Abstract
Astroblastoma is a rarely diagnosed primary brain neoplasm whose histogenesis has been clarified recently. It occurs in children and young adults and presents as a well circumscribed, contrast-enhancing lesion in the cerebral hemisphere. We present a case of 20-year-old woman with an astroblastoma in the left temporal convexity that was treated with total tumor resection alone. We thought the mass was extra-axial neoplasm based on radiological findings of computed tomography and magnetic resonance imaging initially, but later, we obtained angiographic findings suggesting an intra-axial neoplasm. The patient is doing well even two years after surgery. The characteristic radiological and histopathological features of this case are described with a literature review. An astroblastoma should be included in the differential diagnosis of a superficially located tumor presenting with the findings of an extra-axial mass, especially in a young patient.
Keywords: Astroblastoma, Intra-axial neoplasm
INTRODUCTION
Astroblastoma is a rare and controversial brain neoplasm accounting for 0.45 to 2.8% of brain gliomas12). Although its histogenesis has been clarified recently, controversies exist regarding its cellular origin and validity as a distinct entity. Astroblastoma occurs mainly during 10-30 years of age, with a female predominance of 63%4,11). It is well-defined, solid or cystic, often situated superficially in the cerebral hemisphere, and exhibits characteristic astroblastic pseudorosettes and perivascular hyalinization4,9). This report describes the case of a young woman with a large cerebral astroblastoma in the left temporal convexity with its clinical, radiological, and pathological features.
CASE REPORT
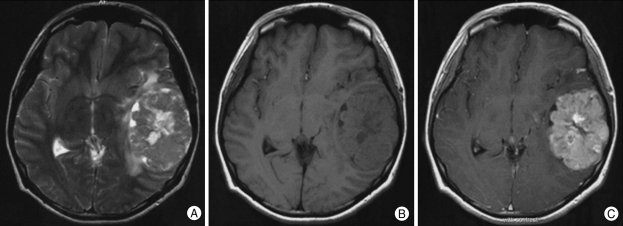
In May 2005, a 20-year-old woman presented with an 8-month history of headache. Neurological examination revealed no abnormalities. Cranial computed tomography (CT) revealed a large, isodense, superficial, heterogeneously enhanced mass with broad base on the left temporal convexity. It contained a multifocal cystic component (Fig. 1). Cranial magnetic resonance imaging (MRI) revealed a 6 × 5 × 4 cm well demarcated mass that was isointense with partially high signal intensity (bubble-like appearance) on T2-weighted images and isointense on T1-weighted images with cleft like area in medial border of the tumor. After contrast injection, a strong heterogeneous contrast enhancement was observed (Fig. 2). There was little evidence of peritumoral edema. CT and MRI findings suggested the diagnosis of an extra-axial neoplasm. Angiographic findings revealed a hypervascular intra-axial mass vascularized by the distal branches of the left middle cerebral artery and severe tumor-induced vascular displacements and stretching (Fig. 3). The patient underwent left frontotemporoparietal craniotomy with removal of tumor in the supine position at 10 days postadmission. The tumor located beneath the dura mater was lobulated, soft, pinkish, well circumscribed, and suckable, and the cyst contained dark-yellow fluid. It was completely removed macroscopically. Postoperative recovery was uneventful, and she was discharged on the 18th postoperative day. Histopathological examination revealed perivascular pseudorosettes on hematoxylin and eosin staining, a typical finding of astroblastoma, and hyaline thickening and mild sclerosis of some vessel walls (Fig. 4). The nuclei were round, contained compact chromatin, and showed infrequent mitosis. Immunohistochemical analysis revealed positive staining for glial fibrillary acidic protein (GFAP), S-100 protein, vimentin, epithelial membrane antigen, neuron-specific enolase, and cytokeratin (Fig. 5). Ki-67 labeling index was 16% and p53 positivity was 14.8%. Pathological diagnosis was confirmed as an astroblastoma. Follow-up MRI at 19 months postoperatively did not reveal tumor recurrence. The patient is doing well 2 years postoperatively.
Fig. 1.
Cranial computed tomography scans showing a large, isodense, superficial, heterogeneously enhanced mass with broad base on the left temporal convexity (A, B). Note the multifocal cystic component within the tumor.
Fig. 2.
Cranial magnetic resonance imaging showing an isointense mass with partially high signal intensity (bubble-like appearance) and little evidence of peritumoral edema on T2-weighted images (A) and T1-weighted images (B) with cleft like area in medial border of the tumor. After contrast injection, a strong heterogeneous contrast enhancement is observed (C).
Fig. 3.
Anteroposterior (A) and lateral view (B) of left internal carotid artery angiograpy showing a hypervascular intra-axial mass vascularized by the distal branches of the left middle cerebral artery and tumor-induced vascular displacements and stretching.
Fig. 4.
Histopathological examination of the surgical specimen (A, B) showing perivascular pseudorosettes, hyaline thickening and mild sclerosis of some vessel walls (H & E, ×400).
Fig. 5.
Photomicrographs of immunostained tissue samples showing different degrees of immunoreactivity with antibodies. The tumor cells were positive for glial fibrillary acidic protein (A), S-100 protein (B), vimentin (C), epithelial membrane antigen (D), neuron-specific enolase (E), and cytokeratin (F).
DISCUSSION
Bailey and Cushing initially described astroblastoma in 19263), and their data were supported by Bailey and Bucy in 19302). Astroblastoma is classified under neuroepithelial tumors of uncertain origin by the 2000 World Health Organization (WHO) classification10). Its histogenesis remains controversial. Kubota T et al.9) assumed that astroblastomas are of astrocytic origin or come from the ependymal lineage. Because astroblastoma exhibits a highly variable biological behavior, a WHO grade has not been established yet. Based on morphology, astroblastomas are classified into prognostically favorable 'low-grade/well-differentiated' and unfavorable 'high-grade/anaplastic' groups. The former shows perivascular pseudorosettes, low to moderate number of mitotic figures, minimal cellular atypia, little or no vascular endothelial proliferation, and predominant sclerosis of vascular walls. The latter exhibits cytologic atypias, compact cellularity, perivascular cells, high mitotic rates, and vascular endothelial hypertropy5).
MRI typically reveals a large, well-delineated, lobulated superficial mass with cystic and solid components, frequently demonstrating a multicystic 'bubbly' appearance. The 'bubbly' appearance likely results from the angioarchitecture of tumor causing signal voids on MRI4). It is hyperintense to white matter on fast fluid-attenuated inversion recovery (FLAIR) images and T2-weighted images and hypointense to isointense on T1-weighted images4). The lesion has a relatively low peritumoral T2 hyperintensity despite of their potentially large size, suggesting no local infiltration in the surrounding brain tissue4). It shows heterogeneous and rim enhancements on both contrast-enhanced T1-weighted and CT images. The rim enhancement is probably due to peripheral displacement of vessels with tumor enlargement. Occasionally, astroblastomas resemble an extra-axial neoplasm on MRI, leading to their preoperative misdiagnosis13). Astroblastoma has been reported as a hypervascular mass on the cerebral convexity1,8). We should have looked at the CT and MRI findings more carefully. We misinterpreted the lesion as an extra-axial mass with broad base on the left temporal convexity and with cleft like area in medial border of the tumor. However, cerebrovascular angiographic findings suggested an intra-axial mass, and these findings are beneficial for preoperative preparations.
Astroblastomas are relatively well circumscribed and grow by expansion rather than infiltration. Such a growth pattern cannot distinguish them from hemispheric cerebral ependymomas. The distinguishing features of astroblastomas are short and thick cytoplasmic processes of perivascular pseudorosettes, hylanized or even sclerosed blood vessels, rarefied spaces between pseudorosettes, and the presence of an abundant fibrillary pattern9). A recent study demonstrated that astroblastomas have characteristic chromosomal aberrations because they exhibit gain of chromosomes 19 and 20, suggesting that astroblastoma is a distinct entity rather than a variant of ependymoma6).
Since astroblastomas are rare, optimal treatment protocols have not been established. Their natural history seems to be between astrocytomas and glioblastomas1). Several investigators have found that its prognosis may be predicted by the histology and extent of resection1,5,7). Low-grade astroblastomas may be successfully treated with gross total resection, without requiring adjuvant therapy; their prognosis is similar to that of low-grade gliomas11). Treatment of high-grade astroblastomas, which involves surgery, remains controversial with ill-defined roles of adjuvant chemotherapy and radiation. Its prognosis corresponds to that of anaplastic astrocytomas5,11).
CONCLUSION
Astroblastomas should be considered in the differential diagnosis of a superficially located tumors with MRI findings of an extra-axial mass, particularly in young adults. Preoperative cerebral angiography may help in preventing the misdiagnosis as extra-axial tumor.
Acknowledgement
This paper was supported by Wonkwang University in 2006.
References
- 1.Alaraj A, Chan M, Oh S, Michals E, Valyi-Nagy T, Hersonsky T. Astroblastoma presenting with intracerebral hemorrhage misdiagnosed as dural arteriovenous fistula : review of a rare entity. Surg Neurol. 2007;67:308–313. doi: 10.1016/j.surneu.2006.05.050. [DOI] [PubMed] [Google Scholar]
- 2.Bailey P, Bucy PC. Astroblastomas of the brain. Acta Psychiatr Neurol. 1930;5:439–461. [Google Scholar]
- 3.Bailey P, Cushing H. A classification of the Tumors of the Glioma Group on a Histogenetic Basis with a Correlated Study of Prognosis. Philadelphia: J.B. Lippincott Company; 1926. pp. 83–84.pp. 134–167. [Google Scholar]
- 4.Bell JW, Osborn AG, Salzman KL, Blaser SI, Jones BV, Chin SS. Neuroradiologic characteristics of astroblastoma. Neuroradiology. 2007;49:203–209. doi: 10.1007/s00234-006-0182-0. [DOI] [PubMed] [Google Scholar]
- 5.Bonnin JM, Rubinstein LJ. Astroblastomas : a pathological study of 23 tumors, with a postoperative follow-up in 13 patients. Neurosurgery. 1989;25:6–13. [PubMed] [Google Scholar]
- 6.Brat DJ, Hirose Y, Cohen KJ, Feuerstein BG, Burger PC. Astroblastoma : clinicopathologic features and chromosomal abnormalities defined by comparative genomic hybridization. Brain Pathol. 2000;10:342–352. doi: 10.1111/j.1750-3639.2000.tb00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caroli E, Salvati M, Esposito V, Orlando ER, Giangaspero F. Cerebral astroblastoma. Acta Neurochir (Wien) 2004;146:629–633. doi: 10.1007/s00701-004-0230-7. [DOI] [PubMed] [Google Scholar]
- 8.Han YM, Kim JT, Chung DS, Park YS. A Case of Astroblastoma. J Korean Neurosurg Soc. 2006;40:373–376. [Google Scholar]
- 9.Kubota T, Sato K, Arishima H, Takeuchi H, Kitai R, Nakagawa T. Astroblastoma : immunohistochemical and ultrastructural study of distinctive epithelial and probable tanycytic differentiation. Neuropathology. 2006;26:72–81. doi: 10.1111/j.1440-1789.2006.00636.x. [DOI] [PubMed] [Google Scholar]
- 10.Lantos PL, Rosenblum MK. Astroblastoma, Pathology and genetics. In: Kleihues P, Cavenee WK, editors. Tumours of the Nervous System. World Health Organization Classification of Tumours. Lyon: International Agency for Research on Cancer Press; 2000. pp. 88–89. [Google Scholar]
- 11.Navarro R, Reitman AJ, de León GA, Goldman S, Marymont M, Tomita T. Astroblastoma in childhood : pathological and clinical analysis. Childs Nerv Syst. 2005;21:211–220. doi: 10.1007/s00381-004-1055-7. [DOI] [PubMed] [Google Scholar]
- 12.Pizer BL, Moss T, Oakhill A, Webb D, Coakham HB. Congenital astroblastoma : an immunohistochemical study. Case report. J Neurosurg. 1995;83:550–555. doi: 10.3171/jns.1995.83.3.0550. [DOI] [PubMed] [Google Scholar]
- 13.Yunten N, Ersahin Y, Demirtas E, Yalman O, Sener RN. Cerebral astroblastoma resembling an extra-axial neoplasm. J Neuroradiol. 1996;23:38–40. [PubMed] [Google Scholar]