Abstract
Objective
Cerebral vasospasm still remains a major cause of the morbidity and mortality, despite the developments in treatment of aneurysmal subarachnoid hemorrhage. The authors measured the utility and benefits of external lumbar cerebrospinal fluid (CSF) drainage to prevent the clinical vasospasm and its sequelae after endovascular coiling on aneurysmal subarachnoid hemorrhage in this randomized study.
Methods
Between January 2004 and March 2006, 280 patients with aneurysmal subarachnoid hemorrhage were treated at our institution. Among them, 107 patients met our study criteria. The treatment group consisted of 47 patients who underwent lumbar CSF drainage during vasospasm risk period (about for 14 days after SAH), whereas the control group consisted of 60 patients who received the management according to conventional protocol without lumbar CSF drainage. We created our new modified Fisher grade on the basis of initial brain computed tomography (CT) scan at admission. The authors established five outcome criteria as follows : 1) clinical vasospasm; 2) GOS score at 1-month to 6-month follow-up; 3) shunt procedures for hydrocephalus; 4) the duration of stay in the ICU and total hospital stay; 5) mortality rate.
Results
The incidence of clinical vasospasm in the lumbar drain group showed 23.4% compared with 63.3% of individuals in the control group. Moreover, the risk of death in the lumbar drain group showed 2.1% compared with 15% of individuals in the control group. Within individual modified Fisher grade, there were similar favorable results. Also, lumbar drain group had twice more patients than the control group in good GOS score of 5. However, there were no statistical significances in mean hospital stay and shunt procedures between the two groups. IVH was an important factor for delayed hydrocephalus regardless of lumbar drain.
Conclusion
Lumbar CSF drainage remains to play a prominent role to prevent clinical vasospasm and its sequelae after endovascular coiling on aneurysmal subarachnoid hemorrhage. Also, this technique shows favorable effects on numerous neurological outcomes and prognosis. The results of this study warrant clinical trials after endovascular treatment in patients with aneurysmal SAH.
Keywords: Aneurysm, Subarachnoid hemorrhage, Lumbar drain, Cerebral vasospasm, Coil embolization
INTRODUCTION
The management of patients with aneurysmal subarachnoid hemorrhage (SAH) should achieve two main goals; prevention of rebleeding and treatment of cerebral vasospasm26). Early surgical clipping prevents the subsequent aneurysm bleeding. Recently advanced endovascular coiling also prevents the rebleeding. Despite the developments in management of aneurysmal subarachnoid hemorrhage, cerebral vasospasm still remains an important cause of the morbidity and mortality. Cerebral vasospasm usually develops 3-4 days after SAH, continues for 10-14 days.
Various pathophysiologic mechanisms which explain about cerebral vasospasm have been proposed in the previous literatures, but it is not yet clear that the definite mechanism which induces cerebral vasospasm. Optimal management for the cerebral vasospasm thus is not fully established.
Considerable clinical and experimental evidences have been reported that the volume and duration of subarachnoid blood clots are directly related to development and severity of cerebral vasospasm10,12,37). Moreover, the removal of the blood clots and irrigation of the cisterns performed by surgery have been reported to reduce the risk of cerebral vasospasm13,18,23). Nevertheless, it is difficult to completely remove all blood clots performed by surgical clipping. And, in contrast to surgical clipping, the endovascular coiling does not allow to remove the subarachnoid blood clots directly26). Several reports17,20,28) have emphasized that it is effective for preventing cerebral vasospasm to perform additional method, in particular, external lumbar cerebrospinal fluid (CSF) drainage after surgical clipping. But, there is no report on the effect of external lumbar CSF drainage after endovascular coiling on aneurysmal subarachnoid hemorrhage. In this study, we examined the utility and benefits of external lumbar CSF drainage to prevent the clinical vasospasm and its sequelaes after endovascular coiling on aneurysmal subarachnoid hemorrhage.
MATERIALS AND METHODS
Patients demographic findings
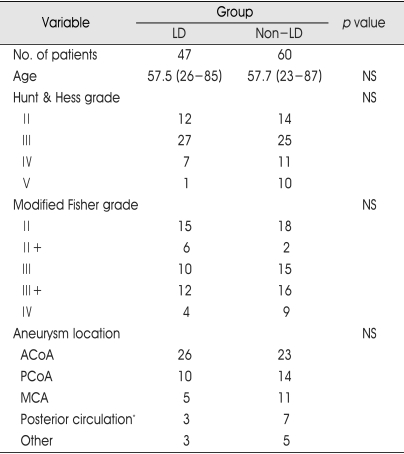
Between January 2004 and March 2006, 280 patients with aneurysmal subarachnoid hemorrhage underwent surgical clipping or endovascular coiling at our institution. The patients who underwent surgical clipping and the patients with unruptured aneurysm were excluded from this study. We also excluded the patients who presented in severe neurological state at admission so that could not underwent any surgical clipping and endovascular coiling and the patients who underwent coiling, but died early. Also, the asymptomatic patients with Fisher grade 1 were excluded because SAH was not visible on brain computed tomography (CT) scan with the patients so that we supposed the effect of lumbar drain was minimal. This left 107 patients who were evaluated by Hunt and Hess grade at admission. Furthermore, all were classified by our modified Fisher grade according to the initial brain CT scan. The our modified Fisher grade creates new categories as grade 2+ and grade 3+ for patients accompany with intraventricular hemorrhage (IVH) for the purpose of identifying the influences of IVH on vasospasm and its sequelae. And, the patients with intracranial hemorrhage of 5 ml or more were classified to Fisher grade 4. Among them, we performed lumbar drain to 47 patients and the others did not receive lumbar drain. The characteristics of 107 patients are displayed in Table 1. Clinical records from admission to discharge were available and reviewed.
Table 1.
Characteristics of the lumbar drain and non-lumbar drain groups in 107 patients with aneurysmal subarachnoid hemorrhage underwent endovascualr coiling
LD : lumbar drain, ACoA : anterior communicating artery, PCoA : posterior communicating artery, MCA : middle cerebral artery, NS : not significant. *Includes basilar, vertebral, superior cerebellar, posterior inferior cerebellar, posterior cerebral arteries.
Clinical managements and lumbar drain method
On arrival, all patients were evaluated neurologic exam and checked initial brain CT scan. In addition, all received appropriate medications and procedures, if needed, underwent intubation, central venous catheter insertion, sedation and ventilation therapy. Then, all patients were admitted to the intensive care units (ICU) and received management according to the conventional protocol. This protocol included nimodipine administration, rigorous monitoring, anticonvulsant administration, correction of electrolyte imbalance, ICP control medication, pain control and so on. All patients underwent endovascular coiling as soon as possible, mostly within 1 day after admission. Soon after endovascular coiling, the patients were evaluated with brain CT scan and lumbar X-ray films. When we considered it was feasible and secure, lumbar CSF drains (Integra Neurosciences, USA) were typically placed (mainly punctured at L4-5 level) and maintained throughout the vasospasm risk period (about for 14 days after SAH). The drain rate was set at 5 to 10 ml/hour. Lumbar drain was discontinued when the CSF was no longer visible blood and the vasospasm risk period passed. We examined CSF every 3 days for detecting CSF infection.
Definition of vasospasm
We diagnosed the clinical vasospasm using the following all criteria : 1) newly developed neurological deficits such as confusion, disorientation, increased sleeping tendency focal motor or speech deficits and pupil reflex change; 2) no other cause of neurological deficits such as hyponatremia, hypoxia, infection, pulmonary edema, drug toxicity; 3) negative findings on brain CT scan of secondary hemorrhage, cerebral edema, hydrocephalus.
Outcome criteria
We established five outcome criteria in this study : 1) clinical vasospasm; 2) Glasgow Outcome Scale (GOS) score at 1-month to 6-month follow-up; 3) shunt procedures for hydrocephalus; 4) the duration of stay in the ICU and total hospital stay; and 5) mortality rate.
Statistical analysis
Tests of associations between the outcome criteria and the lumbar CSF drainage were performed using Pearson chi-square method and Mantel-Haenzel chi-sqaure method. Statistical significance was considered at probability values of less than 0.05. Subgroup specific analysis was also performed. Potential confounding factors were evaluated using multivariate logistic regression analysis. The ORs and their 95% CIs obtained from logistic regression models are reported. All statistical analysis was performed using commercially available software (version 12.0, SPSS Institute.).
RESULTS
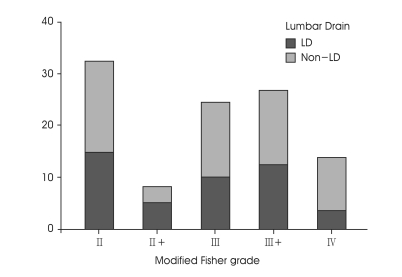
The patients were classified according to our modified Fisher grade (Fig. 1). Regardless of treatment group, 33 patients (30.8%) of Fisher grade 2, 8 patients (7.5%) of Fisher grade 2+, 25 patients (23.4%) of Fisher grade 3, 28 patients (26.2%) of Fisher grade 3+ and 13 patients (12.1%) of Fisher grade 4 were assigned. There were no significant differences in patients age, Hunt and Hess grade, aneurysm location and modified Fisher grade between the two groups (Table 1).
Fig. 1.
Bar graphs showing the proportion of the patients based on our modified Fisher grade. LD : lumbar drain.
Five outcomes analysis
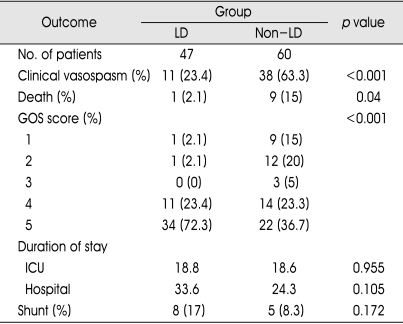
As illustrated in Table 2, lumbar CSF drainage made favorable results on our numerous outcome criteria. That is, the incidence of clinical vasospasm in the lumbar drain group showed 23.4% compared with 63.3% of individuals in the non-lumbar drain group. Moreover, the risk of death in the lumbar drain group showed 2.1% compared with 15% of individuals in the non-lumbar drain group. Those patients who did not receive the lumbar drain had a 2.7-, and 7.1-fold greater risk of suffering clinical vasospasm and aggravating the death than those in whom a lumbar drain was placed. In addition, lumbar drain group had a benefits on GOS score at 1-month to 6-month follow-up compared with the control group. Lumbar drain group had twice more patients than the control group in good GOS score of 5. Reversely, lumbar drain group had from 7 times to 10 times less patients than the control group in poor GOS score of 1 or 2.
Table 2.
Outcomes of the lumbar drain and non-lumbar drain groups in 107 patients with aneurysmal subarachnoid hemorrhage underwent endovascualr coiling
LD : lumbar drain, GOS : Glasgow outcome scale, ICU : intensive care units
However, there were statistically no differences in mean ICU or hospital stay between the two groups. Preferably, the lumbar drain group was longer than the control group in total hospital stay. Among 107 patients, 8 patients (17%) of lumbar drain group and 5 patients (8.3%) of non-lumbar drain group received shunt procedures. This difference also did not reach statistical significance (p=0.172).
Multivariate logistic regression analysis
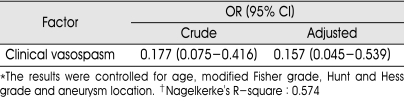
For multivariate logistic regression analysis, the following factors were tested: the presence of lumbar drain, age, our modified Fisher grade, Hunt and Hess grade, aneurysm location. In our study, covariates were considered useful in predicting the outcome strictly if their probability value was less than 0.15 although it seems to be often considered useful if less than 0.25. The factors that were found to predict the clinical vasospasm were as following: the presence of lumbar drain (p<0.001), our modified Fisher grade (p=0.001), anterior circulation aneurysm (p=0.147), Hunt and Hess grade (p=0.068). And, according to independent T-test, mean age of patients suffering clinical vasospasm was 60.6 years, which was older than the patients without clinical vasospasm (mean age : 55.1 years, p=0.029). Table 3 describes the crude ORs for the outcomes and the adjusted ORs from the logistic regression models and their respective 95%. The benefits of lumbar drain with respective outcomes did not change meaningful when controlled for age, modified Fisher grade, Hunt and Hess grade and aneurysm location.
Table 3.
Result of multivariate logistic regression analysis
*The results were controlled for age, modified Fisher grade, Hunt and Hess grade and aneurysm location. †Nagelkerke's R-square : 0.574
Subgroup analysis
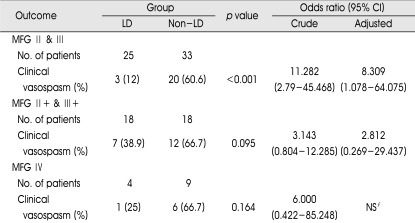
We carried out a subgroup analysis to validate definitely the effects of lumbar CSF drainage within our modified Fisher grade respectively (Table 4). Within our modified Fisher grade 2 and 3, the incidence of clinical vasospasm in the lumbar drain group showed 12% compared with 60.6% of individuals in the non-lumbar drain group. Although the difference did not reach statistical significance, the incidence of clinical vasospasm in the lumbar drain group showed 38.9% compared with 66.7% of individuals in the non-lumbar drain group within our modified Fisher grade 2+ and 3+. That is, regardless of modified Fisher grade, the incidence of clinical vasospasm in the lumbar drain group was less than that in the non-lumbar drain group. However, the incidence of clinical vasospasm in lumbar drain group accompanied with IVH (modified Fisher grade 2+ and 3+) was more than that in the lumbar drain group without IVH (modified Fisher grade 2 and 3). As seen in Table 4, adjusted ORs about clinical vasospasm between the two groups showed that individuals suffering clinical vasospasm in the non-lumbar drain group were more than that in the lumbar drain group when controlled for age, Hunt and Hess grade, and aneurysm location. This means the risk of clinical vasospasm in the lumbar drain group was lower than that in the non-lumbar drain group. Neverthless, adjusted ORs with IVH was lower than that without IVH. Consequently, we considered that IVH would decrease the favorable effect of lumbar drain. On the other hand, there is no statistically significance although lumbar drain brought down the relative risk of clinical vasospasm within modified Fisher grade 4.
Table 4.
Subgroup analaysis in 107 patients with aneurysmal subarachnoid hemorrhage underwent endovascualr coiling
MFG : modified Fisher grade, LD : lumbar drain. NS : not significant due to small cases. *The adjusted ORs were controlled for age, Hunt and Hess grade, and aneurysm location
Correlation between hydrocephalus and IVH
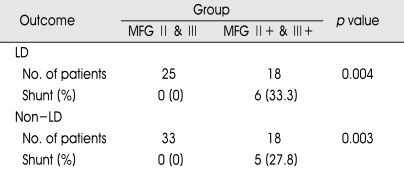
We performed another unique analysis for the purpose of revealing the correlation between hydrocephalus and intraventricular hemorrhage (IVH). We divided additional two groups into the patients with IVH and the patients without IVH except for our modified Fisher grade 4. As seen in Table 5, patients without IVH did not develop delayed hydrocephalus regardless of lumbar drain. But, 33.3% of individuals with IVH in lumbar drain group developed delayed hydrocephalus and received shunt procedures (p=0.004). Likewise, 27.8% of individuals with IVH in non-lumbar drain group developed hydrocephalus (p=0.003). This shows a strong correlation between the development of hydrocephalus and the presence of IVH regardless of lumbar drain.
Table 5.
Subgroup analysis of correlation between shunt procedures and the patients with intraventricualr hemorrhage
MFG : modified Fisher grade, LD : lumbar drain.
DISCUSSION
Although the improvements in treatment of aneurysmal subarachnoid hemorrhage, cerebral vasospasm is the main cause of morbidity and mortality16). Its exact pathophysiologic mechanisms are not fully established and multi-factorial processes that contain oxygen free radicals, endothelin, vascular endothelial growth factor, arachidonic acid derivatives, lipid peroxidation, inactivation of nitrous oxide (NO), activation of protein kinase C system released from blood clots in the subarachnoid spaces and cisterns, are known to be related to cerebral vasospasm3,10,12,14,15,19,21,22,24,31,35-37). There are numerous clinical studies that early surgical removal of blood clots and massive irrigation may reduce the risk of cerebral vasospasm13,18,23). However, it is difficult to remove completely blood colts in subarachnoid spaces and cisterns by surgery. There is no definite evidence that early surgery significantly decrease the occurrence and severity of cerebral vasospasm. In addition, it is impossible to remove the blood clots in subarachnoid spaces and cisterns directly after endovascular coiling for ruptured aneurysm.
Introduction of triple-H (3H) therapy in the early 1980s and calcium channel blocker such as nimodipine in later years decreased the patients with severe cerebral vasospasm from 30 to 20%4,16,20,22,25,30). In the late 1980s, endovascular angioplasty and injection of intra-arterial chemical vasodilators such as papaverine enabled to improve their overall outcome2,6-9,26,32,33). However, because these medications and endovascular intervention are unable to eliminate the blood clots, cerebral vasospasm still contributes to poor outcome in approximately 10 to 40% of patients with dense SAH.
The utility and benefits of lumbar CSF drainage
The theoretical foundation on draining CSF with blood clots from subarachnoid spaces and cisterns prevents and improves the severity of cerebral vasospasm is based on the fact that various substances derived from subarachnoid hemorrhage are related to mechanisms of cerebral vasospasm. Therefore, lumbar CSF drainage seems to be a simple, easy and effective method to wash out the blood clots from subarachnoid spaces and cisterns, consequently protect the clinically cerebral vasospasm. Moreover, lumbar CSF drainage would be expected to promote circulation of clear and newly generated CSF from cerebral ventricles through subarachnoid spaces and also elimination of blood clots from intrathecal space20). In particular, several authors proposed that lumbar CSF drainage after surgical clipping show positive influences on incidence of cerebral vasospasm, GOS score, cerebral infarct, mean hospital stay17,20,28). This study is the first description which reported the effects of lumbar drain after endovascular coiling on aneurysmal SAH. In this study, we also obtained the good results that lumbar drain showed prominent favorable effects on reducing the incidence of clinical vasospasm, preventing the death and recovery to fine GOS score. Within respective our modified Fisher grades except for grade 4, the good effects of lumbar drain were substantiated again. But, the incidence of clinical vasospasm in the lumbar drain group with IVH was more frequently developed comparing with the lumbar drain group without IVH. Consequently, it means that IVH would decrease the favorable effect of lumbar drain. On the other hand, we did not find statistical significant effects on mean hospital stay and hydrocephalus. Instead, our study showed IVH was the powerful factor of delayed hydrocephalus regardless of lumbar drain (Table 5).
Results from several reports have indicated that direct ventricular CSF drainage resulted in preventing the vasospasm after aneurysmal SAH13,17,18,29,34). On the other hand, direct CSF draining from lateral ventricle may cause stasis blood clots within subarachnoid spaces, so that ventricular drainage such like extraventricular drainage (EVD) may deteriorate cerebral vasospasm in fact. Klimo and colleagues20) presented the lumbar CSF drainage was superior than ventricular CSF drainage in the management of vasospasm. In comparison with ventricular CSF drainage, lumbar CSF drainage is more safe without being a direct cause of brain parenchymal damage like intracranial hemorrhage.
Ochiai et al28) claimed the necessity of sedating the patients to maintain the lumbar CSF drainage. But, we did not sedate routinely the patients with lumbar drain because of close surveillance and an experienced team together with neurologically trained intensive care nurses.
Complications of lumbar CSF drainage
Lumbar drain is recently used in many neurosurgical procedures that may be associated with various complications. Spinal nerve root injury, vital organ injury around the spinal cord and post-dural puncture headache are reported as complicatons of lumbar CSF drainage20). In addition, cerebral herniation caused to death or severe neurological impairment are the most serious complication of lumbar CSF dainage, especially in patients with increased intracranial pressure. For the purpose of avoiding these complications, we have identified brain CT scan and lumbar x-ray films before lumbar drain. Although several reports have been referred to infections associated with lumbar drain, Klimo and colleagues20) advocated ventricular drainage such like EVD and lumbar drain were equal in occurrence and possibility of infection. In our study, none of these complications were evident.
CONCLUSION
In this study, lumbar CSF drainage surprisingly showed the reduction of clinical vasospasm following endovascular coiling on aneurysmal subarachnoid hemorrhage. Also, this method favorably influenced the GOS score at 1-month to 6-month follow-up. Lumbar drain may wash out the various substances derived from subarachnoid hemorrhage and related to mechanisms of cerebral vasospasm. However, there were no statistically differences between the two groups in the mean stay of ICU, total hospital stay and shunt procedures. On the other hand, the risk of delayed hydrocephalus depended upon the presence of IVH regardless of lumbar drain.
References
- 1.Adams HP, Jr, Kassel NF, Torner JC, Haley EC., Jr Predicting cerebral ischemia after aneurysmal subarachnoid hemorrhage : influences of clinical condition, CT results, and antifibrinolytic therapy. A report of the Cooperative Aneurysm Study. Neurology. 1987;37:1586–1591. doi: 10.1212/wnl.37.10.1586. [DOI] [PubMed] [Google Scholar]
- 2.Andaluz N, Tomsick TA, Tew JM, Jr, van Loveren HR, Yeh HS, Zuccarello M. Indication for endovascular therapy for refractory vasospasm after aneurysmal subarachnoid hemorrhage : experience at the University of Cincinnati. Surg Neurol. 2002;58:131–138. doi: 10.1016/s0090-3019(02)00789-9. discussion 138. [DOI] [PubMed] [Google Scholar]
- 3.Asano T, Takakura K, Sano K, Kikuchi H, Nagai H, Saito I, et al. Effects of a hydroxyl radical scavenger on delayed ischemic neurological deficits following aneurysmal subarachnoid hemorrhage : results of a multicenter, placebo-controlled double-blind trial. J Neurosurg. 1996;84:792–803. doi: 10.3171/jns.1996.84.5.0792. [DOI] [PubMed] [Google Scholar]
- 4.Awad IA, Carter LP, Spetzler RF, Medina M, Williams FC., Jr Clinical vasospasm after subarachnoid hemorrhage : response to hypervolemic hemodilution and arterial hypertension. Stroke. 1987;18:365–372. doi: 10.1161/01.str.18.2.365. [DOI] [PubMed] [Google Scholar]
- 5.Corsten L, Raja A, Gruppy K, Roitberg B, Misra M, Charbel F, et al. Contemporary management of subarachnoid hemorrhage and vasospasm : the UIC experience. Surg Neurol. 2001;56:140–148. doi: 10.1016/s0090-3019(01)00513-4. discussion 148-150. [DOI] [PubMed] [Google Scholar]
- 6.Dalbasti T, Karabiyikoglu M, Ozdamar N, Oktar N, Cagli S. Efficacy of controlled-release papaverine pellets in preventing symptomatic cerebral vasospasm. J Neurosurg. 2001;95:44–50. doi: 10.3171/jns.2001.95.1.0044. [DOI] [PubMed] [Google Scholar]
- 7.Elliott JP, Newell DW, Lam DJ, Eskridge JM, Douville CM, Le Roux PD, et al. Comparison of balloon angioplasty and papaverine infusion for the treatment of vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg. 1998;88:277–284. doi: 10.3171/jns.1998.88.2.0277. [DOI] [PubMed] [Google Scholar]
- 8.Eskridge JM, McAuliffe W, Song JK, Deliganis AV, Newell DW, Lewis DH, et al. Balloon angioplasty for the treatment of vasospasm : results of first 50 cases. Neurosurgery. 1998;42:510–516. doi: 10.1097/00006123-199803000-00016. discussion 516-517. [DOI] [PubMed] [Google Scholar]
- 9.Firlik KS, Kaufmann AM, Firlik AD, Jungreis CA, Yonas H. Intra-arterial papaverine for the treatment of cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Surg Neurol. 1999;51:66–74. doi: 10.1016/s0090-3019(97)00370-4. [DOI] [PubMed] [Google Scholar]
- 10.Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by CT scanning. Neurosurgery. 1980;6:1–9. doi: 10.1227/00006123-198001000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Graves VB, Strother CM, Duff TA, Perl J., 2nd Early treatment of ruptured aneurysms with Guglielmi detachable coils : effect of subsequent bleeding. Neurosurgery. 1995;37:640–647. doi: 10.1227/00006123-199510000-00006. discussion 647-648. [DOI] [PubMed] [Google Scholar]
- 12.Handa Y, Weir BK, Nosko M, Mosewich R, Tsuji T, Grace M. The effect of timing of clot removal on chronic vasospasm in primate model. J Neurosurg. 1987;67:558–564. doi: 10.3171/jns.1987.67.4.0558. [DOI] [PubMed] [Google Scholar]
- 13.Inagawa T, Kamiya K, Matsuda T. Effect of continuous cisternal drainage on cerebral vasospasm. Acta Neurochir (Wien) 1991;112:28–36. doi: 10.1007/BF01402451. [DOI] [PubMed] [Google Scholar]
- 14.Juvela S. Plasma endothelin and big endothelin concentrations and serum endothelin-converting enzyme activity following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2002;97:1287–1293. doi: 10.3171/jns.2002.97.6.1287. [DOI] [PubMed] [Google Scholar]
- 15.Kamezaki T, Yanaka K, Nagase S. Increased levels of lipid peroxidase as predictive of symptomatic vasospasm and poor outcome after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2002;97:1302–1305. doi: 10.3171/jns.2002.97.6.1302. [DOI] [PubMed] [Google Scholar]
- 16.Kassell NF, Torner JC, Haley EC, Jr, Jane JA, Adams HP, Kongable GL. The International Cooperative Study on the Timing of Aneurysm Surgery. Part 1 : overall management results. J Neurosurg. 1990;73:18–36. doi: 10.3171/jns.1990.73.1.0018. [DOI] [PubMed] [Google Scholar]
- 17.Kasuya H, Schimizu T, Kawaga M. The effect of continuous drainage of cerebrospinal fluid in patients with subarachnoid hemorrhage : a retrospective analysis of 108 patients. Neurosurgery. 1991;28:56–59. doi: 10.1097/00006123-199101000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Kawakami Y, Shimamura Y. Cisternal drainage after early operation of ruptured intracranial aneurysm. Neurosurgery. 1987;20:8–14. doi: 10.1227/00006123-198701000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Kim DE, Suh YS, Lee MS, Kim KY, Lee JH, Hong KW, et al. Vascular NAD(P)H oxidase trigers delayed cerebral vasospasm after subarachnoid hemorrhage in rats. Stroke. 2002;33:2687–2691. doi: 10.1161/01.str.0000033071.99143.9e. [DOI] [PubMed] [Google Scholar]
- 20.Klimo P, Jr, Kestle RW, MacDonald JD, Schmidt RH. Marked reductiopn of cerebral vasospasm with lumbar drainage of cerebrospinal fluid after subarachnoid hemorrhage. J Neurosurg. 2004;100:215–224. doi: 10.3171/jns.2004.100.2.0215. [DOI] [PubMed] [Google Scholar]
- 21.Kodama N, Sasaki T, Kawakami M, Sato M, Asari J. Cisternal irrigation therapy with urokinase and ascorbic acid for prevention of vasospasm after aneurysmal subarachnoid hemorrhage : outcome in 217 patients. Surg Neurol. 2000;53:110–117. doi: 10.1016/s0090-3019(99)00183-4. discussion 117-118. [DOI] [PubMed] [Google Scholar]
- 22.McGirt MJ, Lynch JR, Parra A. Simvastatin increase endothelial nitric oxide synthase and ameliorates cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 2002;33:2950–2956. doi: 10.1161/01.str.0000038986.68044.39. [DOI] [PubMed] [Google Scholar]
- 23.Mizukami M, Kawase T, Usami T, Tazawa T. Prevention of vasospasm by early operation with removal of subarachnoid blood. Neurosurgery. 1982;10:301–307. doi: 10.1227/00006123-198203000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Mocco J, Mack WJ, Kim GH, Lozier AP, Laufer I, Kreiter KT, et al. Rise in serum soluble intercellular adhesion molecule-1 levels with vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2002;97:537–541. doi: 10.3171/jns.2002.97.3.0537. [DOI] [PubMed] [Google Scholar]
- 25.Mori K, Arai H, Nakajima K, Tajima A, Maeda M. Hemorheological and hemodynamic analysis of hypervolemic hemodilution therapy for cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 1995;26:1620–1626. doi: 10.1161/01.str.26.9.1620. [DOI] [PubMed] [Google Scholar]
- 26.Murayama Y, Malisch T, Guglielmi G, Mawad ME, Vinuela F, Duckwiler GR, et al. Incidence of cerebral vasospasm after endovascular treatment of acutely ruptured aneurysms : report on 69 cases. J Neurosurg. 1997;87:830–835. doi: 10.3171/jns.1997.87.6.0830. [DOI] [PubMed] [Google Scholar]
- 27.Murayama Y, Song JK, Uda K, Gobin YP, Duckwiler GR, Tateshima S, et al. Combined endovascular treatment for both intracranial aneurysm and symptomatic vasospasm. AJNR Am J Neuroradiol. 2003;24:133–139. [PMC free article] [PubMed] [Google Scholar]
- 28.Ochiai H, Yamakawa Y. Continuous lumbar drainage for the preoperative management of patients with aneurysmal subarachnoid hemorrhage. Neurol Med Chir (Tokyo) 2001;41:576–580. doi: 10.2176/nmc.41.576. discussion 581. [DOI] [PubMed] [Google Scholar]
- 29.Ogura K, Hara M, Tosaki F, Hirai N. Effect of cisternal drainage after early operation for ruptured intracranial aneurysms. Surg Neurol. 1988;30:441–444. doi: 10.1016/0090-3019(88)90028-6. [DOI] [PubMed] [Google Scholar]
- 30.Ohman J, Servo A, Heiskanen O. Long-term effects of nimodipine on cerebral infarcts and outcome after aneurysmal subarachnoid hemorrhage and surgery. J Neurosurg. 1991;74:8–13. doi: 10.3171/jns.1991.74.1.0008. [DOI] [PubMed] [Google Scholar]
- 31.Pilitsis JG, Coplin WM, O'Regan MH, Wellwood JM, Diaz FG, Fairfax MR, et al. Free fatty acids in human cerebrospinal fluid following subarachnoid hemorrhage and their potential role in vasospasm : a preliminary observation. J Neurosurg. 2002;97:272–279. doi: 10.3171/jns.2002.97.2.0272. [DOI] [PubMed] [Google Scholar]
- 32.Polin RS, Coenen VA, Hansen CA, Shin P, Baskaya MK, Nanda A, et al. Efficacy of transluminal angioplasty for the management of symptomatic cerebral vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2000;92:284–290. doi: 10.3171/jns.2000.92.2.0284. [DOI] [PubMed] [Google Scholar]
- 33.Rosenwasser RH, Armonda RA, Thomas JE, Benitez RP, Gannon PM, Harrop J. Therapeutic modalities for the management of cerebral vasospasm : timing of endovascular options. Neurosurgery. 1999;44:975–980. doi: 10.1097/00006123-199905000-00022. [DOI] [PubMed] [Google Scholar]
- 34.Sakaki S, Ohta S, Kuwabara H, Shiraishi M. The role of ventricular and cisternal drainage in the early operation for ruptured intracranial aneurysm. Acta Neurochir (Wien) 1987;88:87–94. doi: 10.1007/BF01404143. [DOI] [PubMed] [Google Scholar]
- 35.Sasaki T, Wakai S, Asano T, Watanabe T, Kirino T, Sano K. The effect of a lipid hydroperoxide of arachidonic acid on the canine basilar artery : an experimental study on cerebral vasospasm. J Neurosurg. 1981;54:357–365. doi: 10.3171/jns.1981.54.3.0357. [DOI] [PubMed] [Google Scholar]
- 36.Vatter H, Mursch K, Zimmermann M, Zilliken P, Kolenda H, Seifert V, et al. Endothelin-converting enzyme activity in human cerebral circulation. Neurosurgery. 2002;51:445–451. discussion 451-452. [PubMed] [Google Scholar]
- 37.Zabramski JM, Spetzler RF, Bonstelle C. Chronic cerebral vasospasm : effect of volume and timing of hemorrhage in a canine model. Neurosurgery. 1986;18:1–6. doi: 10.1227/00006123-198601000-00001. [DOI] [PubMed] [Google Scholar]