Abstract
When a tear occurs in one of the major cervicocerebral arteries and allows blood to enter the wall of the artery and split its layers, the result is either stenosis or aneurysmal dilatation of the vessel. Vertebral artery dissection (VAD) is an infrequent occurrence but is a leading cause of stroke in young and otherwise healthy patients. This article discusses recent developments in understanding of the epidemiology and pathogenesis of VAD and the various clinical manifestations, methods of diagnosis, and approaches to treatment.
Keywords: Stenosis, Aneurysmal dilatation, Vertebral artery dissection, Diagnosis, Treatment
INTRODUCTION
The term dissection implies a tear in the wall of a major artery leading to the intrusion of blood within the layers of an arterial wall (intramural hematoma). This causes stenosis of the lumen when blood collects between the intima and media or an aneurysmal dilatation of the artery when the hematoma predominantly involves the media and adventitia40). This process was long thought to be a rare cause of stroke, particularly in the absence of trauma, and the diagnosis was usually not made until the postmortem examination11,30). It was the work of Fisher et al.11) in the late 1970s that led to the recognition of the clinical and radiologic features of dissection syndromes facilitating their antemortem diagnosis. This review focuses on the pathogenesis, natural history, clinical features, and therapeutic considerations of vertebral artery dissection (VAD).
EPIDEMIOLOGIC FEATURES
The overall incidence of VAD is approximately 1-1.5 per 100,0005). Spontaneous dissections of the carotid and vertebral artery account for only about 2 percent of all ischemic strokes3,12,30,34), but they are an important cause of ischemic stroke in young and middle-aged patients and account for 10 to 25 percent of such cases.
Spontaneous dissections of the vertebral arteries affect all age groups, including children, but there is a distinct peak in the fifth decade of life3,34,35). Although there is no overall sex-based predilection, women are on average about five years younger than men at the time of the dissection34).
PATHOLOGIC FEATURES
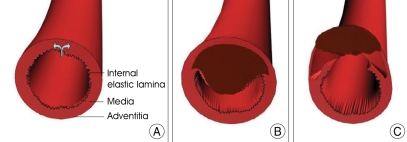
An arterial wall consists of three layers-intima (the innermost layer), media (the middle muscular layer), and adventitia (the outermost layer). It is generally agreed that a tear in the wall of an artery leads to a collection of blood between the layers of the artery, leading to formation of an intramural hematoma (Fig. 1A, B).
Fig. 1.
Classic type of vessel wall disruption during dissection. A normal vertebral arterial wall consists of three layers-intima (the innermost layer), media (the middle muscular layer), and adventitia (the outermost layer) (A). When a tear occurs in the arterial wall (arrow in A) and allows blood to enter the wall of the artery and split its layers, the result is the intrusion of blood within the layers of an arterial wall (intramural hematoma). This causes stenosis of the lumen when blood collects between the intima and media (B) or an aneurysmal dilatation of the artery when the hematoma predominantly involves the media and adventitia (C).
However, there is no universal agreement as to which wall is the primary site of dissection. Some authorities consider a rupture within the connective tissue and vasa vasorum of the media as the most probable initial event in dissection. The intramural hematoma may then later penetrate the intima and reconnect with the true arterial lumen. Others think that an intimal tear occurs30), which allows blood under arterial pressure to enter the wall of the artery.
Initial tears are not always easy to diagnose on pathological specimens. Even in the carefully selected patients it is not always possible to show a communication between the intramural hematoma (the so called false lumen) and the true lumen, suggesting that some dissections of vertebral arteries may be caused by a primary intramural hematoma (Fig. 1C).
Genetic Factors
Patients with a spontaneous dissection of the vertebral artery are thought to have an underlying structural defect of the arterial wall, although the exact type of arteriopathy remains elusive in most cases30). Foremost among the heritable connective tissue disorders that are associated with an increased risk of spontaneous dissections of the vertebral arteries is Ehlers-Danlos syndrome type IV33). Others include Marfan's syndrome, autosomal dominant polycystic kidney disease, and osteogenesis imperfecta type I32,33). Although these well-characterized heritable connective tissue disorders have been identified in only 1 to 5 percent of patients with spontaneous dissection of the vertebral artery, one-fifth of patients have a clinically apparent but as yet unnamed connective tissue disorder38).
Environmental Factors
A history of a minor precipitating event is frequently elicited in patients with a spontaneous dissection of the vertebral artery11,30). Some precipitating events associated with hyperextension or rotation of the neck include practicing yoga, painting a ceiling, coughing, vomiting, sneezing, the receipt of anesthesia, and the act of resuscitation11). Such neck movements, particularly when they are sudden, may injure the artery as a result of mechanical stretching.
Chiropractic manipulation of the neck has been associated with VAD14). It has been estimated that as many as 1 in 20,000 spinal manipulations causes a stroke.
A recent history of a respiratory tract infection is a risk factor for spontaneous dissections of the vertebral artery13). The possibility of an infectious trigger is supported by the finding of a seasonal variation in the incidence of spontaneous dissections of the vertebral arteries, with a peak incidence in the fall.
A potential link with common risk factors for vascular disease, such as tobacco use, hypertension, and the use of oral contraceptives, has not been systematically evaluated. One case-control study suggested migraine as a risk factor for dissection9).
NATURAL HISTORY - HEALING PROCESS
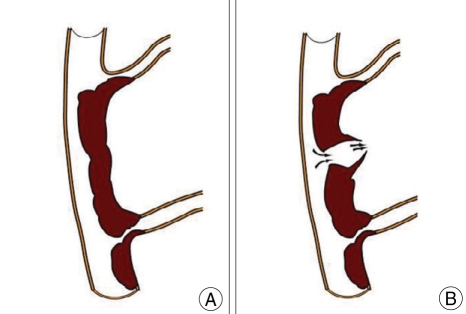
Spontaneous VAD is mainly divided into two types : 1) the ischemic type, which is manifest by ischemic symptoms and/or infarction of the vertebrobasilar circulation due to arterial narrowing and thromboembolism; and 2) the hemorrhagic type, which presents as a subarachnoid hemorrhage (SAH) caused by rupture of an intradural vertebral artery dissecting aneurysm (Fig. 2).
Fig. 2.
Schematic represention of dissecting process in the VAD. Vertebral artery dissection is mainly divided into two types : (A) ischemic type, which is manifest by ischemic symptoms and/or infarction of the vertebrobasilar circulation due to perforating artery occlusion, thromboembolism, and hemodynamic accident; and (B) hemorrhagic type, which presents as a subarachnoid hemorrhage caused by adventitial rupture of an intradural vertebral artery dissecting aneurysm.
In the series of Mokri and coworkers25) follow-up angiography was performed in 16 patients with 21 lesions. These authors reported that 13 stenotic lesions (61.9%) observed on initial angiography studies exhibited resolution on subsequent angiography studies, whereas only one vessel became occluded. A higher rate of vascular healing in cases of extracranial VAD has been demonstrated. Occlusive changes do not always result in poor outcome in patients unless the contralateral vertebral artery is hypoplastic44). Recanalization occurs in vessels with occlusion, although this is not common17,27). Thus, follow-up angiography studies should be considered, even if the affected vessel appears to be occluded on the initial angiography examination.
Acutely ruptured dissections are unstable and have a tendency to rebleed. The rebleeding rate has been reported to be as high as 71.4% in a group of 42 untreated patients21,45). The mortality rate of these rebleeds was high, being 46.7% in this series. As a rule, the shorter the time since the initial hemorrhage, the higher risk of rebleeding in the acute phase. In the study conducted by Mizutani21), 70% of rebleeds occurred within the first 24 h after the initial SAH and 80% in the next week. Fortunately, as the time passes, the risk of rebleeding decreases considerably. Yamaura et al.41) proposed that a ruptured dissecting aneurysm enters into a healing stage approximately 1 month after the initial SAH. In Mizutani's series21), only 10% of rebleeding occurred more than one month after the initial hemorrhage. In their discussion of the histopathology of the healing response following dissection, Mizutani et al.22), proposed that vessel wall repair is completed after the neointima covers the entire area of the arterial wall. This repair occurs from the disrupted ends of the media toward the ruptured portion. As they pointed out, this healing mechanism may be delayed under several circumstances such when there is an extensive defect of the aneurysmal wall in the ruptured portion (i.e. large aneurysms), aneurysms with abundant thrombus in the ruptured portion (since neointima may appear along with retraction of the thrombus), or aneurysms in which the media is completely separated from the adventitia. This vessel wall reaction, however, seems to be unpredictable.
CLASSIFICATION
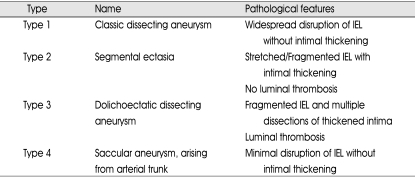
Several groups have proposed patho-anatomical classifications of intracranial dissecting aneurysms24,28). Mizutani et al.24) found that nonatherosclerotic aneurysms unrelated to the branching zones had several lesional patterns of the internal elastic lamina (IEL) and intima, and they classified them into four types. There was a strong relationship between the pathological features of the aneurysms and their clinical courses. Type 1 corresponded to classic dissecting aneurysms, the pathogenesis of which was characterized by acute widespread disruption of the IEL without intimal thickening. Patients with Type 1 aneurysms had an ominous clinical course, and many presented with sudden subarachnoid hemorrhage with frequent rebleeding. Type 2 aneurysms were segmental ectasias, which had an extended and/or fragmented IEL with intimal thickening. Weakness of the arterial wall caused by the damaged IEL was assumed to be compensated by the intimal thickening. The luminal surface of the thickened intima was smooth without thrombus formation. The patients with Type 2 aneurysms had a placid clinical course. Type 3 aneurysms were dolichoectatic dissecting aneurysms, pathologically characterized by fragmentation of the IEL, multiple dissections of thickened intima, and organized thrombus in the lumen. Most of them were symptomatic and progressively enlarged over time. Type 4 aneurysms were saccular aneurysms unrelated to the branching zones. They arose in areas with minimally disrupted IEL without intimal thickening, and there was a risk of rupture. In another study, Mizutani23) proposed a classification of the connection between true and false lumen. Accordingly, the primary mechanism by which a cerebral dissecting aneurysm is created is by the sudden disruption of the IEL. The plane of dissection extends through the media. The majority of aneurysms have one entrance into the pseudolumen (entry-only type). This type is apparently associated with an unstable clinical course. Some cerebral dissecting aneurysms have both an entrance and exit (entry-exit type). This type of aneurysm occasionally contains a constant flow of blood through the pseudolumen and is clinically more stable than entry-only aneurysms.
CLINICAL FEATURES
Extracranial VADs
Neck trauma may clearly precede an extracranial VAD. The vertebral artery is most mobile and thus most vulnerable to mechanical injury at C1 to C2 as it leaves the transverse foramen of the axis vertebra and suddenly turns to enter the intracranial cavity. Clinical manifestations include severe neck pain mostly in the occipitocervical area followed after a variable interval by ischemic symptoms. In some patients there may not be any ischemic symptoms. Dizziness, vertigo, double vision, ataxia, and dysarthria are common clinical features. Lateral medullary (Wallenberg syndrome) and cerebellar infarctions are the most common types of strokes. Occasionally, spinal cord infarctions occur because of the involvement of branches of the extracranial vertebral artery that supply the cervical spinal cord.
Intracranial VADs
More than 50% of intracranial VADs are associated with subarachnoid hemorrhage (SAH)40). Since Yonas et al.43) described the pathological and radiographic features of intracranial dissecting aneurysm as a cause for SAH, it has been increasingly recognized as a cause of SAH with a unfavorable prognosis and a high rate of rebleeding. Some 1-10% of all intracranial non-traumatic SAH are caused by ruptured intracranial dissection, and in children, the rate may be even higher. The majority of hemorrhagic intracranial arterial dissection is located in the posterior circulation most likely reflecting it's structure since histological studies29) have shown that the intradural vertebral artery has a thin media and adventitia with fewer elastic fibers, so dissections of the intradural vertebral artery are prone to result in SAH, in contrast to dissections of other vessels1). Brain stem infarctions and aneurysmal arteries presenting as space occupying lesions are other manifestations.
In the past, intracranial dissections were considered neurologically devastating or fatal, but modern technology has led to increased recognition that patients with intracranial dissections also may have only minor symptoms6).
DIAGNOSIS
Catheter angiography has been the "gold standard" of diagnosing arterial dissections. The commonest finding on angiography is the so called "string sign"-a long segment of narrowed lumen. The pathognomonic features of dissection, such as an intimal flap or a double lumen, are found in less than 10% of cases40). The artery may show sudden tapering because of occlusion of the lumen, aneurysmal dilatations are also found in some cases.
Magnetic resonance techniques are now replacing conventional angiography as the gold standard in the diagnosis of dissections of the vertebral arteries, because the resolution of magnetic resonance angiography now approaches that of conventional angiography, and magnetic resonance imaging can show the intramural hematoma itself. The intramural hematoma characteristically has a crescentic shape adjacent to the vessel lumen and often spirals along the length of the artery. Fat suppression techniques are important to differentiate small intramural hematomas from the surrounding soft tissues. Magnetic resonance imaging is superior to angiography in the diagnosis of dissection without associated luminal abnormalities or in cases resulting in nonspecific occlusion30). Magnetic resornance imaging can also be used for follow up monitoring of the dissections.
CT angiography has been shown to have a very high sensitivity for vertebral artery dissection, but there is only limited experience with the technique. Unlike the other modalities, changes are apparent very soon after ictus.
PROGNOSIS
Extracranial VADs generally carry a good prognosis. A literature review reports 50% of cases having no neurological deficit, 21% mild deficits only, and 25% moderate to severe deficits, the remaining 4% having died40). In a recent study looking at the relation between recanalization rate and neurological outcome, no relation was found between these two variables7), the neurological outcome was dependent on the lesion localization and the presence of good collaterals.
Intracranial dissections are usually associated with severe neurological deficits or SAH and carry a poor prognosis. The risk of a recurrent dissection in an initially unaffected artery is about 2 percent during the first month but then decreases to a rate of only about 1 percent per year34). However, the increased risk persists for at least a decade and possibly longer38). The risk of a recurrence is higher in young patients with a heritable arteriopathy36). Only rarely do dissections recur in the same artery3,34).
THERAPEUTIC CONSIDERATIONS
Medical Treatment
To prevent thromboembolic complications, anticoagulation with intravenous heparin followed by oral warfarin has been recommended for all patients with acute dissections of the vertebral artery, regardless of the type of symptoms, unless there are contraindications such as the presence of a large infarct with associated mass effect, hemorrhagic transformation of the infarcted area, an intracranial aneurysm, and intracranial extension of the dissection31). Although antithrombotic treatment has been advocated since the 1970s11,30), no randomized trials have been reported, and the validity of such treatment has never been proved. However, there are some indirect evidence of the appropriateness of anticoagulation. Imaging studies suggest that more than 90 percent of infarcts due to dissection are thromboembolic rather than hemodynamic in origin18), and transcranial Doppler studies show a high frequency of intracranial microemboli39). Anticoagulation with a target international normalized ratio (INR) of 2.0 to 3.0 is generally used for three to six months.
One approach is to obtain a magnetic resonance angiogram (MRA) after three months, continue anticoagulant therapy for three more months if luminal irregularities are found, and then repeat the magnetic resonance study and change to antiplatelet therapy if the luminal irregularities are still present. The rationale behind this approach is the high rate of recanalization within the first three months after the dissection and the observation that, after the discontinuation of anticoagulation, symptoms occasionally recur within three to six months after the onset of dissection but rarely after six months. The fear that anticoagulant therapy or intravenous thrombolysis with tissue-plasminogen activator will extend the dissection appears to be unfounded30). Nevertheless, anticoagulation is not innocuous, and some patients are treated with antiplatelet therapy alone, particularly those who have no symptoms of ischemia.
Surgical Interventions
Most VADs heal spontaneously30). However, an urgent surgical intervention may be required in patients presenting with SAH. Symptomatic aneurysmal dilatation of the artery may also warrant surgery. Chronic VADs have also been treated by surgical reconstruction to prevent further ischemic or thromboembolic complications, if medical treatment with six month anticoagulation fails or if the dissecting aneurysms and/or high grade stenosis persist26,40). Surgical interventions include endovascular treatment and the arterial repair.
Endovascular Therapy
Endovascular treatment has largely supplanted surgery as the initial therapy of choice once medical therapy fails or is contraindicated4,8,10,20,31).
Endovascular occlusion of the parent artery
A saccular aneurysm will persist unless treated by surgical clipping or endovascular embolization. In contrast, reflecting the intrinsic mechanism of healing, a dissection can resolve spontaneously. The aims of treatment are, first, to reduce the "hemodynamic stress" on the vessel wall that could produce rebleeding and, second, to provide a suitable environment for healing. Both goals can be achieved by eliminating or reversing the flow within the dissection through sacrificing the parent artery close to or even far proximal to the dissection45). If the dissection is not completely excluded from the antegrade arterial circulation following proximal occlusion, the potential for rebleeding still exists42). Rebleeding can be anticipated when the dissection cavity increases in size after proximal occlusion2). Rebleeding from a "caecum-like" dissection usually occurs within several hours after proximal occlusion, presumably as result of hemodynamic changes in its lumen.
Endovascular trapping
Because histopathology shows that the rupture point is in close proximity to the entrance into the dissection23), an option in treatment is double catheterization and simultaneous embolization of the proximal and distal portions of the dissection15). Endovascular trapping does not cross the dissected segment (which should be avoided). However, ischemia may occur in the territory of brainstem perforators arising from the healthy vessel distal to the dissected portion that will be occluded. Therefore, it is not likely that trapping is superior to occlusion of the proximal parent vessel45). This is because the theoretical benefits (exclusion of the diseased vessel segment) are outweighed by the risk of occluding brain stem perforators in the occluded distal healthy vessel.
Intracranial stenting
Stent implantation has the advantage of preserving the patency of the parent vessel and remodeling blood flow. Nevertheless, there are unnecessary difficulties and hazards. These are that the stent has to be navigated through the dissection, inducing the risk of further dissecting the wall, that uncovered stents that do not fully exclude the dissection from the circulation may lead to early rebleeding, and that stent-assisted coiling of the dissection (as well as coiling of the dissection itself) might cause rupture of the dissection or lead to recanalization if the coil migrates through the dissected vessel wall, so not completely securing the situation19). Finally, the use of stent grafts in the intracranial circulation is experimental, and likely to occlude perforators to the brain stem and even may induce a secondarily symptomatic excessive neointimal proliferation.
Surgery
Surgical treatment should be considered for patients with persistent ischemic symptoms refractory to optimal medical care who are not candidates for endovascular treatment37). Arterial ligation can be a very safe and simple treatment, assuming the presence of adequate collateral blood flow has been established. However, delayed ischemia because of propagation of thrombus or embolization is a potential threat in these patients in the early postoperative period. Trapping of the dissected segment of vertebral artery with or without extracranial-intracranial arterial bypass also can be a curative treatment option.
Rebleeding after surgical proximal occlusion has been reported2,16), whereas trapping excludes the dissection from the circulation thus eliminating the risk of rebleeding which has not been reported after surgical trapping for VADs. However, the location of dissection makes the surgical approach technically demanding with a high risk of cranial nerve damage. Rigorous comparison between surgical and endovascular treatment is difficult, because most reports of surgical treatment include less than 10 patients and their features are not comparable.
SUMMARY
VAD implies a tear in the wall of the artery leading to the intrusion of blood within the layers of an arterial wall. The important pathologic feature of VAD is characterized by acute widespread disruption of the IEL. VAD is mainly divided into two types : the ischemic type, which is manifest by ischemic symptoms and/or infarction of the vertebrobasilar circulation due to arterial narrowing and thromboembolism; and the hemorrhagic type, which presents as an SAH caused by rupture of an intradural vertebral artery dissecting aneurysm. Most dissections of the vertebral arteries heal spontaneously and especially, extracranial VADs generally carry a good prognosis. However, intracranial VADs are usually associated with severe neurological deficits or SAH and carry a poor prognosis, so an urgent surgical intervention may be required in patients presenting with hemorrhage.
Table 1.
Summary of types of aneurysm24)
References
- 1.Anxionnat R, de Melo Neto JF, Bracard S, Lacour JC, Pinelli C, Civit T, et al. Treatment of hemorrhagic intracranial dissections. Neurosurgery. 2003;53:289–300. doi: 10.1227/01.neu.0000073417.01297.93. discussion 300-301. [DOI] [PubMed] [Google Scholar]
- 2.Aoki N, Sakai T. Rebleeding from intracranial dissecting aneurysm in the vertebral artery. Stroke. 1990;21:1628–1631. doi: 10.1161/01.str.21.11.1628. [DOI] [PubMed] [Google Scholar]
- 3.Bassetti C, Carruzzo A, Sturzenegger M, Tuncdogan E. Recurrence of cervical artery dissection. A prospective study of 81 patients. Stroke. 1996;27:1804–1807. doi: 10.1161/01.str.27.10.1804. [DOI] [PubMed] [Google Scholar]
- 4.Bejjani GK, Monsein LH, Laird JR, Satler LF, Starnes BW, Aulisi EF. Treatment of symptomatic cervical carotid dissections with endovascular stents. Neurosurgery. 1999;44:755–760. doi: 10.1097/00006123-199904000-00037. discussion 760-761. [DOI] [PubMed] [Google Scholar]
- 5.Bogousslavsky J, Regli F. Ischemic stroke in adults younger than 30 years of age. Cause and prognosis. Arch Neurol. 1987;44:479–482. doi: 10.1001/archneur.1987.00520170009012. [DOI] [PubMed] [Google Scholar]
- 6.Caplan LR, Biousse V. Cervicocranial arterial dissections. J Neuroophthalmol. 2004;24:299–305. doi: 10.1097/00041327-200412000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Caso V, Paciaroni M, Corea F, Hamam M, Milia P, Pelliccioli GP, et al. Recanalization of cervical artery dissection : influencing factors and role in neurological outcome. Cerebrovasc Dis. 2004;17:93–97. doi: 10.1159/000075775. [DOI] [PubMed] [Google Scholar]
- 8.Coric D, Wilson JA, Regan JD, Bell DA. Primary stenting of the extracranial internal carotid artery in a patient with multiple cervical dissections : technical case report. Neurosurgery. 1998;43:956–959. doi: 10.1097/00006123-199810000-00139. [DOI] [PubMed] [Google Scholar]
- 9.D'Anglejan-Chatillon J, Ribeiro V, Mas JL, Youl BD, Bousser MG. Migraine--a risk factor for dissection of cervical arteries. Headache. 1989;29:560–561. doi: 10.1111/j.1526-4610.1989.hed2909560.x. [DOI] [PubMed] [Google Scholar]
- 10.DeOcampo J, Brillman J, Levy DI. Stenting : a new approach to carotid dissection. J Neuroimaging. 1997;7:187–190. doi: 10.1111/jon199773187. [DOI] [PubMed] [Google Scholar]
- 11.Fisher CM, Ojemann RG, Roberson GH. Spontaneous dissection of cervico-cerebral arteries. Can J Neurol Sci. 1978;5:9–19. [PubMed] [Google Scholar]
- 12.Giroud M, Fayolle H, Andre N, Dumas R, Becker F, Martin D, et al. Incidence of internal carotid artery dissection in the community of Dijon. J Neurol Neurosurg Psychiatry. 1994;57:1443. doi: 10.1136/jnnp.57.11.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grau AJ, Brandt T, Buggle F, Orberk E, Mytilineos J, Werle E, et al. Association of cervical artery dissection with recent infection. Arch Neurol. 1999;56:851–856. doi: 10.1001/archneur.56.7.851. [DOI] [PubMed] [Google Scholar]
- 14.Hufnagel A, Hammers A, Schonle PW, Bohm KD, Leonhardt G. Stroke following chiropractic manipulation of the cervical spine. J Neurol. 1999;246:683–688. doi: 10.1007/s004150050432. [DOI] [PubMed] [Google Scholar]
- 15.Kai Y, Hamada JI, Morioka M, Todaka T, Mizuno T, Ushio Y. Endovascular coil trapping for ruptured vertebral artery dissecting aneurysms by using double microcatheters technique in the acute stage. Acta Neurochir(Wien) 2003;145:447–451. doi: 10.1007/s00701-003-0012-7. discussion 451. [DOI] [PubMed] [Google Scholar]
- 16.Kawamata T, Tanikawa T, Takeshita M, Onda H, Takakura K, Toyoda C. Rebleeding of intracranial dissecting aneurysm in the vertebral artery following proximal clipping. Neurol Res. 1994;16:141–144. doi: 10.1080/01616412.1994.11740213. [DOI] [PubMed] [Google Scholar]
- 17.Leclerc X, Lucas C, Godefroy O, Nicol L, Moretti A, Leys D, et al. Preliminary experience using contrast-enhanced MR angiography to assess vertebral artery structure for the follow-up of suspected dissection. AJNR Am J Neuroradiol. 1999;20:1482–1490. [PMC free article] [PubMed] [Google Scholar]
- 18.Lucas C, Moulin T, Deplanque D, Tatu L, Chavot D. Stroke patterns of internal carotid artery dissection in 40 patients. Stroke. 1998;29:2646–2648. doi: 10.1161/01.str.29.12.2646. [DOI] [PubMed] [Google Scholar]
- 19.MacKay CI, Han PP, Albuquerque FC, McDougall CG. Recurrence of a vertebral artery dissecting pseudoaneurysm after successful stentsupported coil embolization : case report. Neurosurgery. 2003;53:754–759. doi: 10.1227/01.neu.0000080065.49651.48. discussion 760-761. [DOI] [PubMed] [Google Scholar]
- 20.Marks MP, Dake MD, Steinberg GK, Norbash AM, Lane B. Stent placement for arterial and venous cerebrovascular disease : preliminary experience. Radiology. 1994;191:441–446. doi: 10.1148/radiology.191.2.8153318. [DOI] [PubMed] [Google Scholar]
- 21.Mizutani T, Aruga T, Kirino T, Miki Y, Saito I, Tsuchida T. Recurrent subarachnoid hemorrhage from untreated ruptured vertebrobasilar dissecting aneurysms. Neurosurgery. 1995;36:905–911. doi: 10.1227/00006123-199505000-00003. discussion 912-913. [DOI] [PubMed] [Google Scholar]
- 22.Mizutani T, Kojima H, Asamoto S. Healing process for cerebral dissecting aneurysms presenting with subarachnoid hemorrhage. Neurosurgery. 2004;54:342–347. doi: 10.1227/01.neu.0000103449.80484.7e. discussion 347-348. [DOI] [PubMed] [Google Scholar]
- 23.Mizutani T, Kojima H, Asamoto S, Miki Y. Pathological mechanism and three-dimensional structure of cerebral dissecting aneurysms. J Neurosurg. 2001;94:712–717. doi: 10.3171/jns.2001.94.5.0712. [DOI] [PubMed] [Google Scholar]
- 24.Mizutani T, Miki Y, Kojima H, Suzuki H. Proposed classification of nonatherosclerotic cerebral fusiform and dissecting aneurysms. Neurosurgery. 1999;45:253–259. doi: 10.1097/00006123-199908000-00010. discussion 259-260. [DOI] [PubMed] [Google Scholar]
- 25.Mokri B, Houser OW, Sandok BA, Piepgras DG. Spontaneous dissections of the vertebral arteries. Neurology. 1988;38:880–885. doi: 10.1212/wnl.38.6.880. [DOI] [PubMed] [Google Scholar]
- 26.Muller BT, Luther B, Hort W, Neumann-Haefelin T, Aulich A, Sandmann W. Surgical treatment of 50 carotid dissections : indications and results. J Vasc Surg. 2000;31:980–988. doi: 10.1067/mva.2000.104586. [DOI] [PubMed] [Google Scholar]
- 27.Pozzati E, Andreoli A, Limoni P, Casmiro M. Dissecting aneurysms of the vertebrobasilar system : study of 16 cases. Surg Neurol. 1994;41:119–124. doi: 10.1016/0090-3019(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 28.Sano H, Kato Y, Okuma I, Yamaguchi S, Ninomiya T, Arunkumar R, et al. Classification and treatment of vertebral dissecting aneurysm. Surg Neurol. 1997;48:598–605. doi: 10.1016/s0090-3019(97)00022-0. [DOI] [PubMed] [Google Scholar]
- 29.Sasaki O, Ogawa H, Koike T, Koizumi T, Tanaka R. A clinicopathological study of dissecting aneurysms of the intracranial vertebral artery. J Neurosurg. 1991;75:874–882. doi: 10.3171/jns.1991.75.6.0874. [DOI] [PubMed] [Google Scholar]
- 30.Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344:898–906. doi: 10.1056/NEJM200103223441206. [DOI] [PubMed] [Google Scholar]
- 31.Schievink WI. The treatment of spontaneous carotid and vertebral artery dissections. Curr Opin Cardiol. 2000;15:316–321. doi: 10.1097/00001573-200009000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Schievink WI, Bjornsson J, Piepgras DG. Coexistence of fibromuscular dysplasia and cystic medial necrosis in a patient with Marfan's syndrome and bilateral carotid artery dissections. Stroke. 1994;25:2492–2496. doi: 10.1161/01.str.25.12.2492. [DOI] [PubMed] [Google Scholar]
- 33.Schievink WI, Michels VV, Piepgras DG. Neurovascular manifestations of heritable connective tissue disorders. A review. Stroke. 1994;25:889–903. doi: 10.1161/01.str.25.4.889. [DOI] [PubMed] [Google Scholar]
- 34.Schievink WI, Mokri B, O'Fallon WM. Recurrent spontaneous cervical-artery dissection. N Engl J Med. 1994;330:393–397. doi: 10.1056/NEJM199402103300604. [DOI] [PubMed] [Google Scholar]
- 35.Schievink WI, Mokri B, Piepgras DG. Spontaneous dissections of cervicocephalic arteries in childhood and adolescence. Neurology. 1994;44:1607–1612. doi: 10.1212/wnl.44.9.1607. [DOI] [PubMed] [Google Scholar]
- 36.Schievink WI, Mokri B, Piepgras DG, Kuiper JD. Recurrent spontaneous arterial dissections : risk in familial versus nonfamilial disease. Stroke. 1996;27:622–624. doi: 10.1161/01.str.27.4.622. [DOI] [PubMed] [Google Scholar]
- 37.Schievink WI, Piepgras DG, McCaffrey TV, Mokri B. Surgical treatment of extracranial internal carotid artery dissecting aneurysms. Neurosurgery. 1994;35:809–815. doi: 10.1227/00006123-199411000-00002. discussion 815-816. [DOI] [PubMed] [Google Scholar]
- 38.Schievink WI, Wijdicks EF, Michels VV, Vockley J, Godfrey M. Heritable connective tissue disorders in cervical artery dissections : a prospective study. Neurology. 1998;50:1166–1169. doi: 10.1212/wnl.50.4.1166. [DOI] [PubMed] [Google Scholar]
- 39.Srinivasan J, Newell DW, Sturzenegger M, Mayberg MR, Winn HR. Transcranial Doppler in the evaluation of internal carotid artery dissection. Stroke. 1996;27:1226–1230. doi: 10.1161/01.str.27.7.1226. [DOI] [PubMed] [Google Scholar]
- 40.Thanvi B, Munshi SK, Dawson SL, Robinson TG. Carotid and vertebral artery dissection syndromes. Postgrad Med J. 2005;81:383–388. doi: 10.1136/pgmj.2003.016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamaura A, Watanabe Y, Saeki N. Dissecting aneurysms of the intracranial vertebral artery. J Neurosurg. 1990;72:183–188. doi: 10.3171/jns.1990.72.2.0183. [DOI] [PubMed] [Google Scholar]
- 42.Yasui T, Komiyama M, Nishikawa M, Nakajima H. Subarachnoid hemorrhage from vertebral artery dissecting aneurysms involving the origin of the posteroinferior cerebellar artery : report of two cases and review of the literature. Neurosurgery. 2000;46:196–200. discussion 200-201. [PubMed] [Google Scholar]
- 43.Yonas H, Agamanolis D, Takaoka Y, White RJ. Dissecting intracranial aneurysms. Surg Neurol. 1977;8:407–415. [PubMed] [Google Scholar]
- 44.Yoshimoto Y, Wakai S. Unruptured intracranial vertebral artery dissection. Clinical course and serial radiographic imagings. Stroke. 1997;28:370–374. doi: 10.1161/01.str.28.2.370. [DOI] [PubMed] [Google Scholar]
- 45.Zhao WY, Krings T, Alvarez H, Ozanne A, Holmin S, Lasjaunias P. Management of spontaneous haemorrhagic intracranial vertebrobasilar dissection: review of 21 consecutive cases. Acta Neurochir(Wien) 2007;149:585–596. doi: 10.1007/s00701-007-1161-x. discussion 596. [DOI] [PubMed] [Google Scholar]