Abstract
Objective
Hydrocephalus is a common sequelae of aneurysmal subarachnoid hemorrhage (SAH) and patients who develop hydrocephalus after SAH typically have a worse prognosis than those who do not. This study was designed to identify factors predictive of shunt-dependent chronic hydrocephalus among patients with aneurysmal SAH, and patients who require permanent cerebrospinal fluid diversion.
Methods
Seven-hundred-and-thirty-four patients with aneurysmal SAH who were treated surgically between 1990 and 2006 were retrospectively studied. Three stages of hydrocephalus have been categorized in this paper, i.e., acute (0-3 days after SAH), subacute (4-13 days after SAH), chronic (≥14 days after SAH). Criteria indicating the occurrence of hydrocephalus were the presence of significantly enlarged temporal horns or ratio of frontal horn to maximal biparietal diameter more than 30% in computerized tomography.
Results
Overall, 66 of the 734 patients (8.9%) underwent shunting procedures for the treatment of chronic hydrocephalus. Statistically significant associations among the following factors and shunt-dependent chronic hydrocephalus were observed. (1) Increased age (p < 0.05), (2) poor Hunt and Hess grade at admission (p < 0.05), (3) intraventricular hemorrhage (p < 0.05), (4) Fisher grade III, IV at admission (p < 0.05), (5) radiological hydrocephalus at admission (p < 0.05), and (6) post surgery meningitis (p < 0.05) did affect development of chronic hydrocephalus. However the presence of intracerebral hemorrhage, multiple aneurysms, vasospasm, and gender did not influence on the development of shunt-dependent chronic hydrocephalus. In addition, the location of the ruptured aneurysms in posterior cerebral circulation did not correlate with the development of shunt-dependent chronic hydrocephalus.
Conclusion
Hydrocephalus after aneurysmal SAH seems to have a multifactorial etiology. Understanding predisposing factors related to the shunt-dependent chronic hydrocephalus may help to guide neurosurgeons for better treatment outcomes.
Keywords: Subarachnoid hemorrhage, Ventriculoperitoneal shunt, Chronic hydrocephalus, Related factor
INTRODUCTION
It is known that hydrocephalus is a relatively common complication in aneurysmal subarachnoid hemorrhage (SAH) patients. The prognosis is poor in SAH case complicated by hydrocephalus, and the potential factors affecting the development of hydrocephalus in SAH patient still remain incompletely understood.
Relating factors that have been suggested are the age of patient, sex, intraventricular hemorrhage, Fisher grade, clinical vasospasm, initial mental status, history of hypertension, and site of ruptured aneurysm, and so on8,18,22,28).
As there are still many opinions posed for possible risk factors associating the development of hydrocephalus, this study was designed to analysis various factors in aneurysmal SAH cases, particularly in cases requiring shunt operation and not requiring it, and forecast potential factors affecting the development of hydrocephalus to provide certain indices helpful for treatment of hydrocephalus cases.
MATERIALS AND METHODS
A total of 734 patients were treated with surgical clipping from 1990 to 2006 were surveyed. They were diagnosed by brain computerized tomography (CT) with 3-dimensional angiography and digital substraction angiography (DSA). Patients with unruptured cerebral aneurysm and patients treated with intra-vascular intervention procedure were excluded in this study.
Digital substraction angiography before operation and brain CT before and after operation were taken. Fisher grade, presence of intraventricular hemorrhage (IVH) and intracerebral hemorrhage (ICH) were checked by pre-operative brain CT. Hunt and Hess grade was measured with initial neurological examination.
This study classified hydrocephalus to acute (0-3 days after SAH), subacute (4-13 days after SAH) and chronic (≥ 14 days after SAH) by brain CT26). The criteria indicating hydrocephalus was the presence of significantly enlarged temporal horns or ratio of frontal horn to maximal biparietal diameter more than 30% in brain CT.
The site of ruptured aneurysm was detected by pre-operative DSA. We compared various factors to confirm statistical significance in operated cases and non-operated cases. Those factors are as follows ; (1) age, (2) gender, (3) initial Hunt and Hess grade, (4) initial IVH, (5) initial ICH, (6) initial Fisher grade I, II or III, IV, (7) radiological hydrocephalus at the time of admission, (8) location of ruptured aneurysm, (9) presence of multiple aneurysms, (10) presence of clinical vasospasm and (11) presence of postoperative meningitis.
Data were analized using statistical package SPSS software for windows, version 12.0.
Significance for intergroup difference was assessed by chi-square test for categorical variables and independent-sample t-test for continuous variables.
Logistic regression analysis was performed to determine the factors related to shunt dependent hydrocephalus.
RESULTS
During research period, a total of 734 cases were treated with surgical clipping, 8.9% of them needed shunt operation. Several patients who showed no improvement of symptoms after LP shunt operation were treated again with VP shunt operation.
Eleven factors were compared and analyzed between cases requiring shunt operation and not requiring it, and the results are outlined as follows.
Age
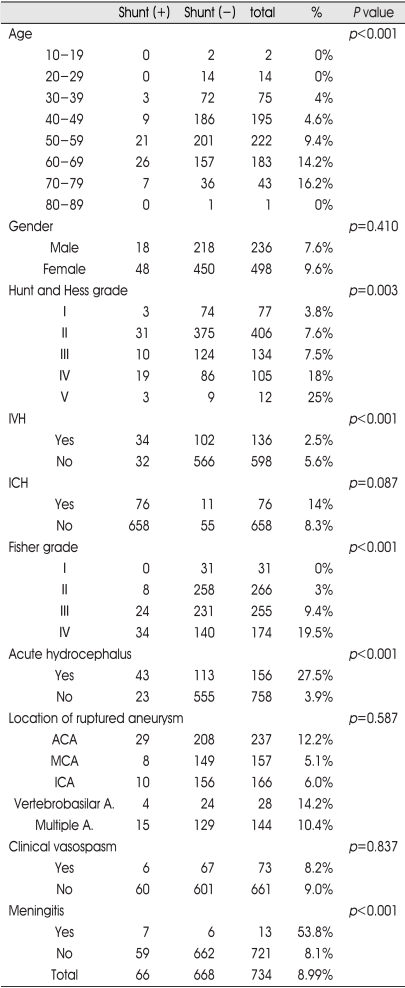
The mean age of the patients was 53 (ranging from 17 to 82). It was found that the mean age of shunt operation cases was 58.4, while that of control was 52.3. That indicates, shunt operation cases were older than control (p<0.001). The older age groups were treated with shunt operation more often (Table 1).
Table 1.
Factors related to shunt dependent hydrocephalus
IVH : intraventricular hemorrhage, ICH : intracerebral hemorrhage, ACA : anterior cerebral artery, MCA : middle cerebral artery, ICA : internal carotid artery, A : artery
Gender
Female group (498 cases) comprised significantly higher ratio of all cerebral aneurismal SAH cases treated with operation than male group (236 cases). Fourty-eight female (9.6%) and 18 male (7.6%) patients were treated with shunt operation. The female group comprised higher ratio of shunt operation cases than the male group (p=0.410) (Table 1).
Hunt and Hess grade
Hunt and Hess grade IV or V group had an shunt operation more frequently than Hunt and Hess grade I or II group (p=0.003) (Table 1).
Intraventricular hemorrhage in initial brain CT
Twenty-five percent of patients with intraventricular hemorrhage (IVH) in initial brain CT were treated with shunt operation. In comparison, 5.6% of patients without IVH in initial brain CT were treated with shunt operation(p<0.001)(Table 1).
Intracerebral hemorrhage in initial brain CT
Fourteen percent of patients with intracerebral hemorrhage (ICH) in initial brain CT were treated with shunt operation. On the other hand, 8.3% of patients without ICH were treated with shunt operation (p=0.087) (Table 1).
Fisher grade in initial brain CT
The Fisher grade I, II group underwent shunt operation by 0% and 3% compared to the Fisher grade III, IV group by 9.4% and 19.5%. This indicates the Fisher grade III, IV group underwent shunt operation much more than the Fisher grade I, II group (p<0.001) (Table 1).
Hydrocephalus in initial brain CT
Twenty-seven-and-five percent of patients with hydrocephalus on initial brain CT were treated with shunt operation. On the other hand, 3.9% of patients without hydrocephalus were treated with shunt operation (p<0.001) (Table 1).
Site of ruptured aneurysm
The number of shunt operation according to the location of the aneurysms detected by pre-operation DSA are as follows ; 4 cases (14.2%) located in posterior circulation, 29 cases (12.2%) located in anterior cerebral artery including anterior communicating artery, 8 cases (5%) located in middle cerebral artery, 10 cases (6%) located at internal carotid artery were requiring shunt operation, and 15 cases (10.4%) located in multiple sites. The 15 cases of aneurysms at multiple sites were excluded in this study because we couldn't detect ruptured sites clearly (Table 1).
There was no significant relationship between location or presence of multiple aneurysms and the requirement of shunt operation in statistical analysis (p=0.587) (Table 1).
Clinical vasospasm
Seventy-three patients who clinical vasospasm were treated with 3H (hypertension, hypervolemia, hemodilution) therapy and 6 cases (8.2%) were treated with shunt operation (p=0.837) (Table 1).
Meningitis after clipping surgery
Meningitis diagnosed by cerebrospinal fluid examination was detected in 13 patients during research period and 7 cases (53.8%) were treated with shunt operation(p<0.001) (Table 1).
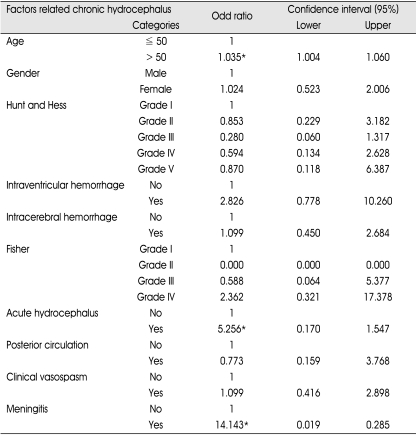
The logistic regression analysis showed that only three factors, age of patient, initial acute hydrocephalus, and postoperative encephalitis, have statistical significance in needing shunt operation (Table 2).
Table 2.
Factors related to shunt dependent hydrocephalus (Logistic regression analysis)
*Statistical significance at 95% confidence interval
DISCUSSION
The previous studies have shown that patients with aneurysmal SAH with ruptured cerebral aneurysms developed hydrocephalus after clipping operation from 6% to 67%5,26). In this study, 8.9% were treated with shunt operation due to chronic hydrocephalus. It was reported that the potential risk factors inducing hydrocephalus after SAH includ old age, posterior circulation aneurysm, IVH, hypertension, poor Fisher grade, poor Hunt and Hess grade, symptomatic vasospasm, female sex, low Glasgow coma scale score, focal ischemia, previous use of anti-fibrinolytic agents, and so on8,18,22,28).
This study analyzed potential factors affecting the development of hydrocephalus after aneurysmal SAH by allowing for these factors.
First, we found that the age of patients as a factor for the development of hydrocephalus. As shown in Table 1, the older age led to the higher ratio of shunt operation, which was comparable to the former findings from the analysis on 300 cases22). In addition, Graff-Radford et al.2,8) analyzed age as a potential factor of hydrocephalus after rupture of cerebral aneurysm, and also showed findings similar to those above.
Second, we found though results from more shunt operation was required in female group than in male group. Other previous studies have been controversial4,16,20,27). It is estimated that these differences would be associated with higher onset of SAH in older female group1,7,12,16,20).
The findings of previous studies have shown that Hunt-Hess grade at admission may work as a potential factor for the development of hydrocephalus, and another previous study showed that mentality of patient at admission may work as a potential factor affecting the development of hydrocephalus8). This study also observed a finding which supports that the poorer Hunt-Hess grade would be more likely to work as potential factor of hydrocephalus with statistical significance. However, this results can be influenced by Hunt-Hess grade V which did not need shunt operation because of their low long-term survival rate.
The presence of IVH on CT at admission may work as a potential factor of hydrocephalus, as reported in many studies2,8,10,17,28). It is estimated that this result possibly attributed to IVH-induced disturbance and obstruction of CSF circulation, and our study also showed results comparable to those found in previous studies.
The cases complicated with ICH on CT at admission showed higher onset rate of hydrocephalus requiring shunt operation. But, there was no statistical significance. One study on 47 spontaneous ICH cases found that the rate of hydrocephalus might depend on the site of ICH23), but we didn't analyze the site of ICH.
Many previous studies showed that low initial Fisher grade may be a risk factor for the development of hydrocephalus in aneurysmal SAH cases4,8,9,14,26). In this study, we noted a finding similar to studies previously conducted. But, another study on 897 cerebral aneurysm rupture cases reported that low initial Fisher grade was not a risk factor influencing shunt operation21).
Since hydrocephalus was classified to acute and chronic by Foltz & Ward5,10,27) in 1956, there have been a series of studies on possible mechanisms of hydrocephalus depending on the onset time after SAH. It is suggested that acute hydrocephalus results mainly from hemorrhage-induced obstruction of forth ventricle and basal cistern5,10,27). In our study, SAH cases with acute hydrocephalus showed higher frequency of chronic hydrocephalus later requiring shunt operation, contrary to SAH cases without acute cerebral hydrocephalus. Another study reported that ferritin level in CSF may be useful index to predict the development of ocases at admission showed higher CSF ferritin level than other cases24,25).
The site of ruptured aneurysm can also affect the development of hydrocephalus. According to several previous studies, it seems that ruptured aneurysm in posterior circulations required shunt operation more often5,10,27). This study showed similar result, but there was no statistical significance.
In our study, cases that had clinical vasospasm showed more requirements for shunt operation, but there was also no statistical significance. According to a report on 718 cases by Dorai et al.,5), it was found that 152 cases (21.2%) were treated with shunt operation. One-hundred-and-two cases showed vasospasm symptoms, and 36 cases of them (35.3%) were treated with shunt operation5) and other previous studies reported similar findings3,6,11).
In this study, 13 cases were complicated with meningitis, and 7 cases of them (54%) required shunt operation. It suggested that meningitis was associated with the duration of indwelling catheter due to acute hydrocephalus, and there have been many similar reports13,29,30).
CONCLUSION
There have been a series of studies that are on potential risk factors of hydrocephalus after SAH caused by ruptured cerebral aneurysm requiring shunt operation. It has been often reported that those cases requiring shunt operation show worse in prognosis than other cases that didn't require shunt operation. In this study, we found several statistically significant factors the onset of hydrocephalus. Understanding and awareness of these factors related to and forecasting long-term disease course, it is expected that treatment of SAH will make prognosis better.
References
- 1.Aho K, Fogelholm R. Incidence and early prognosis of stroke in Espoo-Kauniainen area, Finland, in 1972. Stroke. 1974;5:258–261. [PubMed] [Google Scholar]
- 2.Auer LM, Mokry M. Disturbed cerebrospinal fluid circulation after subarachnoid hemorrhage and acute aneurysm surgery. Neurosurgery. 1990;26:804–808. doi: 10.1097/00006123-199005000-00012. discussion 808-809. [DOI] [PubMed] [Google Scholar]
- 3.Black PM. Hydrocephalus and vasospasm after subarachnoid hemorrhage from ruptured intracranial aneurysms. Neurosurgery. 1986;18:12–16. doi: 10.1227/00006123-198601000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Donauer E, Reif J, al-Khalaf B, Mengedoht EF, Faubert C. Intraventricular hemorrhage caused by aneurysms and angiomas. Acta Neurochir (Wien) 1993;122:23–31. doi: 10.1007/BF01446982. [DOI] [PubMed] [Google Scholar]
- 5.Dorai Z, Hynan LS, Kopitnik TA, Samson D. Factors related to hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2003;52:763–769. doi: 10.1227/01.neu.0000053222.74852.2d. discussion 769-771. [DOI] [PubMed] [Google Scholar]
- 6.Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6:1–9. doi: 10.1227/00006123-198001000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Fogelholm R, Hernesniemi J, Vapalahti M. Impact of early surgery on outcome after aneurysmal subarachnoid hemorrhage. Apopulation-based study. Stroke. 1993;24:1649–1654. doi: 10.1161/01.str.24.11.1649. [DOI] [PubMed] [Google Scholar]
- 8.Graff-Radford NR, Torner J, Adams HP, Kassell NF. Factors associated with subarachnoid hemorrhage. A report of the Cooperative Aneurysm Study. Arch Neurol. 1989;46:744–752. doi: 10.1001/archneur.1989.00520430038014. [DOI] [PubMed] [Google Scholar]
- 9.Gruber A, Reinprecht A, Bavinzski G, Czech T, Richling B. Chronic shunt dependent hydrocephalus after early surgical and early endovascular treatment of ruptured intracranial aneurysms. Neurosurgery. 1999;44:503–509. doi: 10.1097/00006123-199903000-00039. discussion 509-512. [DOI] [PubMed] [Google Scholar]
- 10.Hasan D, Tanghe HL. Distribution of cisternal blood in patients with acute hydrocephalus after subarachnoid hemorrhage. Ann Neurol. 1992;31:374–378. doi: 10.1002/ana.410310405. [DOI] [PubMed] [Google Scholar]
- 11.Heros RC, Zervas NT, Varsos V. Cerebral vasospasm after subarachnoid hemorrhage : an update. Ann Neurol. 1983;14:599–608. doi: 10.1002/ana.410140602. [DOI] [PubMed] [Google Scholar]
- 12.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women : Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 13.James HE, Walsh JW, Wilson HD, Connor JD. The management of cerebrospinal fluid shunt infection : a clinical experience. Acta Neurochir (Wien) 1981;59:157–166. doi: 10.1007/BF01406345. [DOI] [PubMed] [Google Scholar]
- 14.Kinugasa K, Kamata I, Hirotsune N, Tokunaga K, Sugiu K, Handa A, et al. Early treatment of subarachnoid hemorrhage after preventing rerupture of an aneurysm. J Neurosurg. 1995;83:34–41. doi: 10.3171/jns.1995.83.1.0034. [DOI] [PubMed] [Google Scholar]
- 15.Kongable GL, Lanzino G, Germanson TP, Truskowski LL, Alves WM, Torner JC, et al. Gender-related differences in aneurysmal subarachnoid hemorrhage. J Neurosurg. 1996;84:43–48. doi: 10.3171/jns.1996.84.1.0043. [DOI] [PubMed] [Google Scholar]
- 16.Longstreth WT, Nelson LM, Koepsell TD, van Belle G. Subarachnoid hemorrhage and hormonal factors in women. A population-based casecontrol study. Ann Intern Med. 1994;121:168–173. doi: 10.7326/0003-4819-121-3-199408010-00002. [DOI] [PubMed] [Google Scholar]
- 17.Mehta V, Holness RO, Connolly K, Walling S, Hall R. Acute hydrocephalus following aneurysmal subarachnoid hemorrhage. Can J Neurol Sci. 1996;23:40–45. doi: 10.1017/s0317167100039160. [DOI] [PubMed] [Google Scholar]
- 18.Pietilä TA, Heimberger KC, Palleske H, Brock M. Influence of aneurysm location on the development of chronic hydrocephalus following SAH. Acta Neurochir (Wien) 1995;137:70–73. doi: 10.1007/BF02188784. [DOI] [PubMed] [Google Scholar]
- 19.Schmieder K, Koch R, Lüke S, Harders A. Factors influencing shunt dependency after aneurysmal subarachnoid haemorrhage. Zentralbl Neurochir. 1999;60:133–140. [PubMed] [Google Scholar]
- 20.Schwartz SM, Petitti DB, Siscovick DS, Longstreth WT, Jr, Sidney S, Raghunathan TE, et al. Stroke and use of low dose oral contraceptives in young women : A pooled analysis of two US studies. Stroke. 1998;29:2277–2284. doi: 10.1161/01.str.29.11.2277. [DOI] [PubMed] [Google Scholar]
- 21.Sheehan JP, Polin RS, Sheenan JM, Baskaya MK, Kassell NF. Factors associated with hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery. 1999;45:1120–1127. doi: 10.1097/00006123-199911000-00021. discussion 1127-1128. [DOI] [PubMed] [Google Scholar]
- 22.Spallone A, Gagliardi FM. Hydrocephalus following aneurysmal SAH. Zentralbl Neurochir. 1983;44:141–150. [PubMed] [Google Scholar]
- 23.Sumer MM, Acikgoz B, Akpinar G. External ventricular drainage for acute obstructive hydrocephalus developing following spontaneous intracerebral haemorrhages. Neurol Sci. 2002;23:29–33. doi: 10.1007/s100720200020. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki H, Muramatsu M, Kojima T, Taki W. Intracranial heme metabolism and cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 2003;34:2796–2800. doi: 10.1161/01.STR.0000103743.62248.12. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki H, Muramatsu M, Tanaka K, Fujiwara H, Kojima T, Taki W. Cerebrospinal fluid ferritin in chronic hydrocephalus after aneurysmal subarachnoid hemorrhage. J Neurol. 2006;253:1170–1176. doi: 10.1007/s00415-006-0184-1. [DOI] [PubMed] [Google Scholar]
- 26.Vale FL, Bradley EL, Fisher WS., 3rd The relationship of subarachnoid hemorrhage and the need for postoperative shunting. J Neurosurg. 1997;86:462–466. doi: 10.3171/jns.1997.86.3.0462. [DOI] [PubMed] [Google Scholar]
- 27.van Gijn J, Hijdra A, Wijdicks EF, Vermeulen M, van Crevel H. Acute hydrocephalus after aneurysmal subarachnoid hemorrhage. J Neurosurg. 1985;63:355–362. doi: 10.3171/jns.1985.63.3.0355. [DOI] [PubMed] [Google Scholar]
- 28.Vermeij FH, Hasan D, Vermeulen M, Tanghe HL, van Gijn J. Predictive factors for deterioration from hydrocephalus after subarachnoid hemorrhage. Neurology. 1994;44:1851–1855. doi: 10.1212/wnl.44.10.1851. [DOI] [PubMed] [Google Scholar]
- 29.Villarejo FJ. Postoperative ventriculitis in hydrocephalus : Treatment with external ventricular drainage. Acta Neurochir (Wien) 1979;48:41–45. doi: 10.1007/BF01406019. [DOI] [PubMed] [Google Scholar]
- 30.Widenka DC, Wolf S, Schurer L, Plev DV, Lumenta CB. Factors leading to hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurol Neurochir Pol. 2000;34(6 Suppl):56–60. [PubMed] [Google Scholar]