Abstract
Objective
Barbiturate coma therapy (BCT) has been known to be an useful method to control increased intracranial pressure (IICP) refractory to medical and surgical treatments. We have used BCT for patients with severe IICP during the past 10 years, and analyzed our results with review of literatures.
Methods
We analyzed 92 semicomatose or comatose patients with Glasgow coma scale (GCS) of 7 or less with severe IICP due to cerebral edema secondary to parenchymal damages irrespective of their causes. Forty patients who had received BCT with ICP monitoring from January 1997 to December 2006 were included in BCT group, and fifty-two patients who had been managed without BCT from January 1991 to December 1995 were divided into control group. We compared outcomes with Glasgow outcome scale (GOS) and survival rate between the two groups.
Results
Good outcome (GOS=4 and 5) rates at 3-month after insult were 27.5% and 5.8% in BCT and control group, respectively (p<0.01). One-year survival rates were 35.9% and 12.5% in BCT and control group, respectively (p<0.01). In BCT group, the mean age of good outcome patients (37.1 ± 14.9) was significantly lower than that of poor outcome patients (48.1 ± 13.5) (p<0.05).
Conclusion
With our 10-year experience, we suggest that BCT is an effective treatment method for severe IICP patients for better survival and GOS, especially for younger patients.
Keywords: Barbiturate coma, Increased intracranial pressure, Survival, Glasgow outcome scale
INTRODUCTION
Increased intracranial pressure (IICP) can be a life-threatening complication of various neurological insults including head trauma and stroke6). High-dose barbiturate administration was recommended to control IICP refractory to maximum standard medical and surgical treatment. However, hemodynamic stability is essential before and during barbiturate therapy9). Barbiturate coma therapy (BCT) for the treatment of IICP has been studied since 1970s with varying outcomes13,20,22). In 1988, investigators reported the results of a five-center randomized controlled trial of high-dose barbiturate therapy for intractable IICP in patients with Glasgow coma scale (GCS) of 4-86). They found that the patients in the barbiturate therapy group had improved in intracranial pressure (ICP) control. But, because of small number of patients and the cross-over of patients between treatments arms, they were unable to comment about the effects of BCT on outcome.
There were only two domestic reports about the effect of BCT for IICP patients in Korea3,18). Although both reports showed favorable outcomes of BCT for intractable IICP patients, they represent fairly old data, reported at least 17 years ago, and were limited because of a small number of patients.
In this study, we summarized and analyzed our results of BCT used for severe IICP patients during the past 10 years, and compared it to those of the severe IICP patients treated without BCT.
MATERIALS AND METHODS
Patients
A retrospective analysis was done in 49 patients who received BCT. They were admitted due to head trauma or stroke between January 1997 and December 2006, and were semicomatose or comatose with GCS of 7 or less at admission. We selected patients for this study who had severe IICP due to cerebral edema following parenchymal damage irrespective of their causative pathologic conditions, head trauma or stroke. Nine patients who died within 3 days after brain insult were excluded because they were considered to die of direct injury of brain stem or other vital organs which could not be affected by BCT. After exclusion of the 9 patients, 40 patients were included in BCT group. Applying the same criteria, we selected 52 patients for control group who were admitted between January 1991 and December 1995 and managed with routine ICP control protocol including mannitol, lasix, and hyperventilation. We didn't use BCT for ICP control during the period (1991-1995).
BCT protocol
All patients in BCT group underwent intraventricular ICP monitoring with Spiegelberg air-pouch ICP monitor (Spiegelberg GmBH & Co. KG, Hambrug, Germany). We followed the BCT protocol from the study of Eisenberg et al.6) Barbiturate coma was induced with pentobarbital at a loading dose of 10 mg/kg over 30 minutes, 5 mg/kg/hour for 3 hours followed by continuous infusion (1 to 3 mg/kg/hour). We adjusted infusion rate for controlling ICP not exceeding 20 mmHg. A bolus intravenous injection of pentobarbital (100 or 200 mg) was used when ICP increased more than 20 mmHg during BCT. If the ICP was stable for more than 3 days, we then started to taper the dose of pentobarbital at a rate of 1 mg/kg/hour/day. Mean duration of BCT was 6.2 ± 2.4 days. Hypotension during the treatment was managed with volume replacement, dopamine and dobutamine. Nevertheless, if hypotension persisted or arrhythmia developed, we ceased BCT.
Data for analysis
Demographic data, initial GCS, the types of brain insult, the types of operative management, the GOS scores at 3 months after insult, and complications were obtained from medical records. We classified GOS into good outcome (GOS=4, 5) and poor outcome (GOS=1, 2, 3). Data such as age, sex ratio, initial ICP, initial GCS, GOS and survival rate, types of brain insult, types of operation were compared between BCT and control group. In BCT group, we analyzed factors related with good outcome. Survival rate was checked monthly until 1 year after insult.
Statistical analysis
Statistical analysis was done with t-test or chi-square test. The Kaplan-Meier method was used to calculate curves for overall survival and to estimate mean survival in both groups. Survival curves were compared using the log-rank test. p value less than 0.05 was considered statistically significant.
RESULTS
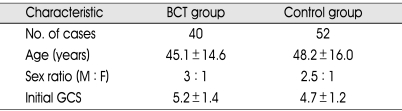
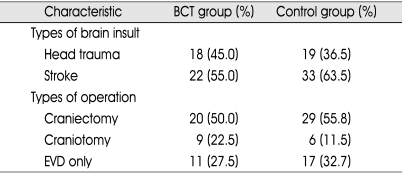
The demographic data and initial GCS were presented in Table 1. Mean ages, male to female ratios, and initial GCS were 45.1 ± 14.6 and 48.2 ± 16.0, 3 : 1 and 2.5 : 1, and 5.2 ± 1.4 and 4.7 ± 1.2 in BCT and control group, respectively. Types of brain insult and operative management were listed in Table 2. The ratio of head trauma to stroke were 9 : 11 and 19 : 33 in BCT and control group, respectively. All patients from BCT and control group underwent various surgical managements such as craniectomy, craniotomy or EVD. There was no statistically significant difference in the data mentioned above between the two groups.
Table 1.
Demographic data
Table 2.
Types of brain insult and operative management
GOS score and survival rate
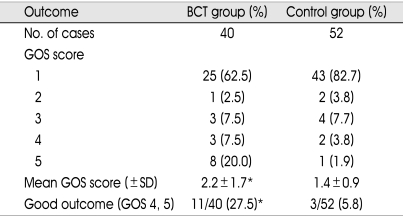
Table 3 lists GOS score at 3 months after insult in the two goups. Mean GOS scores were 2.2 ± 1.7 and 1.4 ± 0.9 in BCT and control group, respectively (p<0.01). Good outcome (GOS 4, 5) rates were 27.5% (11/40) in BCT group and 5.8% (3/52) in control group (p<0.01).
Table 3.
GOS score at 3 months after insult
*p < 0.01
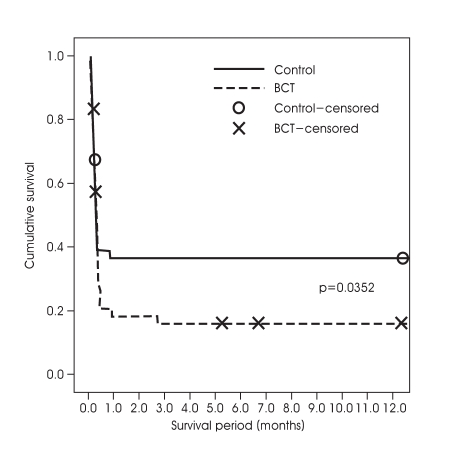
Cumulative survival rates of the two groups using a Kaplan-Meier curve were presented in Fig. 1. One-year survival rates were 35.9% and 12.5% in BCT and control group, respectively (p<0.01). Most of deaths occurred within 1-month after insult, 92.0% in BCT and 95.3% in control group. And, there was significant difference in 1-month survival rate between BCT and control group, 41% and 18% (p<0.05).
Fig. 1.
Kaplan-Meier curve shows cumulative survival rate of two groups. x, o : loss during follow up
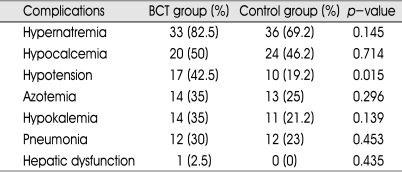
Complications
There were various complications in both groups, such as hypotension, azotemia, pulmonary edema, hepatic dysfunction, and electrolyte imbalance (hypernatremia, hypokalemia, hypocalcemia) (Table 4). Total complication rates were 87.5% (35/40) and 71.2% (37/52) in BCT and control group, respectively, without any statistical difference. Hypernatremia was the most common complication in both BCT (82.5%) and control group (69.2%). Hypotension was found more frequently in BCT group (42.5%) than control group (19.2%) (p<0.05).
Table 4.
Complications
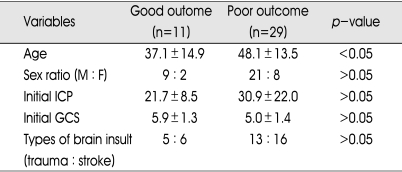
Prognostic factors
Table 5 listed age, sex ratio, initial ICP, initial GCS, types of brain insult and their relationship with the outcome in BCT group. The mean age of good outcome patients in BCT group (34.8 ± 15.4) was significantly lower than that of poor outcome patients (48.1 ± 13.1) (p<0.05). Among 8 patients aged up to 30 years, 5 patients (62.5%) showed good outcome, whereas only 6 (18.8%) out of the 32 patients who were older than 30 years showed good outcome (p<0.05). Other factors were not significantly different between good and poor outcome groups.
Table 5.
Relationship between various factors and outcome in BCT group
DISCUSSION
Since the 1930s, high-dose barbiturate has been known to decrease ICP10). It has been recognized for many years that barbiturate has many pharmacological effects, and it is beneficial for the management of IICP patients6). Theoretical benefits of barbiturate in IICP patients derive from vasoconstriction in normal brain areas (shunting blood to ischemic brain tissue), and decreased metabolic oxygen demand with accompanying reduction of cerebral blood flow12). Other mechanisms by which barbiturate may exert protective effects include stabilization of lysosomal membrane, reduction of intracellular calcium concentration, modification of amino acid and neurotransmitter release, scavenging of free radicals, alteration of fatty acid metabolism, reduction in cerebrospinal fluid production, membrane stabilization, and suppression of seizure1,2,5,17,22).
Sedatives and analgesics such as morphine sulfate, midazolam, fentanyl, sufentanyl, propofol were also common management strategies for ICP control although there is no evidence to support their efficacy in this regard and they have not been shown to positively affect outcome9). In 1999, Kelly et al.11) conducted a double-blind, randomized controlled trial comparing multiple endpoints for patients who received either propofol or morphine sulfate. According to their study, ICP was significantly lower on day 3 in patients receiving propofol without any improvement in mortality and GOS outcome.
The association between the level of ICP and survival is well documented in most of the literatures on this subject6,7,15,16). Survival rate in the patients with adequately controlled ICP is clearly superior to that of the patients with persistently elevated ICP15). Michael et al.15) reported that the patients, whose ICPs were successfully controlled by pentobarbital, had five times greater chance of survival. Our results, a higher survival rate in BCT group, can be comparable with the results of previous studies6,7,16) showing reciprocal relationship between survival and high ICP.
Eisenberg et al.6) reported the results of a five-center randomized controlled trial of high-dose barbiturate therapy for intractable IICP in patients with GCS of 4-8. They found that the patients in the pentobarbital group had improved in ICP control. One-month survival rate of BCT patients in our study (41%) is similar to that of Eisenberg's study, 37.8%6). Furthermore, our study showed higher 1-year survival rate, 35.9%, in BCT group than that of control group, 12.5%. BCT seems to be effective for both short-term and long-term survival in patients with severe IICP.
With the data showing lower mean age of the good outcome patients in BCT group, we could suggest that age might be a meaningful prognostic factor. We had divided age group every 10 years, and we have found age related differences in the outcome between two age groups, aged up to 30 and older than 30 years. In BCT group, 62.5% of patients aged up to 30 years had good outcome, but only 18.8% of patients older than 30 showed good outcome (p<0.05). Etienne et al.6) also reported that mortality rate of the patients received BCT was related to age.
However, well known risks and the ongoing controversies over the benefits of BCT have limited the use of BCT to the most extreme clinical situations9). Complications of BCT were mostly transient and could be adequately resolved in the intensive care unit setting, and there was no significant difference in the complication rate between BCT and control group. In Etienne's study group6), bronchopneumonia was the most common complication. In our study, hypernatremia was the most common complication in both groups. Systemic hypotension, which was more frequently observed in BCT group (42.5%) than control group (19.2%) in our study, is lower than that of other reports showing, 58% in BCT group19). The lower incidence of hypotension in our study seems to be related with concomitant use of dopamine and dobutamine for maintaining adequate blood pressure.
There are some limitations which could affect the reliability of the results of our study. The patient groups include heterogenous conditions, head trauma and stroke. Under the basic idea that traumatic brain injury and stroke can roughly share the common pathophysiological pathway4,8,14), we tried to select patients having brain edema resulting from parenchymal injury at the corresponding brain region. But, it still can have bias in the interpretation of our results. So, we are planning further study using patient groups that include more specified and homogeneous brain conditions if we can collect more number of IICP patients receiving BCT. The different time periods of the BCT and control group might be another bias. Control and BCT group patients were admitted before and after 1996, respectively, and there could be some differences in the IICP treatment between the two time periods. However, there have not been changes in our IICP management protocol since early 1990's except BCT which started in 1997 in our hospital, and it didn't seem to affect the results. One more thing we thought very unfortunate is that we didn't use ICP monitoring before 1997, and there was no chance to compare ICP between BCT and control group.
CONCLUSION
This study, a summary of our 10-year experience of BCT, demonstrates that BCT is useful and relatively safe in the treatment of severe IICP patients, especially in younger age group. However, a larger, randomized and prospective study comparing more homogeneous disease groups must be required for further investigation.
References
- 1.Branston NM, Hope DT, Symon L. Barbiturates in focal ischemia of primate cortex : effects on blood flow distribution, evoked potential, and extracellular potassium. Stroke. 1979;10:647–653. doi: 10.1161/01.str.10.6.647. [DOI] [PubMed] [Google Scholar]
- 2.Cai Z, McCaslin PP. Acute, chronic and differential effects of several anesthetic barbiturates on glutamate receptor activation in neuronal culture. Brain Res. 1993;611:181–186. doi: 10.1016/0006-8993(93)90501-d. [DOI] [PubMed] [Google Scholar]
- 3.Cho MY, Shin JH, Choi CS, Ju MB. The effect of barbiturate coma therapy. J Koryo Gen Hosp. 1991;14:149–154. [Google Scholar]
- 4.DeGraba TJ, Fisher M, Yatsu FM. Atherogenesis and strokes. In: Barnett HJM, Mohr JP, Stein BM, Yatsu FM, editors. Stroke. Pathophysiology, diagnosis, and management. ed 2. New York: Churchill Livingstone; 1992. p. 29. [Google Scholar]
- 5.Demopoulos HB, Flamm ES, Pietronigro DD, Seligman ML. The free radical pathology and the microcirculation in the major central nervous system disorders. Acta Physiol Scand Suppl. 1980;492:91–119. [PubMed] [Google Scholar]
- 6.Eisenberg HM, Frankowski RF, Contant CF, Marshall LF, Walker MD. High dose barbiturate control of elevated intracranial pressure in patients with severe head injury. J Neurosurg. 1988;69:15–23. doi: 10.3171/jns.1988.69.1.0015. [DOI] [PubMed] [Google Scholar]
- 7.Etienne D, Jacques B, Arlette V, Florence L, Jean LV. Barbiturate coma for intracranial hypertension: clinical observations. J Crit Care. 2002;17:58–62. doi: 10.1053/jcrc.2002.33032. [DOI] [PubMed] [Google Scholar]
- 8.Graham DI, Adams JH. Ischemic brain damage in fatal head injuries. Lancet. 1971;1:265–266. doi: 10.1016/s0140-6736(71)91003-8. [DOI] [PubMed] [Google Scholar]
- 9.Guidelines for the management of severe traumatic brain injury 3rd Edition, XI. Anesthetics, Analgesics, and Sedatives. J Neurotrauma. 2007;24(suppl 1):S71–S76. doi: 10.1089/neu.2007.9985. [DOI] [PubMed] [Google Scholar]
- 10.Horsley JS. The intracranial pressure during barbital narcosis. Lancet. 1937;1:141–143. [Google Scholar]
- 11.Kelly DF, Goodale DB, Williams J, Herr DL, Chappell ET, Rosner MJ, et al. Propofol in the treatment of moderate and severe head injury : a randomized, prospective double-blinded pilot trial. J Neurosurg. 1999;90:1042–1052. doi: 10.3171/jns.1999.90.6.1042. [DOI] [PubMed] [Google Scholar]
- 12.Lyons MK, Meyer FB. Cerebrospinal fluid physiology and the management of increased intracranial pressure. Mayo Clin Proc. 1990;65:684–707. doi: 10.1016/s0025-6196(12)65131-3. [DOI] [PubMed] [Google Scholar]
- 13.Marshall LF, Smith RW, Shapiro HM. The outcome with aggressive treatment in severe head injuries. Part II. Acute and chronic barbiturate administration in the management of head injury. J Neurosurg. 1979;50:26–30. doi: 10.3171/jns.1979.50.1.0026. [DOI] [PubMed] [Google Scholar]
- 14.Martin NA, Patwardhan RV, Alexander MJ, Afrik CZ, Lee JH, Shalmon E, et al. Characterization of cerebral hemodynamic phases following severe head trauma: hypoperfusion, hyperemia and vasospasm. J Neurosurg. 1997;87:9–19. doi: 10.3171/jns.1997.87.1.0009. [DOI] [PubMed] [Google Scholar]
- 15.Michael WL, Scott AD, Mary ES, Roger RB, Dan RT. The efficacy of barbiturate coma in the management of uncontrolled intracranial hypertension following neurosurgical trauma. J Neurotrauma. 1994;11:325–331. doi: 10.1089/neu.1994.11.325. [DOI] [PubMed] [Google Scholar]
- 16.Miller JD, Butterworth JF, Gudeman SK, Faulkner JE, Choi SC, Selhorst JB, et al. Further experience in the management of severe head injury. J Neurosurg. 1981;54:289–299. doi: 10.3171/jns.1981.54.3.0289. [DOI] [PubMed] [Google Scholar]
- 17.Morgan WW, Bermudez J, Chang XY. The relative potency of pentobarbital in suppressing the kainic acid- or the N-methyl-D-aspartic acid-induced enhancement of cGMP in cerebellar cells. Eur J Pharmacol. 1991;204:335–338. doi: 10.1016/0014-2999(91)90861-j. [DOI] [PubMed] [Google Scholar]
- 18.Park CK, hang JH, Kim DS, Lee SW, Kim MC, Kang JK, et al. Therapeutic barbiturate coma in uncontrolled intracranial hypertension : Management of patients and effect on outcome. J Korean Neurosurg Soc. 1986;15:381–394. [Google Scholar]
- 19.Schalen W, Messeter K, Nordstrom CH. Complications and side effects during thiopentone therapy in patients with severe head injuries. Acta Anaesthesiol Scand. 1992;36:369–377. doi: 10.1111/j.1399-6576.1992.tb03483.x. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz ML, Tator CH, Rowed DW, Reid SR, Meguro K, Andrews DF. The University of Toronto Head Injury Treatment Study : a prospective, randomized comparison of pentobarbital and mannitol. Can J Neurol Sci. 1984;11:434–440. doi: 10.1017/s0317167100045960. [DOI] [PubMed] [Google Scholar]
- 21.Steen PA, Michenfelder JD. Mechanism of barbiturate protection. Anesthesiology. 1980;53:183–185. [PubMed] [Google Scholar]
- 22.Ward JD, Becker DP, Miller JD, Choi SC, Marmarou A, Wood C, et al. Failure of prophylactic barbiturate coma in the treatment of severe head injury. J Neurosurg. 1985;62:383–388. doi: 10.3171/jns.1985.62.3.0383. [DOI] [PubMed] [Google Scholar]
- 23.Wilberger JE, Harris M, Diamond DL. Acute subdural hematoma : morbidity and mortality related to timing of operative intervention. J Trauma. 1990;30:733–736. [PubMed] [Google Scholar]