Abstract
A 64-year-old woman was referred to our hospital with a one-month history of progressive headache. Magnetic resonance imaging (MRI) showed a hemorrhagic mass adjacent to the left inferior cerebellar hemisphere associated with a peripheral rim of signal void. Angiography demonstrated an avascular mass and the provisional diagnosis was a large cavernous angioma in the cerebellum. Intraoperative findings revealed a thrombosed giant aneurysm of the left distal posterior inferior cerebellar artery (PICA). We report an unusual case of a completely thrombosed giant aneurysm simulating a large cavernous angioma in the cerebellum. The cerebellar cisternal location of the mass may be a clue for the pre-operative diagnosis of an aneurysm.
Keywords: Posterior inferior cerebellar artery (PICA), Thrombosed giant aneurysm, Cavernous angioma
INTRODUCTION
Aneurysms of the vertebrobasilar system account for 5 to 10% of all intracranial aneurysms3,14,17), and they are referred to as giant aneurysms when they exceed 2.5 cm in size. Aneurysms of this size or larger are very rarely found in the vertebrobasilar system, and giant aneurysms of the PICA are also quite rare. Approximately, 50-of all giant aneurysms are thrombosed, but complete obliteration of the aneurysmal sac is uncommon8-11). When they become completely thrombosed, giant aneurysm can simulate a tumor such as a large cavernous angioma. We report an unusual case of a completely thrombosed giant aneurysm.
CASE REPORT
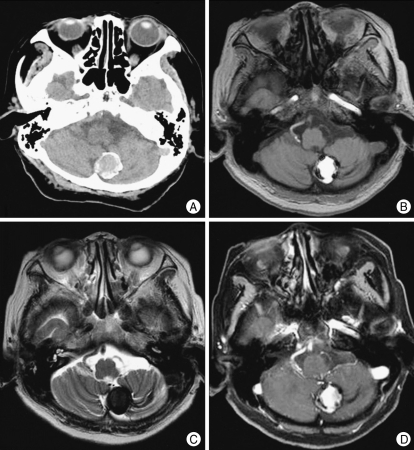
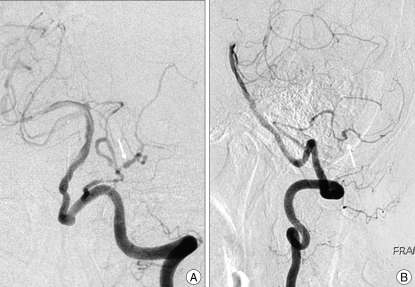
A 64-year-old woman was referred to our hospital with a one-month history of progressive headache. Examination revealed an awake, fully oriented patient with mild hemiparesis on the left side. The patient's vital signs were normal, and there were no cerebellar signs. Computerized tomography scans displayed a large, 2.5-cm in diameter, non-homogeneous, rim-calcified hemorrhagic lesion adjacent to the left cerebellar hemisphere (Fig. 1A). Magnetic resonance images (MRI) demonstrated a well-defined mass, 2.5 cm in size, adjacent to the left inferior cerebellar hemisphere with high signal intensity on T1-weighted images and low signal intensity on T2-weighted images (Fig. 1B, C). The mass showed no enhancement after gadolinium administration (Fig. 1D). The mass was associated with a peripheral signal void rim and not with peri-lesional edema. Angiogram of the left vertebral artery revealed an avascular mass lesion. And there was a vascular diminution in the tonsillomedullary and the telovelotonsillar segment (Fig. 2A, B). The provisional diagnosis was a large cavernous angioma in the left cerebellum.
Fig. 1.
A : Computerized tomography scan shows a 2.5-cm in diameter, rim-calcified hemorrhagic lesion in the left cerebellar hemisphere. B : T1-weighted image reveals a 2.5-cm-sized well-defined hyperintense mass. C : T2-weighted image reveals a peripheral signal void rim and no perilesional edema. D : Gadolinium-enhanced T1-weighted enhancement image showing no contrast enhancement of the lesion.
Fig. 2.
Left vertebral artery angiogram. A : anteroposterior view; B : lateral view showing an angiographic escape of the mass lesion and there is a vascular diminution in the tonsillomedullary and the telovelotonsillar segment.
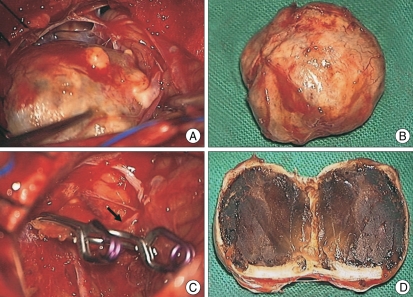
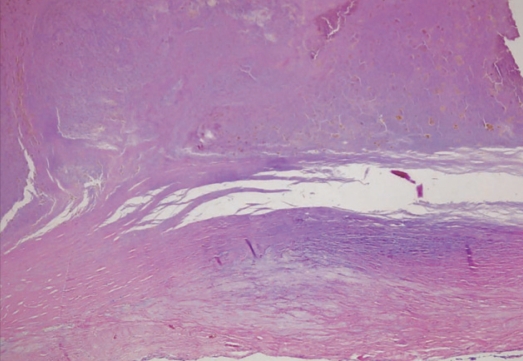
We performed the operation using a midline suboccipital craniotomy under general anesthesia. The cisterna magna was opened, exposing the cerebellar tonsils and the tonsilar loop. The mass was exposed in the cerebellar cistern and adhered to the left interior cerebellar hemisphere (Fig. 3A). The mass was a giant aneurysm which was located at the telovelotonsillar segment of the left distal PICA. There was absence of a definite neck and the multiple vascular channels. After the applications of two Yasagil clips (712&728) to parent artery, the aneurysm was effectively debulked (Fig. 3B) and we confirmed the proper placement of the clip and the good patency of the artery posterior to the clip (Fig. 3C). Subacute thrombus was noted on the inner surface of the aneurysm and was associated with a focal disruption in the thick wall of the aneurysm (Fig. 3D). Microscopic examination showed that the aneurysmal wall was formed of homogeneous fibrous connective tissue, containing intimal thickening, focal myxoid change, dystrophic calcification, and collagenous fibrosis of the vessel wall. The sac was filled with a heterogeneous thrombus (Fig.4). The pathological diagnosis was atherosclerotic aneurysm. The postoperative course was uneventful and the patient's headache and hemiparesis improved. There was no change in the postoperative angiography
Fig. 3.
Operative findings A : The mass is exposed in the cerebellar cistern. B : The aneurysm is effectively debulked. C : After the applications of two clips to parent artery, proper placement of the clip and the good patency of the artery posterior to the clip (arrow) are noted. D : Subacute thrombus is noted on the inner surface of the aneurysm.
Fig. 4.
Pathologic finding of the aneurysmal sac demonstrating homogeneous fibrous connective tissue, containing intimal thickening, focal myxoid change, dystrophic calcification, and collagenous fibrosis of the vessel wall suggesting a atherosclerotic aneurysm.
DISCUSSION
The most common sites from which giant aneurysms arise are the bifurcation of the basilar artery and the origin of the PICA. Giant aneurysms developing from other locations within the posterior cranial fossa are much more uncommon. Giant aneurysms usually present clinically as spaceoccupying lesions and differ from the presentation of the common and medium-sized aneurysms6,19). Thus, giant intracranial aneurysms may occasionally be mistaken for tumors2,3,5,7,21).
Giant aneurysms typically appear on computerized tomography (CT) as rounded or oval-shaped masses with distinct outlines. A significant amount of edema is also frequently found surrounding the aneurysm. Calcifications are not uncommon findings, and the degree of contrast enhancement is strictly dependent on the amount of intra-aneurysmal thrombosis. Accor-dingly, Schubinger and associates have delineated three types of giant intracranial aneurysms : artially thrombosed, completely thrombosed, and nonthrombosed19). Partially thrombosed aneurysms, which are the most common type, show the socalled "target sign", due to the strong enhancement of a part of the aneurysmal cavity. However, completely thrombosed aneurysms usually appear as non- homogeneous masses of increased density that do not enhance with contrast material. CT scans may not be sufficient to confirm the diagnosis of the nature of the lesion, as reported by several authors4,15,22), because they cannot differentiate between these lesions and tumors. In our case, CT scans displayed a large, 2.5-cm in diameter, non-homogeneous, rim-calcified hemorrhagic lesion without peri-lesional edema in the left cerebellar hemisphere, which could have been mistaken for a cerebellar tumor, such as a cavernous angioma.
Cavernous angioma is a benign vascular lesion that may occur at any site within the central nervous system. CT scans are less sensitive than MRI for the detection of cavernous angiomas. T2-weighted MRI is the most sensitive test for intracranial cavernous angiomas and the findings of T2-weighted MRI generally show a mixed-signal core with a low signal rim. The diagnosis is strongly suggested by findings of multiple lesions with these characteristics and a positive family history. In this case, MRI demonstrated a well-defined mass, 2.5 cm in size, with peripheral signal void rim and no contrast enhancing components. Thus, these MR findings were interpreted as a cavernous angioma. However, the mass might not be located in the cerebellar parenchyma, but rather adjacent to the left inferior cerebellar hemisphere. Intraoperatively, the mass was exposed in the cerebellar cistern and adhered to the left inferior cerebellar hemisphere. Therefore, the cerebellar cisternal location of the mass may be a clue for the preoperative diagnosis of an aneurysm.
Angiography was performed in order to shed further light on the nature of the mass. Angiography is still the most important diagnostic tool because it reveals the true nature of the lesion, especially in patients like ours who do not show the signs and symptoms of subarachnoid bleeding and appear to have space-occupying lesions. In our case, angiography revealed an avascular mass lesion that showed similar findings to those of a brain tumor, such as a cavernous angioma. Cavernous angiomas are usually not detected angiographically, and are therefore grouped with the "occult" vascular malformations. It is well known that completely thrombosed giant aneurysms may escape angiographic identification13,18-20). In fact, both CT and MRl scans were inconclusive, and angiography failed to show the aneurysm, probably because the aneurysm was already completely thrombosed. Several authors have suggested criteria for the angiographic recognition of completely thrombosed giant aneurysms16). But, their practical value dose not seem to be exceptionally great, at least with respect to lesions in the posterior fossa.
Surgical treatment of aneurysms of the distal PICA has yielded good results, as demonstrated by the series conducted by Lewis, et al12). Present optimal surgical treatment is clipping of saccular aneurysm with preser-vation of PICA1,23,24). After the operation, our patient showed clincial improvement and there were no deteriorations in the neurological status of the patient.
CONCLUSION
Distal PICA aneurysms can grow to giant proportions and can simulate a tumor, such as a large cavernous angioma, when they become thrombosed. The cerebellar cisternal location of the mass may be a clue for the pre-operative diagnosis of an aneurysm.
Acknowledgement
This research was supported by a grant (M103KV010018-03K2201-01850) of the 21st Century Frontier Research Program funded by the Ministry of Science and Technology of the Republic of Korea.
References
- 1.Ahn JY, Chung YS, Kim OJ, Choi SW. Medial trunk aneurysm of the distal posterior inferior cerebellar artery in the fourth ventricle. J Korean Neurosurg Soc. 2003;34:57–60. [Google Scholar]
- 2.Belec L, Cesaro P, Brugieres P. Tumor simulating giant serpentine aneurysm of the posterior cerebellar artery. Surg Neurol. 1998;29:210–215. doi: 10.1016/0090-3019(88)90008-0. [DOI] [PubMed] [Google Scholar]
- 3.Bull J. Massive aneurysms at the base of the brain. Brain. 1969;92:535–570. doi: 10.1093/brain/92.3.535. [DOI] [PubMed] [Google Scholar]
- 4.Byrd SE, Bentson JR, Winter J, Wilson GH, Joyce PW, O'Connor L. Giant intracranial aneurysms simulating brain neoplasms on computed tomography. J Comput Assist Tomogr. 1978;2:303–307. doi: 10.1097/00004728-197807000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Cantore GP, Ciappetta P, Vagnozzi R, Bozzao L. Giant aneurysm of the anterior inferior cerebellar artery simulating a cerebellopontine angle tumor. Surg Neurol. 1982;18:76–78. doi: 10.1016/0090-3019(82)90025-8. [DOI] [PubMed] [Google Scholar]
- 6.Castel JC, Chaabane M, Guibert-Tranier F, Piton J, Caille JM. Intracranial supratentorial "hypergiant" aneurysms. Diagnostic problems. A study of 8 cases. J Neuroradiol. 1985;12:135–149. [PubMed] [Google Scholar]
- 7.Fisher A, Som PM, Mosesson RE, Lidov M, Liu TH. Giant intracranial aneurysms with skull base erosion and extracranial masses : CT and MR findings. J Comput Assist Tomogr. 1994;18:939–942. doi: 10.1097/00004728-199411000-00018. [DOI] [PubMed] [Google Scholar]
- 8.Katayama Y, Tsubokawa T, Miyazaki S, Furuichi M, Hirayama T, Himi K. Growth of totally thrombosed giant aneurysm within the posterior cranial fossa. Diagnostic and therapeutic considerations. Neuroradiology. 1991;33:168–170. doi: 10.1007/BF00588260. [DOI] [PubMed] [Google Scholar]
- 9.Khurana VG, Wijdicks EF, Parisi JE, Piepgras DG. Acute deterioration from thrombosis and rerupture of a giant intracranial aneurysm. Neurology. 1999;52:1697–1699. doi: 10.1212/wnl.52.8.1697. [DOI] [PubMed] [Google Scholar]
- 10.Krapf H, Schöning M, Petersen D, Küker W. Complete asymptomatic thrombosis and resorption of a congenital giant intracranial aneurysm. J Neurosurg. 2002;97:184–189. doi: 10.3171/jns.2002.97.1.0184. [DOI] [PubMed] [Google Scholar]
- 11.Lee KC, Joo JY, Lee KS, Shin YS. Recanalization of completely thrombosed giant aneursym; case report. Surg Neurol. 1999;51:94–98. doi: 10.1016/s0090-3019(97)00346-7. [DOI] [PubMed] [Google Scholar]
- 12.Lewis SB, Chang DJ, Peace DA, Lafrentz PJ, Day AL. Distal posterior inferior cerebellar artery aneurysms: clinical features and management. J Neurosurg. 2002;97:756–766. doi: 10.3171/jns.2002.97.4.0756. [DOI] [PubMed] [Google Scholar]
- 13.Maxwell RE, Chou SN. Aneurysmal tumors of the basifrontal region. J Neurosurg. 1997;46:438–445. doi: 10.3171/jns.1977.46.4.0438. [DOI] [PubMed] [Google Scholar]
- 14.Mccormik WF, Nofzinger JD. Saccular intracranial aneurysms : an autopsy study. J Neurosurg. 1965;22:155–159. doi: 10.3171/jns.1965.22.2.0155. [DOI] [PubMed] [Google Scholar]
- 15.Nadjmi M, Ratzka M, Wodarz M. Giant aneurysms in CT and angiography. Neuroradiology. 1978;16:284–286. doi: 10.1007/BF00395274. [DOI] [PubMed] [Google Scholar]
- 16.Palmieri A, Ambrosio A, Profeta G, Maggi G. [Intracranial thrombosed aneurysm. Value of some peculiar radiological signs] Ann Radiol (Paris) 1970;13:869–876. [PubMed] [Google Scholar]
- 17.Sahs AL, Perret GE, Locksley HB, Nishioka H. Intracranial Aneurysms and Subarachnoid Hemorrhage. A Cooperative Study. Philadelphia: Lippincott Co; 1969. p. 296. [Google Scholar]
- 18.Sarwar M, Batnitzky S, Schechter MM. Tumorous aneurysms. Neuroradiology. 1976;12:79–97. doi: 10.1007/BF00333123. [DOI] [PubMed] [Google Scholar]
- 19.Schubiger O, Valavanis A, Hayek J. Computed tomography in cerebral aneurysms with special emphasis on giant intracranial aneurysms. J Comput Assist Tomogr. 1980;4:24–32. doi: 10.1097/00004728-198002000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Schunk H. Spontaneous thrombosis of intracranial aneurysms. Am J Roentgenol Radium Ther Nucl Med. 1964;91:1327–1338. [PubMed] [Google Scholar]
- 21.Smith KA, Kraus GE, Johnson BA, Spetzler RF. Giant posterior communicating artery aneurysm presenting as third ventricle mass with obstructive hydrocephalus. Case report. J Neurosurg. 1994;81:299–303. doi: 10.3171/jns.1994.81.2.0299. [DOI] [PubMed] [Google Scholar]
- 22.Thron A, Bockenheimer S. Giant aneurysms of the posterior fossa suspected as neoplasms on computed tomography. Neuroradiology. 1978;18:93–97. doi: 10.1007/BF00344829. [DOI] [PubMed] [Google Scholar]
- 23.Urbach H, Meyer B, Cedzich C, Solymosi L. Posterior inferior cerebellar artery aneurysm in the fourth ventricle. Neuroradiology. 1995;37:267–269. doi: 10.1007/BF00588330. [DOI] [PubMed] [Google Scholar]
- 24.Yamaura A, Isobe K, Nakamura T, Ono J, Makino H. Posterior inferior cerebellar aneurysm in the fourth ventricle. Surg Neurol. 1980;13:297–299. [PubMed] [Google Scholar]