Abstract
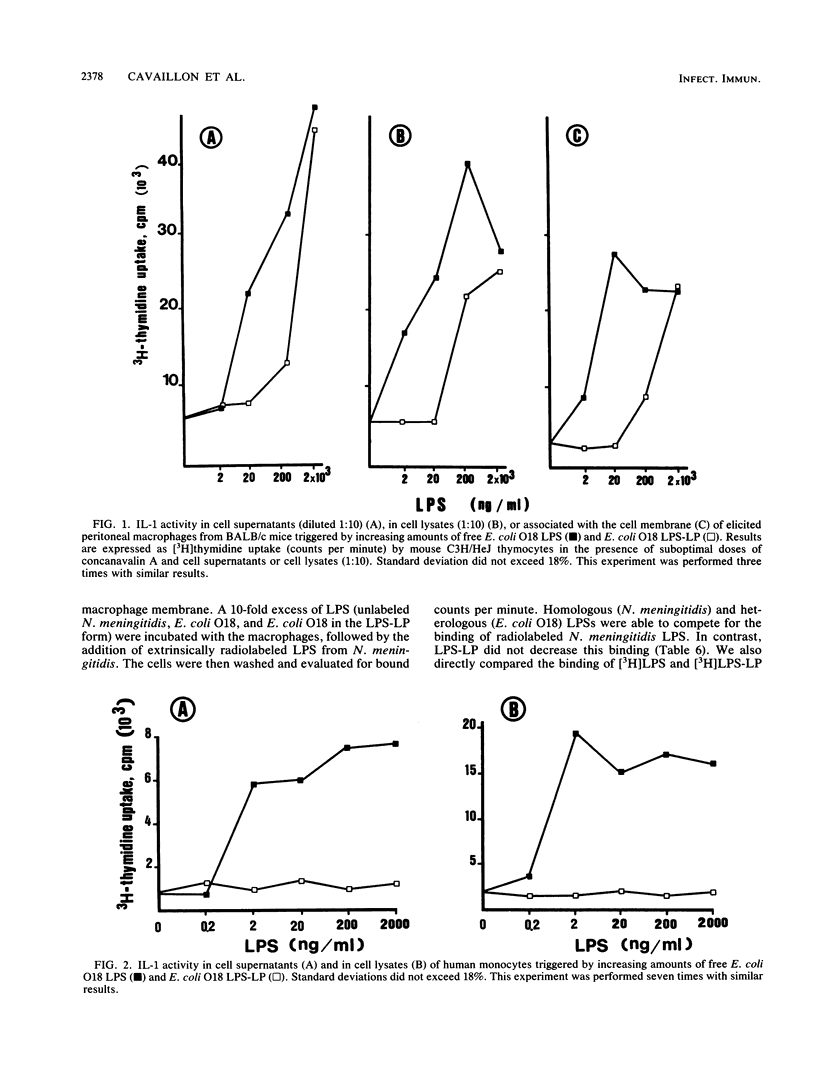
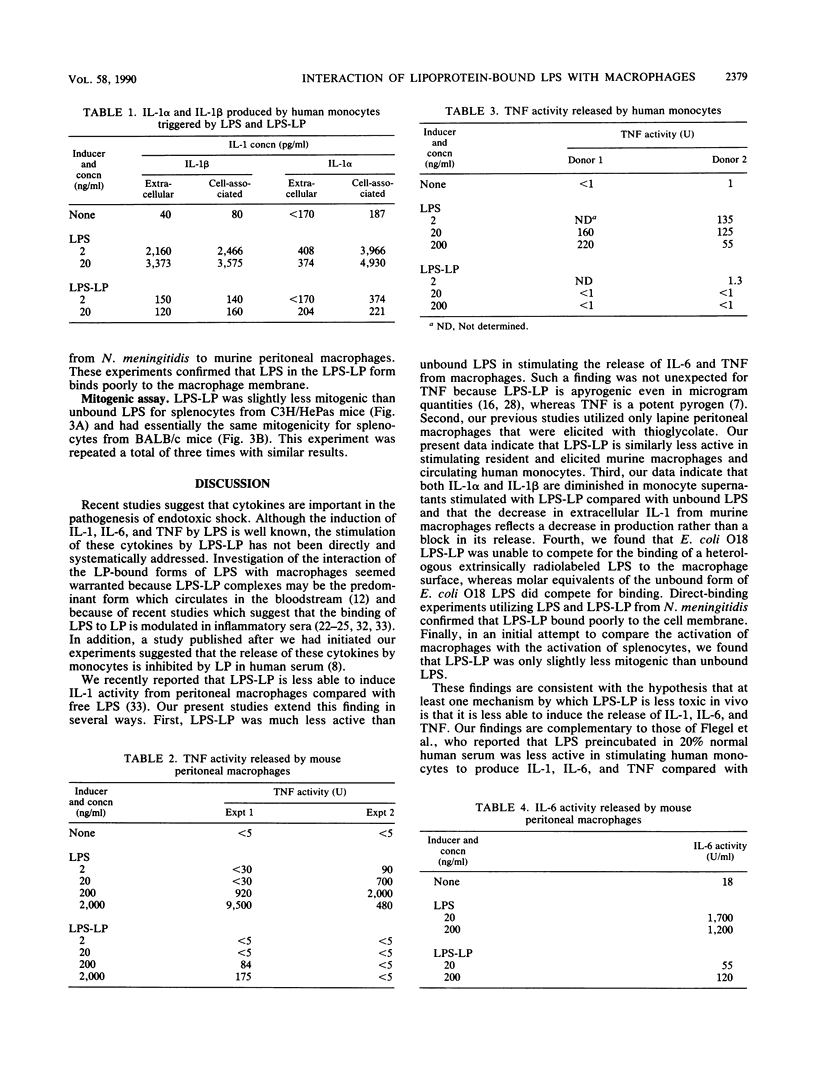
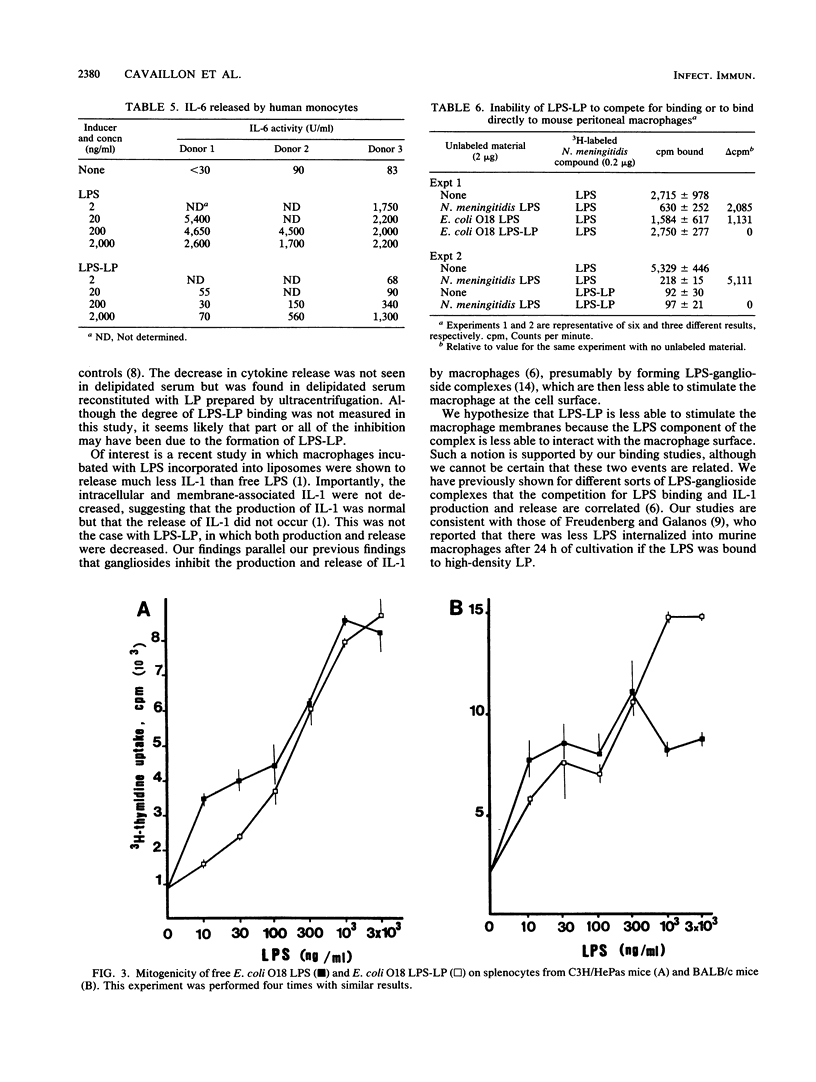
Recent evidence suggests that bacterial lipopolysaccharide binds to serum lipoproteins in vitro and in vivo and that lipopolysaccharide in the form that is bound to lipoprotein is less biologically active in several experimental models. In order to study the mechanism of this apparent detoxification, we compared the ability of free and lipoprotein-bound lipopolysaccharide from Escherichia coli O18 to stimulate interleukin-1, interleukin-6, and tumor necrosis factor from elicited murine peritoneal macrophages and circulating human monocytes. Lipopolysaccharide bound to lipoprotein was 20- to 1,000-fold less active than the unbound form in inducing the release of each cytokine. We also studied the binding of each form of lipopolysaccharide to the macrophage surface. Lipopolysaccharide complexed to lipoprotein was unable to compete for the binding of radiolabeled heterologous lipopolysaccharide to murine macrophages, and radiolabeled lipopolysaccharide-lipoprotein complexes bound poorly compared with molar equivalents of free lipopolysaccharide. Our experiments suggest that in the process of binding to lipoproteins, lipopolysaccharide may be rendered less toxic through a mechanism of decreased ability to induce monocytes and macrophages to release cytokines, perhaps because of an altered interaction at the cell surface.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakouche O., Koff W. C., Brown D. C., Lachman L. B. Interleukin 1 release by human monocytes treated with liposome-encapsulated lipopolysaccharide. J Immunol. 1987 Aug 15;139(4):1120–1126. [PubMed] [Google Scholar]
- Beutler B. A., Milsark I. W., Cerami A. Cachectin/tumor necrosis factor: production, distribution, and metabolic fate in vivo. J Immunol. 1985 Dec;135(6):3972–3977. [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Caroff M., Cavaillon J. M., Fitting C., Haeffner-Cavaillon N. Inability of pyrogenic, purified Bordetella pertussis lipid A to induce interleukin-1 release by human monocytes. Infect Immun. 1986 Nov;54(2):465–471. doi: 10.1128/iai.54.2.465-471.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaillon J. M., Fitting C., Caroff M., Haeffner-Cavaillon N. Dissociation of cell-associated interleukin-1 (IL-1) and IL-1 release induced by lipopolysaccharide and lipid A. Infect Immun. 1989 Mar;57(3):791–797. doi: 10.1128/iai.57.3.791-797.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaillon J. M., Fitting C., Hauttecoeur B., Haeffner-Cavaillon N. Inhibition by gangliosides of the specific binding of lipopolysaccharide (LPS) to human monocytes prevents LPS-induced interleukin-1 production. Cell Immunol. 1987 May;106(2):293–303. doi: 10.1016/0008-8749(87)90173-0. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M., Bernheim H. A., Beutler B., Cerami A., Figari I. S., Palladino M. A., Jr, O'Connor J. V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegel W. A., Wölpl A., Männel D. N., Northoff H. Inhibition of endotoxin-induced activation of human monocytes by human lipoproteins. Infect Immun. 1989 Jul;57(7):2237–2245. doi: 10.1128/iai.57.7.2237-2245.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg M. A., Galanos C. The metabolic fate of endotoxins. Prog Clin Biol Res. 1988;272:63–75. [PubMed] [Google Scholar]
- Haeffner-Cavaillon N., Cavaillon J. M., Etievant M., Lebbar S., Szabo L. Specific binding of endotoxin to human monocytes and mouse macrophages: serum requirement. Cell Immunol. 1985 Mar;91(1):119–131. doi: 10.1016/0008-8749(85)90037-1. [DOI] [PubMed] [Google Scholar]
- Haeffner-Cavaillon N., Cavaillon J. M., Moreau M., Szabó L. Interleukin 1 secretion by human monocytes stimulated by the isolated polysaccharide region of the Bordetella pertussis endotoxin. Mol Immunol. 1984 May;21(5):389–395. doi: 10.1016/0161-5890(84)90036-1. [DOI] [PubMed] [Google Scholar]
- Mathison J. C., Ulevitch R. J. In vivo interaction of bacterial lipopolysaccharide (LPS) with rabbit platelets: modulation by C3 and high density lipoproteins. J Immunol. 1981 Apr;126(4):1575–1580. [PubMed] [Google Scholar]
- Morrison D. C., Brown D. E., Vukajlovich S. W., Ryan J. L. Ganglioside modulation of lipopolysaccharide-initiated complement activation. Mol Immunol. 1985 Oct;22(10):1169–1176. doi: 10.1016/0161-5890(85)90005-7. [DOI] [PubMed] [Google Scholar]
- Munford R. S., Hall C. L., Dietschy J. M. Binding of Salmonella typhimurium lipopolysaccharides to rat high-density lipoproteins. Infect Immun. 1981 Dec;34(3):835–843. doi: 10.1128/iai.34.3.835-843.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munford R. S., Hall C. L., Lipton J. M., Dietschy J. M. Biological activity, lipoprotein-binding behavior, and in vivo disposition of extracted and native forms of Salmonella typhimurium lipopolysaccharides. J Clin Invest. 1982 Oct;70(4):877–888. doi: 10.1172/JCI110684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novitsky T. J., Roslansky P. F., Siber G. R., Warren H. S. Turbidimetric method for quantifying serum inhibition of Limulus amoebocyte lysate. J Clin Microbiol. 1985 Feb;21(2):211–216. doi: 10.1128/jcm.21.2.211-216.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riveau G. R., Novitsky T. J., Roslansky P. F., Dinarello C. A., Warren H. S. Role of interleukin-1 in augmenting serum neutralization of bacterial lipopolysaccharide. J Clin Microbiol. 1987 May;25(5):889–892. doi: 10.1128/jcm.25.5.889-892.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo D., Girard A., Rothfield L. Reconstitution of a functional membrane enzyme system in a monomolecular film. I. Formation of a mixed monolayer of lipopolysaccharide and phospholipid. J Mol Biol. 1970 Nov 14;53(3):475–490. doi: 10.1016/0022-2836(70)90078-1. [DOI] [PubMed] [Google Scholar]
- Rudbach J. A., Akiya F. I., Elin R. J., Hochstein H. D., Luoma M. K., Milner E. C., Milner K. C., Thomas K. R. Preparation and properties of a national reference endotoxin. J Clin Microbiol. 1976 Jan;3(1):21–25. doi: 10.1128/jcm.3.1.21-25.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesh V. L., Morrison D. C. The physical-chemical characterization and biologic activity of serum released lipopolysaccharides. J Immunol. 1988 Nov 15;141(10):3523–3531. [PubMed] [Google Scholar]
- Tobias P. S., McAdam K. P., Soldau K., Ulevitch R. J. Control of lipopolysaccharide-high-density lipoprotein interactions by an acute-phase reactant in human serum. Infect Immun. 1985 Oct;50(1):73–76. doi: 10.1128/iai.50.1.73-76.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias P. S., McAdam K. P., Ulevitch R. J. Interactions of bacterial lipopolysaccharide with acute-phase rabbit serum and isolation of two forms of rabbit serum amyloid A. J Immunol. 1982 Mar;128(3):1420–1427. [PubMed] [Google Scholar]
- Tobias P. S., Soldau K., Ulevitch R. J. Isolation of a lipopolysaccharide-binding acute phase reactant from rabbit serum. J Exp Med. 1986 Sep 1;164(3):777–793. doi: 10.1084/jem.164.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias P. S., Ulevitch R. J. Control of lipopolysaccharide-high density lipoprotein binding by acute phase protein(s). J Immunol. 1983 Oct;131(4):1913–1916. [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Tucker S. B., Pierre R. V., Jordon R. E. Rapid identification of monocytes in a mixed mononuclear cell preparation. J Immunol Methods. 1977;14(3-4):267–269. doi: 10.1016/0022-1759(77)90137-5. [DOI] [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R. The modification of biophysical and endotoxic properties of bacterial lipopolysaccharides by serum. J Clin Invest. 1978 Dec;62(6):1313–1324. doi: 10.1172/JCI109252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R., Weinstein D. B. New function for high density lipoproteins. Their participation in intravascular reactions of bacterial lipopolysaccharides. J Clin Invest. 1979 Nov;64(5):1516–1524. doi: 10.1172/JCI109610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Snick J., Cayphas S., Vink A., Uyttenhove C., Coulie P. G., Rubira M. R., Simpson R. J. Purification and NH2-terminal amino acid sequence of a T-cell-derived lymphokine with growth factor activity for B-cell hybridomas. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9679–9683. doi: 10.1073/pnas.83.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waage A., Brandtzaeg P., Halstensen A., Kierulf P., Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med. 1989 Jan 1;169(1):333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren H. S., Knights C. V., Siber G. R. Neutralization and lipoprotein binding of lipopolysaccharides in tolerant rabbit serum. J Infect Dis. 1986 Nov;154(5):784–791. doi: 10.1093/infdis/154.5.784. [DOI] [PubMed] [Google Scholar]
- Warren H. S., Riveau G. R., de Deckker F. A., Chedid L. A. Control of endotoxin activity and interleukin-1 production through regulation of lipopolysaccharide-lipoprotein binding by a macrophage factor. Infect Immun. 1988 Jan;56(1):204–212. doi: 10.1128/iai.56.1.204-212.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J., Riblet R. Genetic control of responses to bacterial lipopolysaccharides in mice. II. A gene that influences a membrane component involved in the activation of bone marrow-derived lymphocytes by lipipolysaccharides. J Immunol. 1975 May;114(5):1462–1468. [PubMed] [Google Scholar]


