Abstract
Objective
Microscopic and endoscopic transsphenoidal approach (TSA) are major surgical techniques in the treatment of pituitary adenoma. Endoscopic endonasal transsphenoidal approach (EETSA) has been increasingly used for pituitary adenomas, however, its surgical outcome particularly in functioning pituitary adenoma has been debated. Here, we investigated the endocrine outcome of the patients with growth hormone (GH) and adrenocorticotropic hormone (ACTH) secreting pituitary adenoma treated by EETSA.
Methods
We treated 80 patients with pituitary adenoma by EETSA since 2004, of which 12 patients were affected by functioning pituitary adenomas (9 GH, 3 ACTH, 0 PRL; 9 macro, 3 micro). Surgical outcome of those patients treated by EETSA was compared with that of the 11 functioning pituitary adenoma patients (8 GH, 3 ACTH; 8 macro, 3 micro) who underwent sublabial microscopic TSA between 1997 and 2003.
Results
Imaging remission based on postoperative MRI was achieved in 8 (73%) and hormonal remission in 5 (45%) of 11 patients treated by sublabial microscopic TSA. Imaging remission was observed in 10 (83%, p=0.640) and hormonal remission in 10 (83%, p=0.081) of 12 patients by EETSA. CSF leakage was noticed in 2 (17%) of EETSA group and in 2 (18%) of sublabial microscopic TSA group. Panhypopituitarism was observed in 1 (9%) of EETSA group and in 3 (27%) of sublabial microscopic TSA group.
Conclusion
EETSA appears to be an effective and safe method for the treatment of functioning pituitary adenomas.
Keywords: ACTH-secreting pituitary adenoma, GH-secreting pituitary adenoma, Endoscopy, Transsphenoidal approach
INTRODUCTION
The microscopic transsphenoidal approach (TSA) had been a standard in the pituitary adenoma surgery for decades. Since Jankowski et al.10) began to use the endoscope via the endonasal approach for transsphenoidal pituitary surgery in 1992, the use of endoscopic endonasal TSA (EETSA) for sellar and parasellar lesion has been increased5,12,21).
Recent technological advances in specific endoscopic instrumentation, surgical navigation system, and endoscopic image appear to make the EETSA possible as alternative to conventional microscopic TSA. The surgical outcome of EETSA and microscopic TSA showed a wide variability1,4,11,13,19). Despite the increasing use of EETSA, however, debates on its surgical outcome, particularly in functioning pituitary adenomas, have been raised by some microscopic pituitary surgeons unfamiliar to EETSA which has some limiting factors such as two-dimensioned image, less zoom and focus capacity, and longer learning curve to microscopic surgeons1,4,11,13,19). Here, the authors investigated the endocrine outcome of our consecutive cases of growth hormone (GH)-and adrenocorticotropic hormone (ACTH)-producing pituitary adenomas treated by EETSA since 2004 and compared with that of the patients who underwent sublabial microscopic TSA between 1997 and 2003.
MATERIALS AND METHODS
Only the pituitary adenoma patients treated by two surgeons (Jeun SS, Hong YK) were included in this study. Microscopic TSA was performed in 87 patients between 1997 and 2004, and EETSA was undertaken in 80 patients between 2004 and 2007. Among them, 11 patients with functioning pituitary adenoma (3 ACTH-secreting, 8 GH-secreting) underwent sublabial microscopic TSA and 12 functioning pituitary adenoma patients (3 ACTH-secreting, 9 GH-secreting) underwent EETSA. All prolactin (PRL)-secreting adenoma patients had medical treatment as first choice of therapy in EETSA period and were excluded. Medical records of these patients were retrospectively reviewed and their endocrinologic and radiologic outcome and complications were assessed.
Before operation all patients were evaluated by sellar MRI examination including dynamic study, basal hormone level (GH, TSH, prolactin, ACTH, Free T4, cortisol), ophthalmologic and rhinological evaluations. Additionally, GH-producing adenoma patients were evaluated for insulin-like growth factor-1 (IGF-1), oral glucose tolerance test (OGTT), and ACTH-producing adenoma patients were evaluated for 24 h urine free cortisol level. Postoperative hormonal evaluation was done at 3-4 weeks and 3-4 months after the surgery. Postoperative sellar MRI was performed within 72 hours and 2-3 months after the surgery.
Postoperatively, endocinologic remission was defined GH <2.5 ng/ml, GH <1 ng/ml in OGTT and IGF-1 normalization in GH-secreting adenoma, and cortisol <5 µg/dl and undetectable serum ACTH in ACTH-secreting adenoma. Mean follow-up period of EETSA was 12±9.2 months. Statistical analyses were conducted using SPSS 12.0 for Windows (SPSS, Inc., Chicago, IL). All data were analyzed for statistical significance using the Mann-Whitney U test and the chi-square test and p-value less than 0.05 was considered statistically significant.
Surgical techniques of EETSA
We performed nasal swab culture for all patients before operation. In operation room, the patient was positioned supine, with the body trunk raised and the head slightly flexed and rotated towards the surgeon. A three-pin head fixation and neuronavigation system (Styker®, Kalamazoo, MI) was applied intraoperatively. All operations were performed via single nostril. We approached sphenoid ostium via between middle turbinate and nasal septum. After wide opening the sphenoid sinus and sellar floor to see the panoramic view, we introduced endoscope holder and clear vision system (Karl Storz®, Mittelstrasse, Tuttlingen, Germany) and opened the dura and removed the pituitary tumor using the bihands maneuver. We used 0 degree angled endoscope with 4 mm diameter (Karl Storz®). In some cases, we used 30 degrees angled endoscope to see the suprasella area. The video monitor was positioned behind the patient's shoulder directly opposite the surgeon's line of vision and navigation monitor is positioned just lateral side of endoscope monitor. If there was no CSF leakage intraoperatively, sellar reconstruction was not performed. If CSF leakage occurred, sellar reconstruction was done using the abdominal fat graft with or without tailored bioabsorbable graft (Medpor®, Newnan, GA).
RESULTS
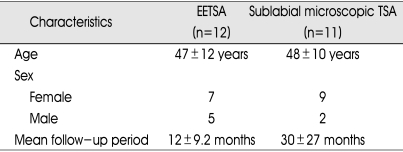
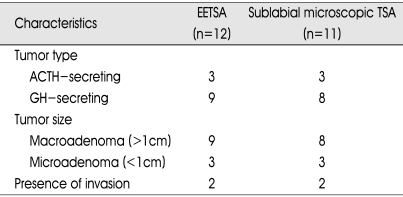
The patient's clinical information is presented in Table 1. There were 16 female and 7 male patients. The mean age was 47±12 years in patients with endoscopic TSA versus 48±10 years in patients with microscopic TSA. The tumor characteristics are depicted in Table 2. Among 23 functioning adenoma cases, 17 cases were macroadenoma (9 in EETSA, 8 in microscopic TSA) and 6 cases were microadenoma (3 in EETSA, 3 in microscopic TSA). Among them, 4 cases were infiltrating type. In EETSA group, one was infiltrating to pituitary stalk and another was infiltrating to left cavernous sinus. In microscopic TSA group one was infiltrating to left cavernous sinus and another was encasing right cavernous ICA.
Table 1.
Clinical characteristics
EETSA : endoscopic endonasal transsphenoidal approach
Table 2.
Tumor characteristics
ACTH : adrenocorticotropic hormone, GH : growth hormone
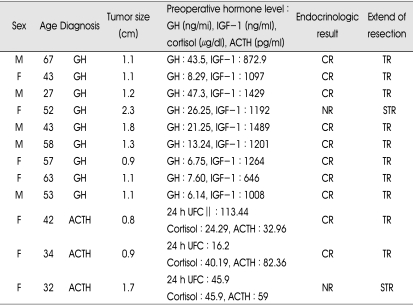
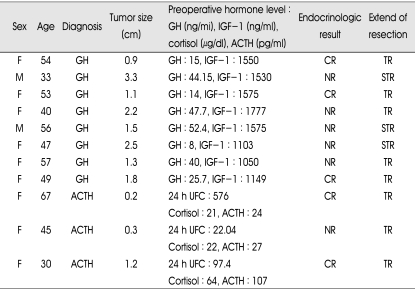
Preoperatively, serum hormone levels were as follows (EETSA group vs. microscopic TSA group): serum GH (20.04±15.98 vs. 30.86±17.29) (p=0.124), serum IGF-1 (1142.19±261.47 vs. 1379.05±288.35) (p=0.101), serum ACTH (58.12±24.75 vs. 52.67±47.12) (p=0.827), serum cortisol (36.79±11.20 vs. 35.66±24.54) (p=0.513) and 24 hours urine cortisol (58.51±49.82 vs. 58.90±37.51) (p=0.513). Each preoperative hormone levels were no statistically different between the EETSA group and microscopic TSA group. In EETSA group, hormonal complete remission was achieved in all except in a patient with GH-secreting adenoma and a patient with ACTH-secreting adenoma (overall, 83%). In each case, postoperative MRI showed remaining mass in pituitary stalk and left cavernous sinus, respectively. In microscopic TSA, imaging remission was observed in 8 of 11 patients (73%) but hormonal remission was observed in 5 of 11 patients (45%). Among 6 patients who did not achieve hormonal remission, follow-up was lost in one patient and the other 5 patients were treated by adjuvant therapy such as bromocriptine, octreotide, external radiation and cyberknife radiosurgery. Table 3 and 4 summarized the overall postoperative results for patients who underwent EETSA and microscopic TSA. The hormonal remission rate appeared to be comparable or superior in EETSA group to microscopic TSA group (p=0.081, by chi-square independent test). The extend of tumor resection based on postoperative MRI was not different between the two groups (p=0.640). Complete hormonal remission was observed in 7 (78%) of 9 macroadenomas of EETSA group and in 3 (38%) of 8 macroadenomas of microscopic TSA group. In microadenoma cases, we achieved 100% hormonal remission rate (3 of 3 patients) in EETSA group and 67% (2 of 3 patients) in microscopic TSA group.
Table 3.
Preoperative status and postoperative results in EETSA
CR : complete remission, NR : no remission, TR : total removal, STR : subtotal removal, UFC : urine free cortisol, IGF-1 : insulin-like growth factor-1
Table 4.
Preoperative status and postoperative results in sublabial microscopic TSA
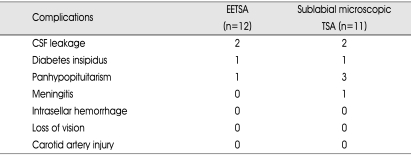
The complications are depicted in Table 5. The usual complications were CSF rhinorrhea, transient diabetes insipidus and postoperative panhypopituitarism. We observed 2 CSF leakage cases (17%) in patients who underwent EETSA group vs. 2 (18%) of 11 patients who underwent microscopic TSA group and 1 panhyphopituitarism (9%) in EETSA group vs. 3 (27%) in microscopic TSA group and no meningitis in EETSA group vs. 1 meningitis in microscopic TSA group.
Table 5.
Postoperative complications
EETSA : endoscopic endonasal transsphenoidal approach
DISCUSSION
In 1886, Pierre Marie first described acromegaly and after that the diagnosis and treatment of pituitary tumor was advanced16). In 1889, Horsley performed first operation for pituitary tumor via transcranial approach9) and Cushing introduced transseptal-transsphenoid approach for pituitary tumor surgery in 19102). This transseptal-transsphenoidal surgery was more advanced in 1960s, because Guiot introduced intraoperative fluoroscopy and Hardy used intraoperative fluoroscopy and microsurgical techniques7,8). These new technologies provided the significant improvement in visualization and reduced the surgical complications.
In early 1990s, technologic advances in specific endoscopic instrumentations, optical lens, monitoring system, holding arms, and small diameter of endoscope provide high-quality panoramic views. Since then many institutes started fully endoscopic TSA and reported comparable or superior postoperative results and less surgical complication rates to microscopic TSA1,4,13). Endocrinologic remission rate was reported to be widely variable : 70-85% in GH-producing adenomas and 68-86% in ACTH-producing adenomas by endoscopic TSA4,11,13,19,22) vs. 54-79% in GH-producing adenomas and 69-86% in ACTH-producing adenomas by microscopic TSA6,14,15,17,18). In our study, EETSA showed comparable or superior surgical outcome as compared to microscopic TSA in tumor removal rate (83% vs. 73%) and endocrinologic remission rate (83% vs. 45%) and the similar trend was observed on separate analysis of macroadenomas and microadenomas. As shown in Table 5, postoperative complication rate, particularly panhypopituitarism, seems to be diminished in endoscopic TSA group. Surgical results of TSA may be influenced by not only surgical techniques and strategy, but also tumor characteristics and the anatomical factors such as growth and invasiveness into the cavernous sinus or surrounding area. Different results of EETSA vs. microscopic TSA in our study may be related to superior illumination and visualization, the well-known advantages of endoscopic TSA. The excellent wide-angle and magnified view of the operative anatomy allows the surgeon to identify the tumor and avoid injury to the normal gland, carotid prominence and other parasellar and suprasellar structures more clearly. In general, a continuous improvement in surgical outcome by increasing one's experience has been documented in a single neurosurgeon's series in pituitary adenomas20). Learning curve may be an important factor related to the surgical outcome of TSA by average surgeons. The different results in our study may result from that the microscopic TSA was performed in the beginning period and the EETSA in later period of pituitary surgery.
In addition to the main advantages of the EETSA such as superior visualization and illumination, the other advantages include reduced postoperative discomfort and shortened hospital stay and operative time which may be related to no nasal packing, no use of speculum, no nasal mucosal destruction and no sublabial incision11). Endonasal microscopic TSA was also known to yield the comparable results to EETSA, we could not tell about that as we moved from sublabial microscopic TSA directly to EETSA without endonasal microscopic TSA experience.
The small number of the patients and not randomized and not prospective investigation limit our study. The true effect of the several advantages of the endoscopic approach over the microscopic approach on the surgical outcome would be defined by direct prospective comparison of outcomes at a single institution. There have been new evolutions in surgical techniques and endoscopic technology to improve the outcome such as two-surgeon technique, vascularized flap for sellar reconstruction and high definition (HD) imaging endoscope system etc.3,23) We agree to the opinion of Frank et al. that the endoscopic endonasal pituitary surgery is not a revolution, but a further step in the evolution of transsphenoidal surgery4).
CONCLUSION
The conventional microscopic TSA had been the standard treatment for almost pituitary adenomas. In our limited series, comparable surgical outcome was achieved by EETSA vs. microscopic TSA in the treatment of GH- and ACTH-secreting pituitary adenomas. The EETSA is a good minimally invasive surgical technique for the functioning pituitary adenomas, and expected to become the gold standard in the treatment of pituitary adenomas by further evolutions in endoscopic technology and surgical technique.
References
- 1.Cho DY, Liau WR. Comparison of endonasal endoscopic surgery and sublabial microsurgery for prolactinomas. Surg Neurol. 2002;58:371–375. doi: 10.1016/s0090-3019(02)00892-3. discussion 375-376. [DOI] [PubMed] [Google Scholar]
- 2.Cushing H. Surgical experience with pituitary disorders. JAMA. 1914;63:1515–1525. [Google Scholar]
- 3.Fortes FSG, Carrau RL, Snyderman CH, Prevedello D, Vescan A, Mintz A, et al. The posterior pedicle inferior turbinate flap : a new vascularized flap for skull base reconstruction. Laryngoscope. 2007;117:1329–1332. doi: 10.1097/mlg.0b013e318062111f. [DOI] [PubMed] [Google Scholar]
- 4.Frank G, Pasquini E, Farneti G, Mazzatenta D, Sciarreta V, Grasso V, et al. The endoscopic versus the traditional approach in pituitary surgery. Neuroendocrinology. 2006;83:240–248. doi: 10.1159/000095534. [DOI] [PubMed] [Google Scholar]
- 5.Gamea A, Fathi M, El-Guindy A. The use of the rigid endoscope in trans-sphenoidal pituitary surgery. J Laryngol Otol. 1994;108:19–22. doi: 10.1017/s0022215100125721. [DOI] [PubMed] [Google Scholar]
- 6.Guilhaume B, Bertagna X, Thomsen M, Bricaire C, Vila-Porcile E, Olivier L, et al. Transsphenoidal pituitary surgery for the treatment of Cushing's disease : Results in 64 patients and long term follow-up studies. J Clin Endocrinol Metab. 1998;66:1056–1064. doi: 10.1210/jcem-66-5-1056. [DOI] [PubMed] [Google Scholar]
- 7.Guiot G, Thebaul B. L'Extirpation des adenomas hypophysaires par voie transsphenoidale. Neuro Chirugie. 1959;1:133. doi: 10.1055/s-0028-1095527. [DOI] [PubMed] [Google Scholar]
- 8.Hardy J. Transsphenoidal microsurgery of the normal and pathological pituitary. Clin Neurosurg. 1969;61:185–217. doi: 10.1093/neurosurgery/16.cn_suppl_1.185. [DOI] [PubMed] [Google Scholar]
- 9.Horsley V. Disease of the pituitary gland. Br Med J. 1906;1:323. [Google Scholar]
- 10.Jankowski R, Auque J, Simon C, Marchal JC, Hepner H, Wayoff M. Endoscopic pituitary tumor surgery. Laryngoscope. 1992;102:198–202. doi: 10.1288/00005537-199202000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Jho HD. Endoscopic transsphenoidal surgery. J Neurooncol. 2001;54:187–195. doi: 10.1023/a:1012969719503. [DOI] [PubMed] [Google Scholar]
- 12.Jho HD, Carrau RL, Ko Y, Daly MA. Endoscopic pituitary surgery : an early experience. Surg Neurol. 1997;47:213–223. doi: 10.1016/s0090-3019(96)00452-1. [DOI] [PubMed] [Google Scholar]
- 13.Kabil MS, Eby JB, Shnhinian HK. Fully endoscopic endonasal vs. transseptal transsphenoidal pituitary surgery. Minim Invas Neurosurg. 2005;48:348–354. doi: 10.1055/s-2005-915635. [DOI] [PubMed] [Google Scholar]
- 14.Kaltsas GA, Isidori AM, Florakis D, Trainer PJ, Camacho-Hubner C, Afshar F, et al. Predictors of the outcome of surgical treatment in acromegaly and the value of the mean growth hormone day curve in assessing postoperative disease activity. J Clin Endocrinol Metab. 2001;86:1645–1652. doi: 10.1210/jcem.86.4.7398. [DOI] [PubMed] [Google Scholar]
- 15.Kreutzer J, Vance ML, Lopes MB, Laws ER., Jr Surgical management of GH-secreting pituitary adenomas : an outcome study using modern remission criteria. J Clin Endocrinol Metab. 2001;86:4072–4077. doi: 10.1210/jcem.86.9.7819. [DOI] [PubMed] [Google Scholar]
- 16.Marie P. Hyertrophie singuliere non congenital des extremites superieures, inferieures et cephalique. Rev Medicine. 1886;6:297–333. [Google Scholar]
- 17.Mortini P, Losa ML, Barzaghi R, Boari N, Giovanelli M. Result of transsphenoidal surgery in a large series of patients with pituitary adenoma. Neurosurgery. 2005;56:1222–1233. doi: 10.1227/01.neu.0000159647.64275.9d. [DOI] [PubMed] [Google Scholar]
- 18.Nakane T, Kuwayama A, Watanabe M, Takahashi T, Kato T, Ichihara K, et al. Long term results of transsphenoidal adenomectomy in patients with Cushing's disease. Neurosurgery. 1987;21:218–222. doi: 10.1227/00006123-198708000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Neal JG, Patel SJ, Kulbersh JS, Osguthorpe JD, Schlosser RJ. Comparison of techniques for transsphenoidal pituitary surgery. Am J Rhinol. 2007;21:203–206. doi: 10.2500/ajr.2007.21.2981. [DOI] [PubMed] [Google Scholar]
- 20.Nomikos P, Buchfelder M, Fahlbusch R. The outcome of surgery in 668 patients with acromegaly using current criteria of biochemical 'cure'. Eur J Endocrinol. 2005;152:379–387. doi: 10.1530/eje.1.01863. [DOI] [PubMed] [Google Scholar]
- 21.Rodziewicz GS, Kelley RT, Kellman RM, Smith MV. Transnasal endoscopic surgery of the pituitary gland : technical note. Neurosurgery. 1996;39:189–193. doi: 10.1097/00006123-199607000-00046. [DOI] [PubMed] [Google Scholar]
- 22.Rudnik A, Zawadzki T, Wojtacha M, Bazowski P, Gamrot J, Galuszka-Ignasiak B, et al. Endoscopic transnasal transsphenoidal treatment of pathology of the sellar region. Minim Invas Neurosurg. 2005;48:101–107. doi: 10.1055/s-2004-830185. [DOI] [PubMed] [Google Scholar]
- 23.Uren B, Vrodos N, Wormald PJ. Fully endoscopic transsphenoidal resection of pituitary tumors: Technique and results. Am J Rhinol. 2007;21:510–514. doi: 10.2500/ajr.2007.21.3059. [DOI] [PubMed] [Google Scholar]