Abstract
Objective
The aim of this study was to analyze the treatment results and prognostic factors in patients with massive cerebral infarction who underwent decompressive craniectomy.
Methods
From January 2000 to December 2005, we performed decompressive craniectomy in 24 patients with massive cerebral infarction. We retrospectively reviewed the medical records, radiological findings, initial clinical assessment using the Glasgow Coma Scale, serial computerized tomography (CT) with measurement of midline and septum pellucidum shift, and cerebral infarction territories. Patients were evaluated based on the following factors : the pre- and post-operative midline shifting on CT scan, infarction area or its dominancy, consciousness level, pupillary light reflex and Glasgow Outcome Scale.
Results
All 24 patients (11 men, 13 women; mean age, 63 years; right middle cerebral artery (MCA) territory, 17 patients; left MCA territory, 7 patients) were treated with large decompressive craniectomy and duroplasty. The average time interval between the onset of symptoms and surgical decompression was 2.5 days. The mean Glasgow Coma Scale was 12.4 on admission and 8.3 preoperatively. Of the 24 surgically treated patients, the good outcome group (Group 2 : GOS 4-5) comprised 9 cases and the poor outcome group (Group1 : GOS 1-3) comprised 15 cases.
Conclusion
We consider decompressive craniectomy for large hemispheric infarction as a life-saving procedure. Good preoperative GCS, late clinical deterioration, small size of the infarction area, absence of anisocoria, and preoperative midline shift less than 11mm were considered to be positive predictors of good outcome. Careful patient selection based on the above-mentioned factors and early operation may improve the functional outcome of surgical management for large hemispheric infarction.
Keywords: Acute cerebral infarction, Brain edema, Brain herniation, Decompressive craniectomy, Intracranial pressure
INTRODUCTION
Acute hemispheric cerebral infarction in the territory of the middle cerebral artery (MCA), and occasionally the anterior cerebral artery (ACA) and posterior cerebral artery (PCA) territories, may cause massive brain swelling that may be medically uncontrolled, known as malignant infarction2,6,9,12,18). Severe postischemic brain edema leading to raised intracranial pressure (ICP), brain herniation, clinical deterioration, coma, and death occurs in approximately 10-15% of patients with large hemispheric infarction13,20). Most early deaths due to large hemispheric infarction are associated with increased intracranial pressure (ICP) and subsequent uncal herniation. The death rate in patients who develop massive hemispheric infarction is as high as 80%, despite aggressive conservative care1,2,6,18). For the patients who suffer massive stroke and continue to deteriorate despite conservative treatment, decompressive surgery may be required. There have been many studies about the results of decompressive surgery3,5,8,9,14,16,19), which have sought several factors such as clinical findings, radiological findings, the degree of increased ICP, and the timing for decompressive surgery5,10,15,21). The goal of our study was to analyze the factors relating to the functional outcome of decompressive craniectomy.
MATERIALS AND METHODS
Our hospital policy dictates that all patients with ischemic stroke are admitted to an acute stroke unit under the care of the Neurology Department. In the event of neurological deterioration and radiological evidence of cerebral edema secondary to the cerebral infarct, neurosurgical consultation would be proceeded. In these settings, unless the patient had an overwhelmhening medical contraindication to surgery, a large decompressive craniectomy has been performed. This study included 24 patients (11 men and 13 women) in whom decompressive craniectomy was performed due to massive MCA infarction at the Department of Neurosurgery between January 2000 and December 2005. All patients were admitted within six hours of the onset of symptoms. Patients older than ninety years, patients with any previous disabling neurological diseases, and patients with extremely poor general conditions such as severe cardiac failure were excluded from this study. We collected data such as age, sex, stroke etiology, distribution of the infarction, affected hemispheric dominancy, presence of anisocoria, preoperative GCS score, functional recovery by GOS score, measurement of the midline shift at the septum pellucidum level, improvement of midline shift after decompressive craniectomy, and duration between the stroke and the operation.
The principal surgical technique was a large decompressive craniectomy with duroplasty. Briefly, a large Dandy skin incision and frontotemporoparietal craniectomy was performed. A large bone flap with a diameter of more than 12 cm of bone was removed. The temporal squama was rongeured out until the floor of the middle cranial fossa was exposed4) to prevent uncal herniation. The dura was opened in a cruciate fashion to allow the brain to expand outward. Duroplasty was then performed. Temporal lobectomy was performed in some cases, when necessary. Postoperatively, all patients were sent to the neurosurgical intensive care unit for close monitoring and management. The patients were mechanically ventilated to maintain mild hyperventilation and received medical management such as intravenous mannitol, steroids, diuretics, and sedative agents.
Using the Glasgow Outcome Scale, 24 patients with massive cerebral infarction were divided into two subgroups. The patients with a GOS score of 1 to 3 were included in the poor outcome group (Group 1), and patients with a GOS score of 4 to 5 were included in the good outcome group (Group 2). All data were expressed as mean±standard deviation. The results were analyzed for statistical significance using the chi-square (χ2) test, the Mann-Whitney rank sum (U) test, and discriminate analysis, with p< 0.005 considered as statistically significant. SPSS 12.0 KO for Windows Release 12.0.1 (SPSS Inc. Illinois, U.S.A) was used for statistical analysis.
RESULTS
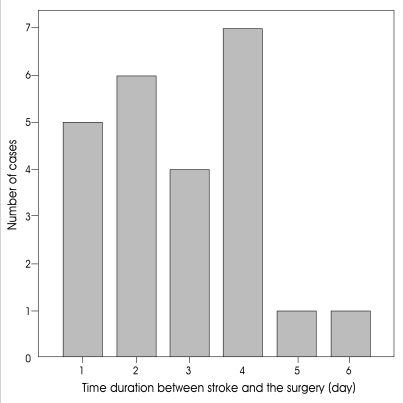
A total of 24 patients were included in this study over a six year period from January 2000. Among these 24 patients, 14 patients experienced long-term survival for a mean follow-up period of 19.3 months (range 9-36 months). Nine patients were mildly and moderately disabled, five patients were severely disabled, and ten out of twenty four patients died despite surgical treatment. There were eleven men and thirteen women whose mean age was 56 years (range, 28-77). Twenty-two patients among twenty-four underwent surgery within 96 hours of their stroke (range, 5-137 hours; median=55.5; mean±SD=58.6±34 hrs.) (Fig. 1). The preoperative GCS ranged from 4 to 11 (median=7.5, mean±SD=7.4±2 points), and the preoperative midline shift ranged from 8 to 21mm (median=11.8;mean±SD=12.4±2.9 mm) in this series.
Fig. 1.
The distribution of duration between stroke onset and surgery.
All patients had MCA infarction (Right MCA : 17 patients; Left MCA : 7 patients); 5 had an additional anterior or posterior cerebral artery territory infarction. Cardio-embolism and atherothrombosis were associated with stroke in 7 cases and 13 cases respectively. The etiology was remained unknown in 4 cases. The following risk factors were present in all patients : hypertension, atrial fibrillation, diabetes mellitus, and ischemic heart disease. There were no surgical complications directly related to the decompression procedure.
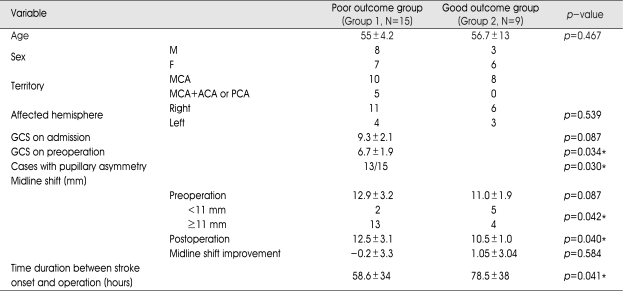
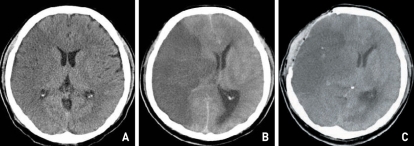
The mean GCS score on diagnosis of the infarction was 9.8±2.2 (7-15). The mean time duration from diagnosis to surgery was 58±34 hours (5-137 hours). The patient characteristics between two groups are listed in Table 1. Group 1 (the poor outcome group) consisted of 8 men and 7 women, whose mean age was 55 years (age ranged from 28 to 77 years). Group 2 (the good outcome group) consisted of 3 men and 6 women. The mean age was 56.7 years (age ranged from 39 to 72 years). In group 1 (N=15), during the follow-up period, 10 out of 15 patients died secondary to transtentorial herniation from increased intracranial pressure (Fig. 5). The remaining 5 patients were severely disabled. In group 2 (N=9), we were able to prevent secondary brain damage in all cases. Three patients were mildly disabled and six patients were moderately disabled (Fig. 6).
Table 1.
Comparison of patient characteristics between good and poor outcome groups in 24 patients with large hemispheric infarctions who underwent decompressive craniectomy
Fisher's exact test was used to compare the proportions of the categorical variables and the Mann Whitney U-test was used to compare continuous variables; *p≤0.05 was considered statistically significant; MCA : Middle cerebral artery, PCA : Posterior cerebral artery, ACA : Anterior cerebral artery
Fig. 5.
Case 7. Serial computerized tomography (CT) scan images of a 38-year-old man with right MCA infarction. A : Initial CT scan, showed minimal right MCA distribution hypodensity several hours after the acute onset of left hemiplegia. B : Preoperative CT scan, showed right MCA infarction and swelling. The midline shift was estimated at about 11.1 cm, and the preoperative Glasgow Coma Scale was seven. C : Progressing midline shift and swelling were seen on postoperative CT scan. The patient showed a unilateral fixed, dilated pupil. He died on third postoperative day.
Fig. 6.
Case 19. Serial computerized tomography (CT) scan images of a 39-year-old woman with MCA infarction. A : Normal CT scan obtained immediately after the symptom onset of left hemiparesis and clear mentality. B : Preoperative CT scan showed right MCA infarction with brain swelling. The midline shift was estimated at about 10.3 cm. The preoperative Glasgow Coma Scale was nine. C : After decompression, the postoperative CT scan showed demarcation of infarction, progressing midline shift and out-bulging through craniectomy site. D : Six months later, improvement of midline shift was seen. E : Postoperative CT scan after cranioplasty without complications. The operation was conducted ninety hours after the onset of symptoms. She survived with GCS score 14 and left hemiparesis.
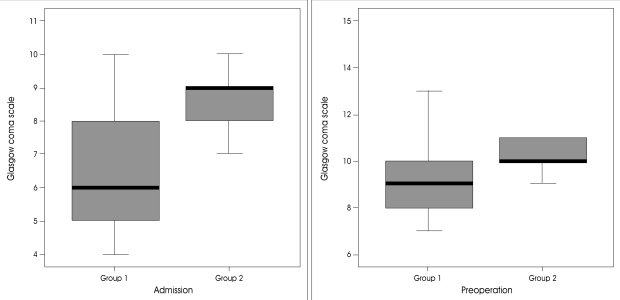
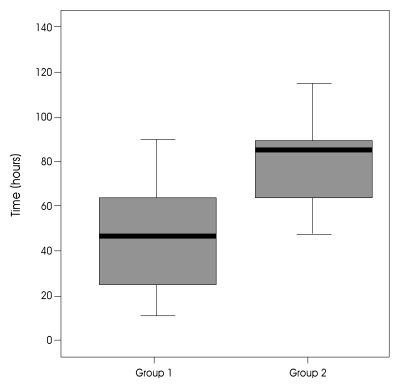
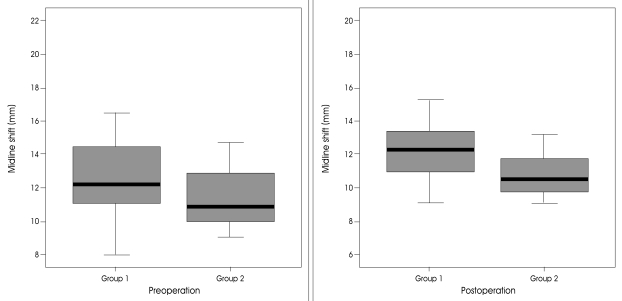
In group 1, the mean GCS score on admission was 9.3 (range 7-14). Except for two patients, they had fixed pupillary light reflexes or anisocorias. In group 2, the mean GCS score on admission was 10.9 (range 8-15) and the preoperative GCS score was 8.6 (range 6-11) (Fig. 3). The mean time interval between symptom onset and operation was 58.6 hours in group 1 and 78.5 hours in group 2 (Fig. 4). The preoperative midline shift of the good outcome group was not significantly less than that of the poor outcome group (p=0.174). But, when 11 mm was chosen as the cut-off point, it was observed that most of patients in the good outcome group had a midline shift less than 11mm (p=0.042) (Fig. 2). The preoperative GCS scores between the two groups were significantly different (p=0.030). In the poor outcome group, the time duration between stroke onset and operation was shorter than that of the good outcome group (58.6±34 vs. 78.5±38 p=0.041). In the poor outcome group, the frequency of preoperative anisocoria was significantly higher (p=0.003). While the frequency of ACA and PCA involvement was higher in the poor outcome group, the difference was not statistically significant (p=0.130). There was no additional ACA or PCA infarction in the good outcome group (Table 1). Other factors including gender, hemispheric dominance, and stroke etiology were similar between the two groups, and there were no significant statistical differences.
Fig. 3.
The distribution of Glasgow Coma Scale on admission and preoperative Glasgow Coma Scale in good outcome and poor outcome groups.
Fig. 4.
The distribution of time interval between symptom onset and operation in good outcome and poor outcome groups.
Fig. 2.
The distribution of midline shift of preoperative and postoperative states in good outcome and poor outcome groups.
DISCUSSION
Over the past 15 years, several studies have shown that decompression surgery is a possible treatment strategy for otherwise medically uncontrollable ICP after massive cerebral infarction3,4,9,16). Surgical decompression seems to be effective in lowering increased ICP and preventing transtentorial herniation. The clinical course of massive hemispheric infarction is typical : a space-occupying mass effect develops rapidly, and the patients deteriorate within the first 2-4 days19). In accordance with the literature, most of the patients in the current series exhibited neurological decline and underwent surgery within the first 4 days after the stroke onset. Many authors propose early intervention before clinical detorioration or appearance of stem injury sign because of mesencephalic ischemia on brain stem3,6,11,18,19). However, a few "late" cases in our series required surgery after 4 days (Fig. 1). From our observation, patients with rapid clinical decline in the early stage of their stroke seemed to have a poor prognosis. In fact, in the poor outcome group, the time duration between stroke onset and operation was shorter than in that of the good outcome group (58 vs. 78 hours) (Fig. 4). This difference showed statistical significance (p=0.041). This difference likely stems from the severity of the infarction, leading to early intervention, and our surgical timing of decompressive surgery, rather than any beneficial effects of late operation. Moreover, early decompressive surgery before signs of herniation has been shown to be superior to delayed surgery in terms of functional outcome19).
Large hemispheric infarction has been associated primarily with atherothrombosis, and secondarily with cardioembolism7,19). We found that these two diseases had almost equal frequency as an etiologic factor. Furthermore, there was no difference of frequency between the good and poor outcome groups. In the current series, the patients with large infarctions including the ACA and PCA territories were more likely to have a poor prognosis, as expected. None of the 9 patients with additional infarction territories was included in the good outcome group. However, the p value was just below the level of statistical significance (p=0.071). This was probably due to the relatively small sample size.
The timing of decompressive surgery has been debated. Very early intervention may lead to unnecessary surgery for the patient who can recover with conservative management. On the other hand, if the decompressive surgery is performed too late, the risk of irreversible damage to the brain stem due to herniation could worsen the outcome. Therefore, patients should be closely observed during medical treatment, and surgical intervention can then be performed if necessary.
The level of consciousness as determined by objective measurement, the amount of midline shift on CT, and the existence of anisocoria should dictate the necessity and timing of surgical intervention. We found that the preoperative GCS score in the good outcome group (8.5) was significantly greater than that in the poor outcome group (6.7) (p=0.030). Severe midline shift over 11 mm was a positive predictor of a poor functional outcome (p=0.042). Similarly, the frequency of anisocoria was significantly higher in the poor outcome group (p=0.03), suggesting that surgery should be performed before pupillary dilatation.
In our study, there were four patients and three patients with left hemisphere involvement in the poor outcome group (N=15) and good outcome group (N=9), respectively. Its difference was just below statistical significance (p=0.539). Thus, we believe that dominant hemispheric infarction should not be considered as an exclusion criterion when deciding to perform this life-saving operation9,17,19).
CONCLUSION
We consider decompressive craniectomy for space occupying large hemispheric infarction as a life-saving procedure that can provide good functional outcomes in selected cases. Our study results indicate that poor preoperative GCS score, the presence of anisocoria, early clinical deterioration and MCA infarction accompanied by additional infarction are considered as the predictors of a poor outcome. Left hemispheric infarction, or dominant hemispheric infarction should not be taken as an exclusion criterion when deciding whether to perform a decompressive operation. In overall, careful patient selection and early operation may improve the functional outcome of surgical management for large hemispheric infarction.
Acknowledgement
This work was supported by Grant from Inje University, 2007.
References
- 1.Berrouschot J, Sterker M, Bettin S, Koster J, Schneider D. Mortality of space-occupying ('malignant') middle cerebral artery infarction under conservative intensive care. Intensive Care Med. 1998;24:620–623. doi: 10.1007/s001340050625. [DOI] [PubMed] [Google Scholar]
- 2.Bounds JV, Wiebers DO, Whisnant JP, Okazaki H. Mechanisms and timing of deaths from cerebral infarction. Stroke. 1981;12:474–477. doi: 10.1161/01.str.12.4.474. [DOI] [PubMed] [Google Scholar]
- 3.Carter BS, Ogilvy CS, Candia GJ, Rosas HD, Buonanno F. One-year outcome after decompressive surgery for massive nondominant hemispheric infarction. Neurosurgery. 1997;40:1168–1175. doi: 10.1097/00006123-199706000-00010. discussion 1175-1176. [DOI] [PubMed] [Google Scholar]
- 4.Delashaw JB, Broaddus WC, Kassell NF, Haley EC, Pendleton GA, Vollmer DG, et al. Treatment of right hemispheric cerebral infarction by hemicraniectomy. Stroke. 1990;21:874–881. doi: 10.1161/01.str.21.6.874. [DOI] [PubMed] [Google Scholar]
- 5.Ganesan V, Ng V, Chong WK, Kirkham FJ, Connelly A. Lesion volume, lesion location, and outcome after middle cerebral artery territory stroke. Arch Dis Child. 1999;81:295–300. doi: 10.1136/adc.81.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R. 'Malignant' middle cerebral artery territory infarction : clinical course and prognostic signs. Arch Neurol. 1996;53:309–315. doi: 10.1001/archneur.1996.00550040037012. [DOI] [PubMed] [Google Scholar]
- 7.Heinsius T, Bogousslavsky J, Van Melle G. Large infarcts in the middle cerebral artery territory. Etiology and outcome patterns. Neurology. 1998;50:341–350. doi: 10.1212/wnl.50.2.341. [DOI] [PubMed] [Google Scholar]
- 8.Holtkamp M, Buchheim K, Unterberg A, Hoffmann O, Schielke E, Weber JR, et al. Hemicraniectomy in elderly patients with space occupying media infarction : improved survival but poor functional outcome. J Neurol Neurosurg Psychiatry. 2001;70:226–228. doi: 10.1136/jnnp.70.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalia KK, Yonas H. An aggressive approach to massive middle cerebral artery infarction. Arch Neurol. 1993;50:1293–1297. doi: 10.1001/archneur.1993.00540120010005. [DOI] [PubMed] [Google Scholar]
- 10.Koh MS, Goh KY, Tung MY, Chan C. Is decompressive craniectomy for acute cerebral infarction of any benefit? Surg Neurol. 2000;53:225–230. doi: 10.1016/s0090-3019(00)00163-4. [DOI] [PubMed] [Google Scholar]
- 11.Kondziolka D, Fazl M. Functional recovery after decompressive craniectomy for cerebral infarction. Neurosurgery. 1988;23:143–147. doi: 10.1227/00006123-198808000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Krieger DW, Demchuk AM, Kasner SE, Jauss M, Hantson L. Early clinical and radiological predictors of fatal brain swelling in ischemic stroke. Stroke. 1999;30:287–292. doi: 10.1161/01.str.30.2.287. [DOI] [PubMed] [Google Scholar]
- 13.Moulin DE, Lo R, Chiang J, Barnett HJ. Prognosis in middle cerebral artery occlusion. Stroke. 1985;16:282–284. doi: 10.1161/01.str.16.2.282. [DOI] [PubMed] [Google Scholar]
- 14.Oppenheim C, Samson Y, Manai R, Lalam T, Vandamme X, Crozier S, et al. Prediction of malignant middle cerebral artery infarction by diffusion-weighted imaging. Stroke. 2000;31:2175–2181. doi: 10.1161/01.str.31.9.2175. [DOI] [PubMed] [Google Scholar]
- 15.Park SQ, Yun IG, Kim BT, Doh JW, Bae HG, Lee KS, et al. Surgical Indications of Decompressive Craniectomy for Middle Cerebral Artery Infarction. J Korean Neurosurg Soc. 1999;28:1588–1593. [Google Scholar]
- 16.Rengachary SS, Batnitzky S, Morantz RA, Arjunan K, Jeffries B. Hemicraniectomy for acute massive cerebral infarction. Neurosurgery. 1981;8:321–328. doi: 10.1227/00006123-198103000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Rieke K, Schwab S, Krieger D, von Kummer R, Aschoff A, Schuchardt V, et al. Decompressive surgery in space-occupying hemispheric infarction : results of an open, prospective trial. Crit Care Med. 1995;23:1576–1587. doi: 10.1097/00003246-199509000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Ropper AH, Shafran B. Brain edema after stroke. Clinical syndrome and intracranial pressure. Arch Neurol. 1984;41:26–29. doi: 10.1001/archneur.1984.04050130032017. [DOI] [PubMed] [Google Scholar]
- 19.Schwab S, Steiner T, Aschoff A, Schwarz S, Steiner HH, Jansen O, et al. Early hemicraniectomy in patients with complete middle cerebral artery infarction. Stroke. 1998;29:1888–1893. doi: 10.1161/01.str.29.9.1888. [DOI] [PubMed] [Google Scholar]
- 20.Silver FL, Norris JW, Lewis AJ, Hachinski VC. Early mortality following stroke : a prospective review. Stroke. 1984;15:492–496. doi: 10.1161/01.str.15.3.492. [DOI] [PubMed] [Google Scholar]
- 21.Steiger HJ. Outcome of acute supratentorial cerebral infarction in patients under 60. Development of a prognostic grading system. Acta Neurochir (Wien) 1991;111:73–79. doi: 10.1007/BF01400491. [DOI] [PubMed] [Google Scholar]