Abstract
Objective
The purpose of this study is to identify the factors affecting the failure of trials (<50% pain reduction in pain for trial period) to improve success rate of spinal cord stimulation (SCS) trial.
Methods
A retrospective review of the failed trials (44 patients, 36.1%) among the patients (n=122) who underwent SCS trial between January 1990 and December 1998 was conducted. We reviewed the causes of failed trial stimulation, age, sex, etiology of pain, type of electrode, and third party support.
Results
Of the 44 patients, 65.9% showed unacceptable pain relief in spite of sufficient paresthesia on the pain area with trial stimulation. Four of six patients felt insufficient paresthesia with stimulation had the lesions of the spinal cord. Seventy five percent of the patients experienced unpleasant or painful sensation during stimulation had allodynia dominant pain. Third-party involvement, sex, age and electrode type had no influence on the outcome.
Conclusion
We conclude that SCS trial is less effective for patients with neuropathic pain of cord lesions, postherpetic neuropathy or post-amputation state. Further, patients with allodynia dominant pain can feel unpleasant or painful during trial stimulation.
Keywords: Spinal cord stimulation, Cord lesion, Allodynia, Paresthesia, Chronic pain
INTRODUCTION
Since 1967, spinal cord stimulation (SCS) has been steadily gaining support as a reversible and nondestructive method for chronic intractable pain10,19,27). In recent years, SCS has been applied with increasing effectiveness due to improvements with patient selection criteria17,19), accuracy in electrode placement16,24-26), and multipolar and multichannel devices17,24-26). SCS has been known to be efficacious for failed-back surgery syndrome (FBSS)16,28,29), peripheral vascular diseases causing rest and claudication pain2,11,18), complex regional pain syndrome (CRPS) I and CRPS II5,7,28,31), peripheral neuropathy16,19), multiple sclerosis17), intractable angina8,9,20,21), postherpetic neuralgia14), and recently in certain cases of visceral pain12,30).
There have been a few reports on the prognostic factors in successful stimulation for long term cases16,17,19,24). But, there has not been any report regarding the analysis of failed trial cases. We have analyzed the factors affecting the failure of trials to improve success rate of SCS trial.
MATERIALS AND METHODS
We performed a retrospective review of the failed trials among the patients (n=122) who underwent SCS trial between January 1990 and December 1998 at one hospital. All patients had used narcotic medication for pain relief before SCS. They were treated in a multidisciplinary pain clinic for a minimum of 6 months and were found to be refractory to conservative modalities of pain relief; after failure, they were then referred for SCS. All patients met the following criteria : 1) A defined non-malignant, organic cause of pain; 2) failure of conservative pain control methods; 3) absence of a major psychiatric disorder; and 4) capacity to give informed consent for the procedure.
Forty-four patients (36.1%) who failed to meet the minimum requirements of 50% pain relief relative to pre-implant levels did not have their systems internalized, were included in this study. This review was done by individuals who had no role in the management of the patients. The analysis was undertaken to reveal the causes of failed SCS trials as well as the pre-operative diagnoses.
A patient's pain was categorized as neuropathic if the patient's history and imaging studies supported a likelihood that roots were involved and if one or more of the followings were associated with pain spreading down the limbs beyond the elbow and/or the knee : sensory loss and/or evoked pain in the distribution of one or more roots at risk of being affected by the known pathology, reflex reduction in the distribution of the root(s), motor weakness in the distribution of such root(s), and causalgic and/or dysesthetic pain. In FBSS patients, the etiology of pain is clearly the case because the pain is located in the distribution of a particular root or roots and neurological deficits implicate damage to these roots. However, many patients with degenerative spinal disease are referred for SCS who did not experience obvious neuropathic pain but have nonspecific pain on their arms of legs that were not in the distribution of any particular nerve root or roots and were not associated with radicular neurologic deficits. So, we have separated FBSS group that was non-specific limb pain and neuropathic pain.
All patients accepted for treatment initially underwent a trial of SCS, as is done at most centers. They underwent a 3-day trial with a Medtronic straight or Sigma monopolar electrode (Medtronic, Inc., Minneapolis, MN), receiving stimulation for 2 hours and resting for 1 hour repeatedly during the 3 days.
The patients' pain levels were recorded on a 0 to 10 VAS3), on which 0 is no pain and 10 is maximum imaginable pain, before and after each 2-hour trial. A "failed SCS trial" was defined as ineffective pain relief (<50%) during the trial stimulation period. We reviewed the clinical predictors of outcome including causes of failed trial stimulation, age, sex, etiology of pain. We used Medtronic Sigma monopolar electrode (Medtronic, Inc., Minneapolis, MN), Medtronic Quad electrode (Medronics, Inc., Minneapolis, MN), and Medtronic Resume electrode (Medronics, Inc., Minneapolis, MN) for SCS, and because there are published outcome differences for different types of equipment15-17,29), we examined the results from the two electrode types separately. We also compared outcomes between patients who received worker's compensation and those who did not. Our statistical analysis was performed using the Fisher's exact test. A p<0.05 was considered statistically significant.
RESULTS
The average patient age was 45.6 years (range, 17-81 yr) and 53.1 years (range, 19-82 yr) in the successful versus failed trial groups, respectively. The successful trial group was 48.7% in male and the failed trial group was 52.3% in male. We observed no differences in outcome related to sex or age. We also did not observe clear differences in outcome related to the use of different stimulation frequencies but did not thoroughly study this matter.
Of the 44 patients, 65.9% showed unacceptable pain relief in spite of sufficient paresthesia on the pain area with trial stimulation (Table 1). Six (13.6%) had insufficient paresthesia, 8 (18.2%) had a painful or unpleasant sense, and one patient could not tolerate internalization of electrode because of oversensitivity to the procedure. Four of six patients felt insufficient paresthesia with stimulation had the lesions of the spinal cord (Table 2). Table 3 shows that 8 patients experienced unpleasant or painful sensation during stimulation. Seventy-five percent of them had allodynia dominant pain. And, two of three cord injury patients felt allodynia dominant pain.
Table 1.
Causes of failed trial stimulation
Table 2.
Insufficient paresthesia with stimulation by diagnosis
PHN : Postherpetic neuralgia
Table 3.
Painful or unpleasant sense during stimulation
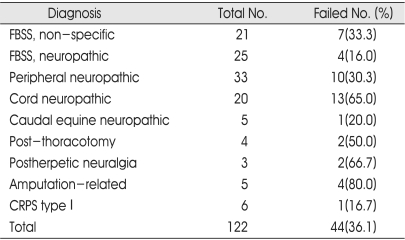
The trial stimulation failed in the 80.0% of the patients with amputation-related pain, 66.7% patients with postherpetic pain, 65.0% of the patients with cord neuropathy, and 50.0% of the patients from a group with post-thoracotomy pain (Table 4). The percentages of successful stimulation trial were similar in patients with root neuropathic pain, nonspecific limb pain and peripheral nerve neuropathic pain.
Table 4.
Number of failed trial stimulation by diagnosis
FBSS : Failed-back surgery syndrome, CRPS : Complex regional pain syndrome
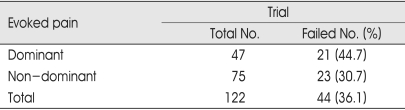
The incidence of failed trial rate was higher in patients with evoked pain dominant (44.7%) than in patients with non-dominant or without evoked pain (30.7%), but the difference was not statistically significant (Table 5).
Table 5.
Evoked pain and failed trial stimulation
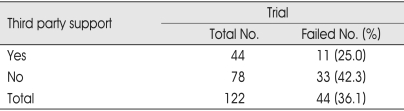
Many of the patients included in this study had received worker's compensation benefits. It has been suggested that third-party payer involvement affects the outcome of pain treatment15-17,24,28,29). Better trial results for our patients who received worker's compensation, but the differences were not statistically significant (Table 6).
Table 6.
Third party support and failed trial stimulation
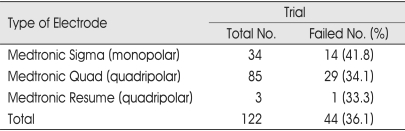
We compared the patient outcome from the stimulation trials when using monopolar versus quadripolar electrodes. No statistically significant difference in outcome between the three electrode types was observed (Table 7).
Table 7.
Type of electrode and failed trial stimulation
DISCUSSION
SCS has become an established treatment option for controlling chronic, benign pain because of its reversibility, minimal invasiveness, low complication rate and effectiveness. A trial of SCS allows the patient and physician to assess the individual benefits before implantation of a permanent device. In most patients, SCS trials are easy to perform, result in low morbidity and exactly emulate the permanent procedure17,22,23). Other diagnostic procedures for clinical pain syndromes, such as nerve blocks to identify candidates for ablative procedures or provocative discography to identify candidates for fusion, do not share these advantages. Additionally, an unsuccessful trial may be terminated by lead removal without a significant risk. Despite advances in the design and production of SCS systems, a failed SCS trial can result of various reasons. But, to our knowledge, no in-depth analysis of failed SCS trials has been undertaken.
We have shown that approximately 36% (44 of 122) of patients fail the stimulation trial in spite of the best efforts in selecting candidates for SCS therapy. This emphasizes the importance of screening before permanent implantation, which is currently not a standard protocol at some centers, despite the very low morbidity rate. This approach reduces the rate of failed permanent implants, and improves cost effectiveness. The stimulation trial also allows for a period of patient adjustment to stimulation-induced paresthesia and counseling by the neurosurgical team. The main disadvantages to this process are that it is an added procedure with associated costs and minimal risks such as root irritation, hematoma, or infection, which may add to the hospital stay.
In this study, we tried to achieve a total overlap of stimulation-induced paresthesia and the area of pain because failure to achieve this leads to less than optimal results. But, majority (65.9%) of the failed trial patients showed unacceptable pain relief in spite of sufficient paresthesia on the pain area with trial stimulation. A recent prospective study by Allegri et al.1) revealed that complete coverage is not necessary to achieve a good outcome.
According to report of long term followed of SCS, the most satisfying pain relief is achieved in patients with FBSS, angina, CRPS, and PVD that was not amenable to revascularization surgery. But, pain caused by cauda equine lesions, paraplegic pain, bone and joint pain, and phantom limb pain responded poorly1,15-17,24). Though this study is the result of trial stimulation, pain originating from FBSS, peripheral neuropathy, cauda equine neuropathy, or CRPS has a better response to SCS trial stimulation, similar to previous long term result. Therefore, trial stimulation might be very important factor for predicting long term result as well as selecting candidate of permanent stimulation. Pain caused by cord lesions, postherpetic pain, post amputation pain responded poorly in our series and these diagnoses continue to be a challenge for this therapy. Failure of SCS trial in patients with cord central pain might result from difficulty in accessing the ideal site of the epidural space because of trauma or previous surgery, difficulty in producing paresthesia over the area of the patient's pain, or dieback of the dorsal columns above a severe cord injury4,29,31).
Reasons for failure of trial stimulation in patients who are otherwise considered to be good candidates for SCS are poorly understood. It is hypothesized that these patients may be an example of a population whose primary pain mechanisms or pathology are unsuitable to SCS or whose anatomy or physiology in some way may prevent accurate electrode placement. Recently, it has been shown that electric stimulation on spinal nerve fibers and the use of somatosensory evoked potentials might lead to better results6). Computer assistance in generating optimal configurations during stimulation trials may also add to success rates13). And, the selection of optimal candidates is the most important factor for increasing successful trial SCS rates. We found no significant influence of age or sex on the failure of a stimulation trial. And, many of our patients, especially those with FBSS, were receiving worker's compensation. The outcome results between patients with and without such coverage were not significantly different.
When we compared our outcome data according to the type of electrode used, we found no difference in the incidence of pain relief between the two groups. Published studies have shown differences in efficacy between different types of equipment16,22,29,31). We were not able to compare outcome data from resume type electrode versus wire type electrode, because the number of case was so small.
CONCLUSION
Understanding why SCS trials fail is the first step toward designing the next generation of successful SCS therapy. According to this study, SCS is less effective in patients with neuropathic pain of cord lesions, postherpetic neuropathy, or post-amputation state. Majority of failed trial stimulation occur in spite of sufficient paresthesia on the pain area with trial stimulation. And, the patients with allodynia dominant pain can feel unpleasant or painful during trial stimulation.
Acknowledgement
We would like to thank Dr. RR Tasker for his devotion toward preparing this manuscript.
References
- 1.Allegri M, Arachi G, Barbieri M, Paulin L, Bettaglio R, Bonetti G, et al. Prospective study of the success and efficacy of spinal cord stimulation. Minerva Anestesiol. 2004;70:117–124. [PubMed] [Google Scholar]
- 2.Broseta J, Barbera J, de Vera JA, Barcia-Salorio JL, Garcia-March G, Gonzalez Darder J, et al. Spinal cord stimulation in peripheral arterial disease. A cooperative study. J Neurosurg. 1986;64:71–80. doi: 10.3171/jns.1986.64.1.0071. [DOI] [PubMed] [Google Scholar]
- 3.Carlsson AM. Assessment of chronic pain. I. Aspects of the reliability and validity of the visual analogue scale. Pain. 1983;16:87–101. doi: 10.1016/0304-3959(83)90088-X. [DOI] [PubMed] [Google Scholar]
- 4.Cioni B, Meglio M, Pentimalli L, Visocchi M. Spinal cord stimulation in the treatment of paraplegic pain. J Neurosurg. 1995;82:35–39. doi: 10.3171/jns.1995.82.1.0035. [DOI] [PubMed] [Google Scholar]
- 5.Forouzanfar T, Kemler MA, Weber WE, Kessels AG, van Kleef M. Spinal cord stimulation in complex regional pain syndrome : cervical and lumbar devices are comparably effective. Br J Anaesth. 2004;92:348–353. doi: 10.1093/bja/aeh072. [DOI] [PubMed] [Google Scholar]
- 6.Gofeld M, Cohen E, Niv D. New approach for spinal cord stimulation trial. Pain Pract. 2005;5:324–326. doi: 10.1111/j.1533-2500.2005.00034.x. [DOI] [PubMed] [Google Scholar]
- 7.Grabow TS, Tella PK, Raja SN. Spinal cord stimulation for complex regional pain syndrome : an evidence-based medicine review of the literature. Clin J Pain. 2003;19:371–383. doi: 10.1097/00002508-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Hautvast RW, Brouwer J, DeJongste MJ, Lie KI. Effect of spinal cord stimulation on heart rate variability and myocardial ischemia in patients with chronic intractable angina pectoris--a prospective ambulatory electrocardiographic study. Clin Cardiol. 1998;21:33–38. doi: 10.1002/clc.4960210107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hautvast RW, DeJongste MJ, Staal MJ, van Gilst WH, Lie KI. Spinal cord stimulation in chronic intractable angina pectoris : a randomized, controlled efficacy study. Am Heart J. 1998;136:1114–1120. doi: 10.1016/s0002-8703(98)70171-1. [DOI] [PubMed] [Google Scholar]
- 10.Holsheimer J. Effectiveness of spinal cord stimulation in the management of chronic pain : analysis of technical drawbacks and solutions. Neurosurgery. 1997;40:990–996. doi: 10.1097/0006123-199705000-00023. discussions 996-999. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs MJ, Jorning PJ, Joshi SR, Kitslaar PJ, Slaaf DW, Reneman RS. Epidural spinal cord electrical stimulation improves microvascular blood flow in severe limb ischemia. Ann Surg. 1988;207:179–183. doi: 10.1097/00000658-198802000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kapural L, Narouze SN, Janicki TI, Mekhail N. Spinal cord stimulation is an effective treatment for the chronic intractable visceral pelvic pain. Pain Med. 2006;7:440–443. doi: 10.1111/j.1526-4637.2006.00165.x. [DOI] [PubMed] [Google Scholar]
- 13.Kenneth MA MJ, Donald LK, Jeffrey HC, Vladmir R. Computer assisted and patient interactive programming of dual octrode spinal cord stimulation in the treatment of chronic pain. Neuromodulation. 1998;1:30–45. doi: 10.1111/j.1525-1403.1998.tb00028.x. [DOI] [PubMed] [Google Scholar]
- 14.Kim JO, Lee MK, Kim JS, Kim DJ. Spinal cord stimulation for intractable postherpetic neuralgia. J Korean Neurosurg Soc. 2003;31:230–233. [Google Scholar]
- 15.Kim SH, Tasker RR, Oh MY. Spinal cord stimulation for nonspecific limb pain versus neuropathic pain and spontaneous versus evoked pain. Neurosurgery. 2001;48:1056–1064. doi: 10.1097/00006123-200105000-00017. discussion 1064-1055. [DOI] [PubMed] [Google Scholar]
- 16.Kumar K, Hunter G, Demeria D. Spinal cord stimulation in treatment of chronic benign pain : challenges in treatment planning and present status, a 22-year experience. Neurosurgery. 2006;58:481–496. doi: 10.1227/01.NEU.0000192162.99567.96. discussion 481-496. [DOI] [PubMed] [Google Scholar]
- 17.Kumar K, Toth C, Nath RK, Laing P. Epidural spinal cord stimulation for treatment of chronic pain--some predictors of success. A 15-year experience. Surg Neurol. 1998;50:110–120. doi: 10.1016/s0090-3019(98)00012-3. discussion 120-111. [DOI] [PubMed] [Google Scholar]
- 18.Kumar K, Toth C, Nath RK, Verma AK, Burgess JJ. Improvement of limb circulation in peripheral vascular disease using epidural spinal cord stimulation : a prospective study. J Neurosurg. 1997;86:662–669. doi: 10.3171/jns.1997.86.4.0662. [DOI] [PubMed] [Google Scholar]
- 19.Long DM, Erickson D, Campbell J, North R. Electrical stimulation of the spinal cord and peripheral nerves for pain control. A 10-year experience. Appl Neurophysiol. 1981;44:207–217. doi: 10.1159/000102203. [DOI] [PubMed] [Google Scholar]
- 20.Mannheimer C, Eliasson T, Augustinsson LE, Blomstrand C, Emanuelsson H, Larsson S, et al. Electrical stimulation versus coronary artery bypass surgery in severe angina pectoris : the ESBY study. Circulation. 1998;97:1157–1163. doi: 10.1161/01.cir.97.12.1157. [DOI] [PubMed] [Google Scholar]
- 21.Murphy DF, Giles KE. Dorsal column stimulation for pain relief from intractable angina pectoris. Pain. 1987;28:365–368. doi: 10.1016/0304-3959(87)90070-4. [DOI] [PubMed] [Google Scholar]
- 22.North RB, Calkins SK, Campbell DS, Sieracki JM, Piantadosi S, Daly MJ, et al. Automated, patient-interactive, spinal cord stimulator adjustment : a randomized controlled trial. Neurosurgery. 2003;52:572–580. doi: 10.1227/01.neu.0000047818.99414.fb. discussion 579-580. [DOI] [PubMed] [Google Scholar]
- 23.North RB, Kidd DH, Olin JC, Sieracki JM. Spinal cord stimulation electrode design : prospective, randomized, controlled trial comparing percutaneous and laminectomy electrodes-part I : technical outcomes. Neurosurgery. 2002;51:381–389. discussion 389-390. [PubMed] [Google Scholar]
- 24.North RB, Kidd DH, Zahurak M, James CS, Long DM. Spinal cord stimulation for chronic, intractable pain : experience over two decades. Neurosurgery. 1993;32:384–394. doi: 10.1227/00006123-199303000-00008. discussion 394-385. [DOI] [PubMed] [Google Scholar]
- 25.Ray CD. Electrical and chemical stimulation of the CNS by direct means for pain control : present and future. Clin Neurosurg. 1981;28:564–588. doi: 10.1093/neurosurgery/28.cn_suppl_1.564. [DOI] [PubMed] [Google Scholar]
- 26.Ray CD, Burton CV, Lifson A. Neurostimulation as used in a large clinical practice. Appl Neurophysiol. 1982;45:160–166. doi: 10.1159/000101592. [DOI] [PubMed] [Google Scholar]
- 27.Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. Anesth Analg. 1967;46:489–491. [PubMed] [Google Scholar]
- 28.Taylor RS. Spinal cord stimulation in complex regional pain syndrome and refractory neuropathic back and leg pain/failed back surgery syndrome : results of a systematic review and meta-analysis. J Pain Symptom Manage. 2006;31:S13–S19. doi: 10.1016/j.jpainsymman.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 29.Taylor RS, Van Buyten JP, Buchser E. Spinal cord stimulation for chronic back and leg pain and failed back surgery syndrome : a systematic review and analysis of prognostic factors. Spine. 2005;30:152–160. doi: 10.1097/01.brs.0000149199.68381.fe. [DOI] [PubMed] [Google Scholar]
- 30.Tiede JM, Ghazi SM, Lamer TJ, Obray JB. The use of spinal cord stimulation in refractory abdominal visceral pain : case reports and literature review. Pain Pract. 2006;6:197–202. doi: 10.1111/j.1533-2500.2006.00085.x. [DOI] [PubMed] [Google Scholar]
- 31.Turner JA, Loeser JD, Deyo RA, Sanders SB. Spinal cord stimulation for patients with failed back surgery syndrome or complex regional pain syndrome : a systematic review of effectiveness and complications. Pain. 2004;108:137–147. doi: 10.1016/j.pain.2003.12.016. [DOI] [PubMed] [Google Scholar]