with a marginal dose of 12.0 Gy±0.7.
It is unclear why the hearing preservation rate is low in comparison with the cranial nerve preservation rate, even when low radiation doses are administered. Several hypotheses have been suggested, but the definite cause is still unknown. One hypothesis is that the hearing apparatus is more sensitive to radiation. One report suggested that unintentional delivery of high doses of radiation to the stria vascularis, the sensory neuroepithelium of the inner ear organs and/or their ganglia, may play a role in the development of post-GK SRS tinnitus, hearing loss, dizziness, vertigo, and imbalance16). Minimizing treatment complications post-GK SRS for vestibular schwannomas requires precise dose planning conformality with the three-dimensional surface of the tumor.
The other hypothesis for the low rate of hearing preservation is that hearing deterioration might be a result of transient changes in the volume of tumors located in internal auditory canal (IAC)19,31). If the IAC portion of the tumor is transiently increased after GK SRS, it compresses the cochlear nerve and eventually results in hearing deterioration. In this study, we found that the majority of hearing deterioration occurs within 2 years after GK SRS. Such a finding suggests that there was a correlation between transient increases in tumor volume and hearing deterioration. However, in statistical analysis, we could not find a statistical significance between transient increases in tumor volume and hearing deterioration. We believe that it is probably due to small numbers of patients in this study.
Furthermore, hearing deterioration occurred in later years in some patients, which indicates that not only transient increases in tumor volume, but other mechanisms may also play important roles in hearing deterioration.
We observed recovery from hearing deterioration in one patient. The hearing level of this patient had deteriorated from G-R grade I to G-R grade V by 9 months after GK SRS. However, the hearing level recovered to G-R grade I at 15 months after GK SRS. The chief complaint of the patient was hemifacial spasm and it was aggravated after GK SRS. Therefore, the patient was treated with a steroid for one month. It was not clear whether the steroid use resulted in hearing recovery. In this study, only one patient showed such a phenomenon, but several studies have found that hearing was improved after administrating steroids to patients who experienced a deterioration in hearing after GK SRS2,28). However, because steroids cause serious side effects, their use should be addressed with caution and should be limited in duration with close monitoring23).
CONCLUSION
Increases in the tumor control rate and reduction of GK SRS-related complications are two major issues associated with GK SRS treatment of vestibular schwannomas. The radiation dose is the most important factor contributing to both issues. However, no definite guideline for determining the appropriate radiation dose for GK SRS has been established. In this article, the treatment of vestibular schwannomas with low-dose GK SRS with a tumor margin of 12 Gy was given and showed good long-term tumor control with effectiveness and safety. However, a satisfactory hearing preservation rate could not be achieved using this method. It is considered that a more sophisticated method is needed to preserve the hearing of patients treated with GK SRS.
References
- 1.Andrews DW, Suarez O, Goldman HW, Downes MB, Bednarz G, Corn BW, et al. Stereotactic radiosurgery and fractionated stereotactic radiotherapy for the treatment of acoustic schwannomas : comparative observations of 125 patients treated at one institution. Int J Radiat Oncol Biol Phys. 2001;50:1265–1278. doi: 10.1016/s0360-3016(01)01559-0. [DOI] [PubMed] [Google Scholar]
- 2.Aronzon A, Ruckenstein MJ, Bigelow DC. The efficacy of corticosteroids in restoring hearing in patients undergoing conservative management of acoustic neuromas. Otol Neurotol. 2003;24:465–468. doi: 10.1097/00129492-200305000-00018. [DOI] [PubMed] [Google Scholar]
- 3.Bertalanffy A, Dietrich W, Aichholzer M, Brix R, Ertl A, Heimberger K, et al. Gamma knife radiosurgery of acoustic neurinomas. Acta Neurochir (Wien) 2001;143:689–695. doi: 10.1007/s007010170047. [DOI] [PubMed] [Google Scholar]
- 4.Cho JH, Paek SH, Chung H-T, Jeong SS, Jung H-W, Kim DG. Hearing Outcome after Gamma Knife Stereotactic Radiosurgery in Vestibular Schwannoma Patients with Serviceable Hearing. J Korean Neurosurg Soc. 2006;40:33–341. [Google Scholar]
- 5.Delsanti C, Tamura M, Galanaud D, Regis J. [Changing radiological results, pitfalls and criteria of failure] Neurochirurgie. 2004;50:312–319. [PubMed] [Google Scholar]
- 6.Flickinger JC, Kondziolka D, Niranjan A, Lunsford LD. Results of acoustic neuroma radiosurgery : an analysis of 5 years' experience using current methods. J Neurosurg. 2001;94:1–6. doi: 10.3171/jns.2001.94.1.0001. [DOI] [PubMed] [Google Scholar]
- 7.Flickinger JC, Lunsford LD, Linskey ME, Duma CM, Kondziolka D. Gamma knife radiosurgery for acoustic tumors : multivariate analysis of four year results. Radiother Oncol. 1993;27:91–98. doi: 10.1016/0167-8140(93)90127-t. [DOI] [PubMed] [Google Scholar]
- 8.Foote RL, Coffey RJ, Swanson JW, Harner SG, Beatty CW, Kline RW, et al. Stereotactic radiosurgery using the gamma knife for acoustic neuromas. Int J Radiat Oncol Biol Phys. 1995;32:1153–1160. doi: 10.1016/0360-3016(94)00454-s. [DOI] [PubMed] [Google Scholar]
- 9.Gardner G, Robertson JH. Hearing preservation in unilateral acoustic neuroma surgery. Ann Otol Rhinol Laryngol. 1988;97:55–66. doi: 10.1177/000348948809700110. [DOI] [PubMed] [Google Scholar]
- 10.Hirato M, Inoue H, Nakamura M, Ohye C, Hirato J, Shibazaki T, et al. Gamma knife radiosurgery for acoustic schwannoma : early effects and preservation of hearing. Neurol Med Chir (Tokyo) 1995;35:737–741. doi: 10.2176/nmc.35.737. [DOI] [PubMed] [Google Scholar]
- 11.Ito K, Kurita H, Sugasawa K, Mizuno M, Sasaki T. Analyses of neuro-otological complications after radiosurgery for acoustic neurinomas. Int J Radiat Oncol Biol Phys. 1997;39:983–988. doi: 10.1016/s0360-3016(97)00507-5. [DOI] [PubMed] [Google Scholar]
- 12.Iwai Y, Yamanaka K, Shiotani M, Uyama T. Radiosurgery for acoustic neuromas : results of low-dose treatment. Neurosurgery. 2003;53:282–287. doi: 10.1227/01.neu.0000073416.22608.b3. discussion 287-288. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi T, Tanaka T, Kida Y. The early effects of gamma knife on 40 cases of acoustic neurinoma. Acta Neurochir (Suppl) 1994;62:93–97. doi: 10.1007/978-3-7091-9371-6_19. [DOI] [PubMed] [Google Scholar]
- 14.Kondziolka D, Lunsford LD, McLaughlin MR, Flickinger JC. Long-term outcomes after radiosurgery for acoustic neuromas. N Engl J Med. 1998;339:1426–1433. doi: 10.1056/NEJM199811123392003. [DOI] [PubMed] [Google Scholar]
- 15.Landy HJ, Markoe AM, Wu X, Patchen SJ, Reis IM, Takita C, et al. Safety and efficacy of tiered limited-dose gamma knife stereotactic radiosurgery for unilateral acoustic neuroma. Stereotact Funct Neurosurg. 2004;82:147–152. doi: 10.1159/000081347. [DOI] [PubMed] [Google Scholar]
- 16.Linskey ME, Johnstone PA, O'Leary M, Goetsch S. Radiation exposure of normal temporal bone structures during stereotactically guided gamma knife surgery for vestibular schwannomas. J Neurosurg. 2003;98:800–806. doi: 10.3171/jns.2003.98.4.0800. [DOI] [PubMed] [Google Scholar]
- 17.Litvack ZN, Noren G, Chougule PB, Zheng Z. Preservation of functional hearing after gamma knife surgery for vestibular schwannoma. Neurosurg Focus. 2003;14:E3. doi: 10.3171/foc.2003.14.5.4. [DOI] [PubMed] [Google Scholar]
- 18.Lunsford LD, Niranjan A, Flickinger JC, Maitz A, Kondziolka D. Radiosurgery of vestibular schwannomas : summary of experience in 829 cases. J Neurosurg. 2005;102(Suppl):195–199. [PubMed] [Google Scholar]
- 19.Nakamura H, Jokura H, Takahashi K, Boku N, Akabane A, Yoshimoto T. Serial follow-up MR imaging after gamma knife radiosurgery for vestibular schwannoma. AJNR Am J Neuroradiol. 2000;21:1540–1546. [PMC free article] [PubMed] [Google Scholar]
- 20.Noren G. Long-term complications following gamma knife radiosurgery of vestibular schwannomas. Stereotact Funct Neurosurg. 1998;70(Suppl 1):65–73. doi: 10.1159/000056408. [DOI] [PubMed] [Google Scholar]
- 21.Noren G, Arndt J, Hindmarsh T. Stereotactic radiosurgery in cases of acoustic neuroma : further experiences. Neurosurgery. 1983;12:12–22. doi: 10.1227/00006123-198307000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Okunaga T, Matsuo T, Hayashi N, Hayashi Y, Shabani HK, Kaminogo M, et al. Linear accelerator radiosurgery for vestibular schwannoma : measuring tumor volume changes on serial three-dimensional spoiled gradient-echo magnetic resonance images. J Neurosurg. 2005;103:53–58. doi: 10.3171/jns.2005.103.1.0053. [DOI] [PubMed] [Google Scholar]
- 23.Paek SH, Chung HT, Jeong SS, Park CK, Kim CY, Kim JE, et al. Hearing preservation after gamma knife stereotactic radiosurgery of vestibular schwannoma. Cancer. 2005;104:580–590. doi: 10.1002/cncr.21190. [DOI] [PubMed] [Google Scholar]
- 24.Park CK, Kim DC, Park SH, Kim JE, Paek SH, Kim DG, et al. Microhemorrhage, a possible mechanism for cyst formation in vestibular schwannomas. J Neurosurg. 2006;105:576–580. doi: 10.3171/jns.2006.105.4.576. [DOI] [PubMed] [Google Scholar]
- 25.Petit JH, Hudes RS, Chen TT, Eisenberg HM, Simard JM, Chin LS. Reduced-dose radiosurgery for vestibular schwannomas. Neurosurgery. 2001;49:1299–1306. doi: 10.1097/00006123-200112000-00003. discussion 1306-1297. [DOI] [PubMed] [Google Scholar]
- 26.Pollock BE. Management of vestibular schwannomas that enlarge after stereotactic radiosurgery : treatment recommendations based on a 15 year experience. Neurosurgery. 2006;58:241–248. doi: 10.1227/01.NEU.0000194833.66593.8B. discussion 241-248. [DOI] [PubMed] [Google Scholar]
- 27.Prasad D, Steiner M, Steiner L. Gamma surgery for vestibular schwannoma. J Neurosurg. 2000;92:745–759. doi: 10.3171/jns.2000.92.5.0745. [DOI] [PubMed] [Google Scholar]
- 28.Sakamoto T, Shirato H, Takeichi N, Aoyama H, Kagei K, Nishioka T, et al. Medication for hearing loss after fractionated stereotactic radiotherapy (SRT) for vestibular schwannoma. Int J Radiat Oncol Biol Phys. 2001;50:1295–1298. doi: 10.1016/s0360-3016(01)01574-7. [DOI] [PubMed] [Google Scholar]
- 29.Unger F, Walch C, Schrottner O, Eustacchio S, Sutter B, Pendl G. Cranial nerve preservation after radiosurgery of vestibular schwannomas. Acta Neurochir Suppl. 2002;84:77–83. doi: 10.1007/978-3-7091-6117-3_9. [DOI] [PubMed] [Google Scholar]
- 30.Weil RS, Cohen JM, Portarena I, Brada M. Optimal dose of stereotactic radiosurgery for acoustic neuromas : A systematic review. Br J Neurosurg. 2006;20:195–202. doi: 10.1080/02688690600886108. [DOI] [PubMed] [Google Scholar]
- 31.Yu CP, Cheung JY, Leung S, Ho R. Sequential volume mapping for confirmation of negative growth in vestibular schwannomas treated by gamma knife radiosurgery. J Neurosurg. 2000;93(Suppl 3):82–89. doi: 10.3171/jns.2000.93.supplement. [DOI] [PubMed] [Google Scholar]