Abstract
Objective
The length of anterior-posterior commissure (AC-PC) in racial groups, age, gender of patients with deep brain stimulation (DBS) and pallidotomy were investigated.
Methods
From January 1996 to December 2003, 211 patients were treated with DBS and pallidotomy. There were 160 (76%) Caucasians, 35 (17%) Hispanics, 12 (5%) Asians and 4 Blacks (2%). There were 88 males and 52 females in DBS-surgery group and 44 males, 27 females in pallidotomy group. Mean age was 58 year-old. There were 19 males and 19 females and mean age was 54.7 years in the control group. Measurements were made on MRI and @Target software.
Results
The average AC-PC distance was 24.89 mm (range 32 to 19), which increased with aging until 75 years old in Caucasian and also increased with aging in Hispanic, but the AC-PC distance peaked at 45 years old in Hispanic. The order of AC-PC distance were 25.2±2 mm in Caucasian, 24.6±2.24 mm in Asian, 24.53 mm in Black, 23.6±1.98 mm in Hispanic. The average AC-PC distance in all groups was 24.22 mm in female who was mean age of 56.35, 25.28 mm in male who was mean age of 60.19 and 24.5±2 mm in control group that was excluded because of the difference of thickness of slice. According to multiple regression analysis, the AC-PC distance was significantly correlated with age, race, and gender.
Conclusion
The AC-PC distance is significantly correlated with age, gender, and race. The atlas of functional stereotaxis would be depended on the variation of indivisual brain that can influenced by aging, gender, and race.
Keywords: Pallidotomy, Anterior commissure, Posterior commissure
INTRODUCTION
There are some differences in size and shape of the skull among races, and age as well as gender. We compared the length of anterior-posterior commissure (AC-PC) depending on the racial groups, age, and gender of patients with deep brain stimulator (DBS) and pallidotomy.
Several centers have employed surgical planning techniques which were already used successfully several decades ago, in particular ventriculography in conjunction with brain atlases14,16,22,23,25). In other centers, CT- and, more recently, MRI-based planning techniques with fused each images have been established for avoiding the risk of geometric inaccuracy due to various image distortion phenomena8,27,29,33,35,38).
History of measurements of AC-PC
Since the transition from animal neurophysiological experiments to the clinical use of stereotaxy in human was made by Spiegel and Wycis in the spring of 1947, neurosurgeons were able to solve the problem of variability in the relation between external skull landmark and intracerebral target by the use of intracerebral reference points proved to be invaluable in the development of human stereotaxis21). Talairach published two atlases34), L'atlas d'anatomie stĺéréotaxique in 1957 and L'exploration stĺéréotaxique du téléncephale in 196726). Talairach and his colleagues also noted that, although cerebral structures do not always have a constant relationship to skull landmarks, the AC-PC line could serve as an accurate guide for identification of intracerebral nuclei. Two years later, the atalas of the human brain by Schaltenbrand and Bailey (1959) simplified this system by taking as axes the AC-PC line, lying in the median (sagittal) plane, and a perpendicular erected in the middle point between the two commissure32).
Modern way of measurements and variability of AC-PC line
Since Brown (1979) was the first to describe and presented a prototype stereotactic frame for use in the CT scanner, Lars Leksell later became the first stereotactic neurosurgeon to adapt his system to utilize CT and MR images. Magnetic Resonance Imaging (MRI) has replaced the usage of CT because it provides a good demonstration of anatomy, permitting direct visualization of the AC-PC line and target. In an effort to overcome the spatial distortion on MR imaging, technique of imaging fusion with CT by using image correlation software on a workstation has been introduced8).
Microelectrode recording has been used to compliment neuroimaging in movement disorder and the update machine like 3.0 tesler MRI has been introduced for better resolution of the nuclei in the brain but there has been still much controversy concerning the reliability of the recording and target of the specific object in the MRI environment36).
MATERIALS AND METHODS
Patients
A total of 211 cases among patients who were treated with deep brain stimulator and pallidotomy for the treatment of movement disorder and pain in the MRI operating room at University of California, Los Angels (UCLA) from January 1996 to December 2003 were reviewed retrospectively to compare the AC-PC distance with age, race, and gender. For the control group, we measured the AC-PC distance of 38 patients with trigeminal neuralgia without intraparenchymal abnormality by Brainlab software (Novalis® from BrainLAB inc., Heimstteten, Germany). There were 160 (76%) Caucasian, 35 (17%) Hispanic, 12 (5%) Asian and 4 (2%) black patients. There were 140 patients with DBS and 71 patients with pallidotomy. Mean age was 58 years old (range; 6.9-85.8). Among the Caucasian, there were 91 males and 69 females. Among the Hispanic, 28 were males and 7 females. Among the Asian, there were 10 males and 2 females. Among the black patients, male were 3 in number and 1 female. In the DBS-surgery group there were 97 patients with Parkinson diseases, 29 patients with essential tremors, 7 patients with dystonia, 3 patients with cerebral palsies and 4 patients with other diagnoses. In the pallidotomy-group there were 51 patients with Parkinson diseases, 4 patients with dystonia, and 11 patients with cerebral palsy and 5 patients with other diagnoses. In the control group, the mean ages was 54.7 years and male were 19 among 38 patients.
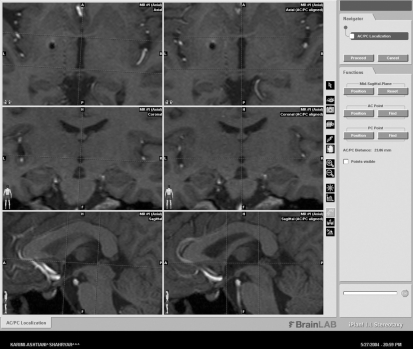
The length of AC-PC lines were obtained through the same sequenced MRI by one specialist using @Target Brain lab software (Fig. 1). However, the AC-PC distance of trigeminal patients was excluded for a statistical analysis because the AC-PC distance of them was measured on 3 mm slice thickness-MRI beyond brain stem-trigeminal nerve level on 1 mm slice thickness-MRI. Nevertheless the exclusion of control group, we were able to make reference to the data.
Fig. 1.
AC-PC distance is calculated through the axial and sagittal images. In axial view, the anterior commissure has the shape of bicycle handlebars, coursing posteriorly, inferiorly and laterally behind the head of caudate nucleus and passes into nucleus of the globus pallidus into the inferior and middle temporal gyri. The anterior commissure is easily recognizable in all plane, at least the posterior commissure and anterior margin of the pineal region were closely related to the superior colliculi as well as the uppermost point of the aqueduct of sylvius3).
How to calculate the length of AC-PC
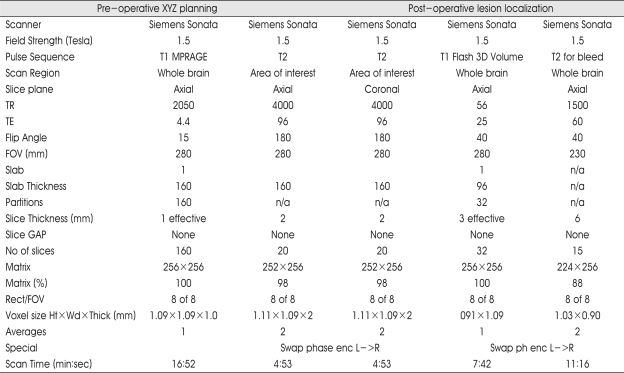
A Leksell stereotactic frame (Elekta, Atlanta, GA) was attached to the patient's head under sterile conditions with local and IV anesthesia in the MRI suite. It was adjusted parallel to the infra-orbito meatal line (Reid's line) to approximate the axial plane of the stereotactic frame to the AC-PC plane of the patient. The 1.5 Tesla MRI (Siemens, Erlangen, Germany) series were obtained by using the Parameters (Table 1). Both T1WI- and T2WI-Images were transformed with the Windows NT-based Brainlab software (Novalis® from BrainLAB inc., Heimstteten, Germany) and also combined with the preoperative T1-weighted MR images and CT scans. Thereafter, AC and PC were identified on T1WI-, T2WI-MRI, and CT scans. In control group, from those who underwent the stereotactic radiosurgery for the treatment of trigeminal neuralgia, the AC-PC distance were measured with the MRI (3 mm slice thickness) (Fig. 1).
Table 1.
Magnetic resonance parameters for pre- and post-operative functional neurosurgery planning
T1W : T1 weighted, T2W : T2 weighted, MPRAGE : Magnetization prepared rapid acquisition gradient echo
Statistical analysis
Analysis was performed with SPSS using the multiple regression analysis as indicated the correlation with variables. Mean and standard deviation of AC-PC distance were compared using ANOVA and t-test.
RESULTS
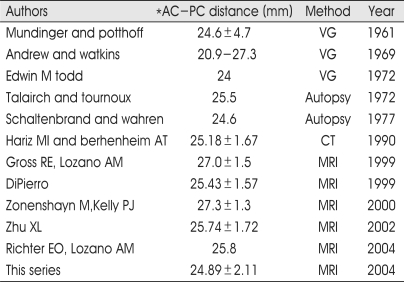
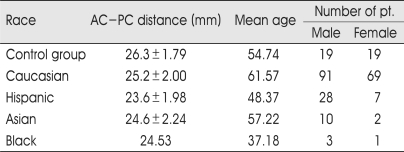
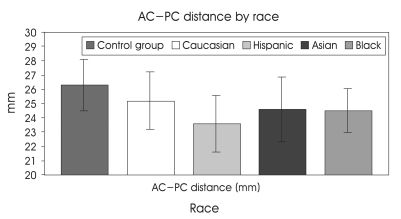
Notably, there was high individual variability of AC-PC distance in our series that is well comparable with standard stereotactic atlases and other previously published studies, particularly with two large series in which MRI and ventriculography have been employed (Table 2). In Table 3 and Fig. 2, the values of AC-PC distance for several variables (age, gender, races; Caucasian, Hispanic, Asian, Black) are summarized and showed. The AC-PC distance increases with aging but decreases in females. Race-related variations have also been detected. The variations show that the Caucasian, Asian, Black, and Hispanic are put in order of the distance decreasing. Black race did not have a stastically significant correlation because the number of patients was only 4. The average AC-PC distance among whole groups was 24.22 mm in female whose mean age were 56.35, 25.28 mm in male whose mean age was 60.19.
Table 2.
AC-PC distance from different sources
VG ventriculography, *from center of anterior commissure to center of posterior commissure
Table 3.
Race-specific analysis of AC-PC distance
Fig. 2.
The vertical line mans standard deviation and the control group is excluded for multiple regression analysis.
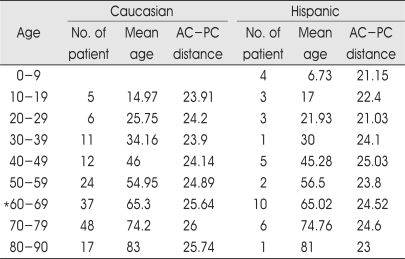
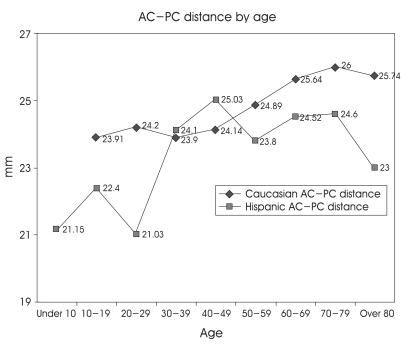
In Table 4, age-related variations have been detected as the strongest variable. The AC-PC distance increases with aging until 75 years old in Caucasian and also increases with aging in Hispanic, but the AC-PC distance peaked at 45 years old in Hispanic (Fig. 3). The data show the AC-PC distance of 37 Caucasians and 10 Hispanics in sixties (mean age; 65 years old). The AC-PC distance was 1.12 mm bigger in Caucasians (p> 0.057). There were 24 males in Caucasian group and 7 males in Hispanic group. The proportion of gender between Caucasian and Hispanic was almost same (male:female=2:1). The etiology was Parkinson disease in 10 Hispanic patients and 30 Parkinson diseases, 5 essential tremors, 1 dystonia, and 1 spasmodic torticollis in 37 Caucasian patients. Otherwise, the mean AC-PC distance of 26 Caucasian male patients in sixties was 26.11 mm whereas that of 7 Hispanic male patients in sixties was 24.86 mm but the AC-PC distance of males among sixties between Caucasian and Hispanic was not also statistically significant (p> 0.05), and the mean AC-PC distance was 24.7 mm in 12 females who were sixties among Caucasian patients. Aforementioned result also has no stastical difference compared with 7 Hispanic male patients (p> 0.3).
Table 4.
Age-specific analysis between caucasian and hispanic
*The AC-PC difference of sixties between caucasian and hispanic was not stastically significant (p>0.05), the AC-PC distance of males among sixties between caucasian and hispanic was not stastically significant (p>0.05)
Fig. 3.
Age-specific differences in the AC-PC distance between caucasian and hispanic. The AC-PC distance increases with aging, until 75 years old in caucasian but increases until 45 years old in hispanic.
In pallidotomy-group among the Caucasian and Hispanic, gender-related variations have been detected. In males, the AC-PC distance was 25±1.7 mm, 23±1.2 mm in females in Caucasian patients and in Hispanic patients were 23±1.7 mm in male, and 22±2.2 mm in female.
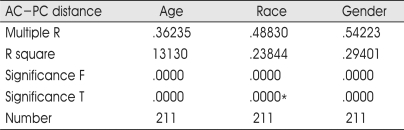
Among the whole 211 patients, multiple regression analysis was also performed to the AC-PC distance. These results imply the AC-PC distance was most heavily correlated with age-predictor. The AC-PC also has significant correlation with gender and race (Table 5).
Table 5.
Multiple regression analysis of the AC-PC distance as dependable variable in the all patients
*p=0.12226, 0.0003 : Black, Asian among races
DISCUSSION
In this study, the AC-PC distance with race, age, and gender of patients has been investigated horizontally, not longitudinally in each individual. With few exceptions, the MRI data were obtained from patients with movement disorder. To what extent this chronic disease and an early-developed disease such as cerebral palsy have been compared with trigeminal neuralgia that was defined without a pathologic condition in intracerebral structure. However, the AC-PC distance of the control group might be bigger than that of DBS and pallidotomy-groups because MRI were undertaken with 3 mm slice thickness at the level of AC-PC line. This was reason why we excluded the control group for stastical analysis.
To review the error of MR images in the field of stereotactic functional neurosurgery, motion artifact and image distortion have been solved by IV anesthesia and fused technique with software. Several quantitative studies have been published in clinical studies comparing MRI with CT or ventriculography6,9,15,16,18,19,22,23,25,28,33). The AC-PC distance has been defined in T1WI-MRI (1 mm slice thickness) since this has allowed for best definition of the commissure. In addition, this sequence did not reveal relevant image distortion and coordinates determined for AC and PC did not differ significantly from CT-derived coordinates7). Whether the targeting has directly been done or not, the AC-PC line became the essential landmark for the localization of the neuroanatomical target in the basal ganglia and diencephalons for relating them to stereotactic atlases. In our series, the AC-PC distance was average 24. 89 mm and its range was 19 to 32 mm. It seems that the AC-PC distance was smaller and wider range than other studies that might be caused by the prevalence of younger and Hispanic patients in California compare with in other medical centers. Our studies have shown that the AC-PC distance was stastically correlated with race, and gender as well as age. The AC-PC distance increased with age and male, and in racial order of the AC-PC distance were Caucasian, Asian, Black, Hispanic and the AC-PC distance showed a significant correlation except the Black race because Black patients were only 4 as a variable. Our data also demonstrated that the AC-PC distance was most seriously influenced by age-predictor. Even though the AC-PC distance of Caucasian was longer than other races and that of male was bigger than female, there was no statistical difference between races, gender if age as the best predictor was situated under same circumstance.
The brain length was correlated with the AC-PC distance but a weak correlation was found between brain width and length as well as between brain width and width of the third ventricle37).
In some dementia studies, regression analysis showed statistically in women, the size of the third ventricle increase by age. Compared to women, the size of the third ventricle in male was bigger. Gender-specific changes in brain size were significantly greater in men than women for the peripheral (sucal) cerebrospinal fluid volume, the lateral (Sylvian) fissure cerebrospinal fluid volume, and the parieto-occipital region area. The main effects of age were observed for all the remaining brain regions examined (cerebral hemisphere volume, frontal region area, temporo-parietal area, lateral ventricular volume, and third ventricular volume). These effects were similar in men and women but asymmetries in brain structures were not by age and gender. At least in some structures, aging effect may be more apparent and rapid in men than women1-5,10-13,17,20,30,31,39). We found out the similar result through analysis of Caucasian DBS group. The length of AC-PC line increased with aging and the peak of AC-PC distance was up to 60 years old in male and 70 years old in female, so the peak of AC-PC distance in female was about 10 years delayed.
A quadratic regression line was also the optimal fit for gray matter volume/white matter volume ratio, supporting earlier observation. There was preferential loss of gray matter between the ages of 20 and 50, with a subsequent predominance of white matter volume loss24). This phenomenon also can be a contributor of changing of AC-PC distance with aging.
CONCLUSION
The AC-PC distance showed significant correlations with age, race, and gender. The distance increased with aging and in cases of male patients. Also, there is a difference between races, as stated above, the Caucasian shows the longest AC-PC distance and the Asian, Black and the Hispanic are put in order of the distance decreasing. This study suggests that caution should be exercised when using the available atlases for functional stereotaxis.
References
- 1.Blanchet B, Roland J, Braun M, Anxionnat R, Moret C. The anatomy and the MRI anatomy of the interhemispheric cerebral commissures. J Neuroradiol. 1995;22:237–251. [PubMed] [Google Scholar]
- 2.Caviness VS, Jr, Filipek PA, Kennedy DN. Quantitative magnetic resonance imaging and studies of degenerative diseases of the developing human brain. Brain Dev. 1992;14(Suppl):S80–S85. [PubMed] [Google Scholar]
- 3.Coffey CE, Lucke JF, Saxton JA, Ratcliff G, Unitas LJ. Sex differences in brain aging : a quantitative magnetic resonance imaging study. Arch Neurol. 1998;55:169–179. doi: 10.1001/archneur.55.2.169. [DOI] [PubMed] [Google Scholar]
- 4.Cowell PE, Allen LS, Kertesz A, Zalatimo NS, Denenberg VH. Human corpus callosum : a stable mathematical model of regional neuroanatomy. Brain Cogn. 1994;25:52–66. doi: 10.1006/brcg.1994.1022. [DOI] [PubMed] [Google Scholar]
- 5.Cowell PE, Turetsky BI, Gur RC, Grossman RI, Shtasel DLl. Sex differences in aging of the human frontal and temporal lobes. J Neurosci. 1994;14:4748–4755. doi: 10.1523/JNEUROSCI.14-08-04748.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cuny E, Guehl D, Burbaud P, Gross C, Dousset V. Lack of agreement between direct magnetic resonance imaging and statistical determination of a subthalamic target : the role of electrophysiological guidance. J Neurosurg. 2002;97:591–597. doi: 10.3171/jns.2002.97.3.0591. [DOI] [PubMed] [Google Scholar]
- 7.De Salles AA, Brekhus SD, De Souza EC, Behnke EJ, Farahani K. Early postoperative appearance of radiofrequency lesions on magnetic resonance imaging. Neurosurgery. 1995;36:932–936. doi: 10.1227/00006123-199505000-00007. discussion 936-937. [DOI] [PubMed] [Google Scholar]
- 8.De Salles AAF GD, Seibel RMM, Lufkin RB. Instrumentation for interventional MRI of the brain. In: De Salles AAF, Lufkin Robert, editors. In minimally Invasive Therapy of the Brain. New York, NY: Thieme; 1997. pp. 108–115. [Google Scholar]
- 9.diPierro CG, Francel PC, Jackson TR, Kamiryo T, Laws ER., Jr Optimizing accuracy in magnetic resonance imaging-guided stereotaxis : a technique with validation based on the anterior commissure-posterior commissure line. J Neurosurg. 1999;90:94–100. doi: 10.3171/jns.1999.90.1.0094. [DOI] [PubMed] [Google Scholar]
- 10.Filipek PA, Kennedy DN, Caviness VS, Jr, Rossnick SL, Spraggins TA. Magnetic resonance imaging-based brain morphometry : development and application to normal subjects. Ann Neurol. 1989;25:61–67. doi: 10.1002/ana.410250110. [DOI] [PubMed] [Google Scholar]
- 11.Fox NC, Jenkins R, Leary SM, Stevenson VL, Losseff NA. Progressive cerebral atrophy in MS-: a serial study using registered, volumetric MRI. Neurology. 2000;54:807–812. doi: 10.1212/wnl.54.4.807. [DOI] [PubMed] [Google Scholar]
- 12.Golomb J, de Leon MJ, Kluger A, George AE, Tarshish C. Hippocampal atrophy in normal aging. An association with recent memory impairment. Arch Neurol. 1993;50:967–973. doi: 10.1001/archneur.1993.00540090066012. [DOI] [PubMed] [Google Scholar]
- 13.Gur RC, Mozley PD, Resnick SM, Gottlieb GL, Kohn M. Gender differences in age effect on brain atrophy measured by magnetic resonance imaging. Proc Natl Acad Sci USA. 1991;88:2845–2849. doi: 10.1073/pnas.88.7.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamel W, Schrader B, Weinert D, Herzog J, Volkmann J. MRI- and skull x-ray-based approaches to evaluate the position of deep brain stimulation electrode contacts--a technical note. Zentralbl Neurochir. 2002;63:65–69. doi: 10.1055/s-2002-33976. [DOI] [PubMed] [Google Scholar]
- 15.Hariz MI. Clinical study on the accuracy of the Laitinen CT-guidance system in functional stereotactic neurosurgery. Stereotact Funct Neurosurg. 1991;56:109–128. doi: 10.1159/000099397. [DOI] [PubMed] [Google Scholar]
- 16.Hariz MI, Bergenheim AT, Fodstad H. Air-ventriculography provokes an anterior displacement of the third ventricle during functional stereotactic procedures. Acta Neurochir (Wien) 1993;123:147–152. doi: 10.1007/BF01401871. [DOI] [PubMed] [Google Scholar]
- 17.Kaye JA, DeCarli C, Luxenberg JS, Rapoport SI. The significance of age-related enlargement of the cerebral ventricles in healthy men and women measured by quantitative computed X-ray tomography. J Am Geriatr Soc. 1992;40:225–231. doi: 10.1111/j.1532-5415.1992.tb02073.x. [DOI] [PubMed] [Google Scholar]
- 18.Laitinen LV. CT-guided ablative stereotaxis without ventriculography. Appl Neurophysiol. 1985;48:18–21. doi: 10.1159/000101091. [DOI] [PubMed] [Google Scholar]
- 19.Levesque MF, Wilson CL, Behnke EJ, Zhang JX. Accuracy of MR-guided stereotactic electrode implantation. 1. Preimplantation correlation with the Talairach system. Stereotact Funct Neurosurg. 1990;54-55:51–55. doi: 10.1159/000100189. [DOI] [PubMed] [Google Scholar]
- 20.Liu RS, Lemieux L, Bell GS, Sisodiya SM, Shorvon SD. A longitudinal study of brain morphometrics using quantitative magnetic resonance imaging and difference image analysis. Neuroimage. 2003;20:22–33. doi: 10.1016/s1053-8119(03)00219-2. [DOI] [PubMed] [Google Scholar]
- 21.Lyons KE, Pahwa R. Deep brain stimulation in Parkinson's disease. Curr Neurol Neurosci Rep. 2004;4:290–295. doi: 10.1007/s11910-004-0054-0. [DOI] [PubMed] [Google Scholar]
- 22.Mandybur G, Morenski J, Kuniyoshi S, Iacono RP. Comparison of MRI and ventriculographic target acquisition for posteroventral pallidotomy. Stereotact Funct Neurosurg. 1995;65:54–59. doi: 10.1159/000098897. [DOI] [PubMed] [Google Scholar]
- 23.Meneses MS, Arruda WO, Hunhevicz SC, Ramina R, Pedrozo AA. Comparison of MRI-guided and ventriculography-based stereotactic surgery for Parkinson's disease. Arq Neuropsiquiatr. 1997;55:547–552. doi: 10.1590/s0004-282x1997000400005. [DOI] [PubMed] [Google Scholar]
- 24.Miller AK, Alston RL, Corsellis JA. Variation with age in the volumes of gray and white matter in the cerebral hemispheres of man : measurements with an image analyser. Neuropath Appl Neurobiol. 1980;6:119–132. doi: 10.1111/j.1365-2990.1980.tb00283.x. [DOI] [PubMed] [Google Scholar]
- 25.Mundinger F, Uhl H. Investigations into possible roentgenographic errors in stereotaxis. Confin Neurol. 1967;29:202–207. doi: 10.1159/000103707. [DOI] [PubMed] [Google Scholar]
- 26.Pecker J. Jean Talairach. Surg Neurol. 1980;14:241–242. [PubMed] [Google Scholar]
- 27.Pollo C, Meuli R, Maeder P, Vingerhoets F, Ghika J. Subthalamic nucleus deep brain stimulation for Parkinson's disease : magnetic resonance imaging targeting using visible anatomical landmarks. Stereotact Funct Neurosurg. 2003;80:76–81. doi: 10.1159/000075163. [DOI] [PubMed] [Google Scholar]
- 28.Rampini P, Egidi M, Zavanone M, Orsi M, Farabola M. Localization accuracy of AC-PC line and functional pallidal target using BRW stereotactic implementation system and axial CT scanning. An experimental study. J Neurosurg Sci. 1998;42:195–201. [PubMed] [Google Scholar]
- 29.Rampini PM, Locatelli M, Alimehmeti R, Tamma F, Caputo E. Multiple sequential image-fusion and direct MRI localisation of the subthalamic nucleus for deep brain stimulation. J Neurosurg Sci. 2003;47:33–39. [PubMed] [Google Scholar]
- 30.Rice RW, Kroning J, Critchlow V. Sex differences in the effects of surgical isolation of the medial basal hypothalamus on linear growth and plasma growth hormone levels in the rat. Endocrinology. 1976;98:982–990. doi: 10.1210/endo-98-4-982. [DOI] [PubMed] [Google Scholar]
- 31.Sabattini L. Evaluation and measurement of the normal ventricular and subarachnoid spaces by CT. Neuroradiology. 1982;23:1–5. doi: 10.1007/BF00399698. [DOI] [PubMed] [Google Scholar]
- 32.Schaltenbrand G, Wahren W. Atlas for Stereotaxy of the human brain. Stuttgart, New York: Thieme; 1977. [Google Scholar]
- 33.Spiegelmann R, Gofman J. CT-target determination in posteroventral pallidotomy : a universal method. Technical note. Acta Neurochir (Wien) 1996;138:732–735. doi: 10.1007/BF01411480. discussion 736. [DOI] [PubMed] [Google Scholar]
- 34.Talairach J. Co-Planar Stereotatic Atlas of the human Bain. Stuttgart: Thieme; 1988. [Google Scholar]
- 35.Uitti RJ, Tsuboi Y, Pooley RA, Putzke JD, Turk MF. Magnetic resonance imaging and deep brain stimulation. Neurosurgery. 2002;51:1423–1428. discussion 1428-1431. [PubMed] [Google Scholar]
- 36.Wolfsberger S, B-S A, Pinker K. Application of three-tesla magnetic resonance imaging for diagnosis and surgery of sellar lesions. J Neurosurg. 2004;100:178–286. doi: 10.3171/jns.2004.100.2.0278. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto T, Katayama Y, Kano T, Kobayashi K, Oshima H. Deep brain stimulation for the treatment of parkinsonian, essential, and poststroke tremor : a suitable stimulation method and changes in effective stimulation intensity. J Neurosurg. 2004;101:201–209. doi: 10.3171/jns.2004.101.2.0201. [DOI] [PubMed] [Google Scholar]
- 38.Yelnik J, Damier P, Demeret S, Gervais D, Bardinet E. Localization of stimulating electrodes in patients with Parkinson disease by using a three-dimensional atlas-magnetic resonance imaging coregistration method. J Neurosurg. 2003;99:89–99. doi: 10.3171/jns.2003.99.1.0089. [DOI] [PubMed] [Google Scholar]
- 39.Zhu XL, Hamel W, Schrader B, Weinert D, Hedderich J. Magnetic resonance imaging-based morphometry and landmark correlation of basal ganglia nuclei. Acta Neurochir (Wien) 2002;144:959–969. doi: 10.1007/s00701-002-0982-x. discussion 968-959. [DOI] [PubMed] [Google Scholar]