Abstract
Objective
There are a few reports on the complications of surgery for epilepsy. We surveyed our data to present complications of epilepsy surgeries from the neurosurgeon's point of view and compare our results with other previous reports.
Methods
A total of 179 surgical procedures for intractable epilepsy (41 diagnostic, 138 therapeutic) were performed in 92 consecutive patients (10 adults, 82 children) during the last 9.2 years (February. 1997-April. 2006). Their medical records and radiological findings were reviewed to identify and analyze the surgical complications.
Results
The diagnostic procedures encompassed various combinations of subdural grid, subdural strips, and depth electrodes. Four minor transient complications developed in 41 diagnostic procedures (4/41=9.8%). A total of 138 therapeutic procedures included 28 anterior temporal lobectomies, 21 other lobectomies, 6 lesionectomies, 21 topectomies, 13 callosotomies, 20 vagus nerve stimulations, 13 multiple subpial transections, and 16 hemispherectomies. Twenty-six complications developed in therapeutic procedures (26/138=18.8%). Out of the 26 complications, 21 complications were transient and reversible (minor; 21/138=15.2%), and 5 were serious complications (major; 5/138=3.6%). Five major complications were one visual field defect, two mortality cases and two vegetative states. There were 2 additional mortality cases which were not related to the surgery itself.
Conclusion
Our results indicate that complication rate was higher than previous other reports in minor complications and was comparable in major complications. However, our results show relatively high frequency of mortality cases and severe morbidity case compared to other previous reports. The authors would like to emphasize the importance of acute postoperative care in young pediatric patients as well as meticulous surgical techniques to reduce morbidity and mortality in epilepsy surgery.
Keywords: Epilepsy, Surgery, Intraoperative complication, Morbidity, Mortality
INTRODUCTION
In past decades, the interests in surgical treatment for drug resistant epilepsy have continuously increased1,4,9,12). The developments of the surgical techniques seemed to decrease the complications of epilepsy surgery and improved the results of operations8,10,11,17). Surgery for intractable epilepsy provides many patients substantial relief from seizures, functional improvement and increased quality of life3,7,15,19).
However, postoperative complications are always accompanied with epilepsy surgeries, like all other neurological surgeries, and the surgeon should always be aware of these complications in order to facilitate assessment of risk/benefit ratio which is essential for planning of epilepsy surgery and counseling patients2,13,18). In this report, we focus our attention on surgical complications of epilepsy surgery from neurosurgeon's point of view, and they are presented in detail regarding minor (transient) and major (permanent) morbidity.
MATERIALS AND METHODS
The present study includes 92 consecutive patients who were operated on the epilepsy center of our hospital for the last 9.2 years; from February 1997 to April 2006. These patients underwent a total of 179 surgical procedures. Among these procedures, 41 were diagnostic and 138 were therapeutic. Out of the 92 patients, 82 patients were children (<18 year old). There were 50 male and 42 female patients. The age of patients at operation ranged between 3 months and 36 years with mean age 9 years. Seven infants were included in the cohort of patients. All patients were followed-up at least more than a year.
The medical records and radiologic studies of the patient were reviewed retrospectively. We defined a complication as an unwanted, unexpected, and uncommon event after diagnostic or therapeutic procedures5,13). Hence, for example, an expected worsened paresis after hemispherectomy was not regarded as a complication2). A complication was defined as minor if it resolves within 3 months without sequelae and major if it lasts longer than 3 months with or without persistent deficits18). Neuropsychological and psychiatric complications as well as the rate of failure to control seizures are not addressed in this article.
RESULTS
Because of relatively predominant activity of pediatric epileptologist of our center, our series have much larger number of pediatric patients than adult patients. The number of temporal lobe epilepsy (TLE) was 22. Out of 22 patients, 15 were children and 7 were adults. In case of 70 extratemporal lobe epilepsy (ETLE), 67 patients were children and 3 patients were adults.
According to the pathologic substrate of epilepsy, there were 25 cases of malformation of cortical development in ETLE. Other representative pathologies were comprised of 7 brain tumors, 4 Sturge-Weber syndromes, and 4 tuberous sclerosis complexes. And, there were 22 cases of Lennox-Gastaut syndrome; it is the most frequent single pathogenic clinical syndrome in pediatric patient.
There were 30 cases of complication after 179 consecutive surgical procedures, so the complication rate of total series was 16.7% (30/179). Among the 30 complications, 4 cases occurred after diagnostic procedure and 26 cases occurred after therapeutic procedure. Therefore, complication rate of diagnostic and therapeutic procedures were 9.8% (4/41) and 18.8% (26/138), respectively.
We excluded the procedure performed for complication itself from the count of procedure (eg. shunt operation for hydrocephalus, hematoma removal for intracranial hemorrhage, etc.).
Diagnostic procedures and complications
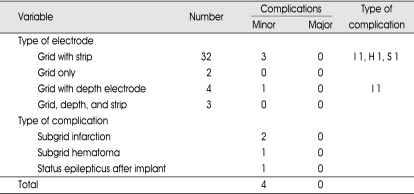
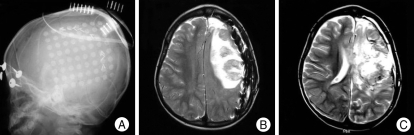
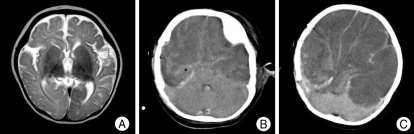
Forty-one diagnostic invasive procedures were performed in 40 patients. The implantation period ranged between 1 to 7 days, with an average of 5 days. The combination of electrodes and types of complication are listed in Table 1. There were two cases of acute cerebral infarction in patients who received subdural grid insertion. A 13-year-old girl underwent single large grid (8×8) insertion on the frontoparietal region, and on the 2nd postoperative day, neurological deterioration was noted. Subgrid infarction and brain swelling were revealed on MRI, and she underwent emergent removal of bone flap and grid. Another case was 5-year-old boy who also underwent single large grid (8×8) insertion. Postoperatively, we could recognize large subgrid infarction on the routine postoperative MRI. However, there was no neurologic deterioration although radiologic finding was more severe than previous case. We performed prescheduled hemispherectomy without emergent decompression (Fig. 1). There was one case of pure subgrid hematoma and it was removed in next therapeutic procedure and no relevant sequelae developed. In one case, the patient dropped into transient status epilepticus attack after electrode implantation. The patient recovered without neurologic deficit and tolerated well during 5 days of monitoring. All four patients did not suffer from permanent sequelae.
Table 1.
Diagnostic procedures and complications
I : infarction, H : hematoma, S : status epilepticus
Fig. 1.
A single large grid (8×8) is inserted on the left temporoparietal lobe (A). On the 3rd postoperative day, a significant subgrid infarction on the routine follow-up magnetic resonance imaging is noted. But, there is no significant mass effect by infarction (B) and no clinical deterioration was noted. Magnetic resonance imaging taken after peri-insular hemispherotomy shows no mass effect (C).
Therapeutic procedures and complications
A total of 138 therapeutic surgical procedures were performed in 92 consecutive patients. These procedures and resultant complications are listed in Table 2 and 3. After 138 procedures, 26 complications developed (18.8%). Among them, 21 cases (15.2%) were minor complications, and 5 cases (3.6%) were major complications.
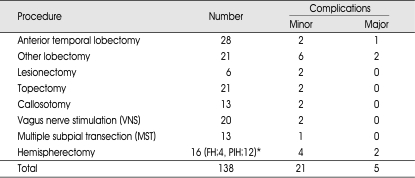
Table 2.
Therapeutic procedures of epilepsy surgery and resultant complications
*16 procedures in 8 patients. FH : functional hemispherectomy, PIH : peri-insular hemispherotomy
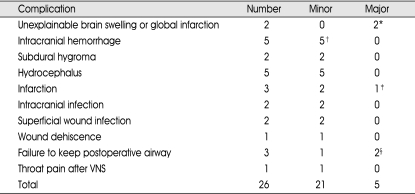
Table 3.
Complications after 138 therapeutic procedures
*dead 1, vegetative state 1, †ICH 2, SDH 2, EDH 1, ‡homonymous hemianopsia due to occipital lobe infarction, §dead 1, vegetative state 1, VNS : vagus nerve stimulation
The therapeutic procedure with the highest rate of complication was hemispherectomy (6/16=37.5%) and it was followed by 'other lobectomies' (7/21=33.3%). 'Other lobectomy' means single or multiple lobectomy except typical anterior temporal lobectomy (ATL) and it was done usually for resection of cortical dysplasia in frontal, parietal, occipital and temporal lobe also. Among the 16 hemispherectomies, 4 were functional hemispherectomies (FH) and 12 were peri-insular hemispherotomies (PIH). All hemispherectomies were performed as intentional two-stage procedures except 2 cases of PIH (Table 2).
The most common complications were intracranial hemorrhage and hydrocephalus (both n; 5=19.2% respectively). Five cases of intracranial hemorrhage encompassed 2 cases of intracerebral hemorrhages (ICH), 2 cases of subdural hematomas (SDH), and 1 case of epidural hematoma (EDH). Of the two ICHs, one is the small intracerebellar hemorrhage developed after ATL. It did not need operation. The other ICH was peculiar bilateral multiple ICHs after ATL and concomitant topectomy. The patient was recovered without neurologic deficit (Fig. 2). Two SDHs and an EDH needed operations for evacuation. All five cases of hydrocephalus occurred after hemispherectomies and other wide resective surgeries, and they needed shunt operations. Among 3 postoperative infarctions, a case of major complication has developed; contralateral homonymous hemianopsia after posteriorly extended temporal lobectomy to remove cortical dysplasia. Other two infarctions were minor ones.
Fig. 2.
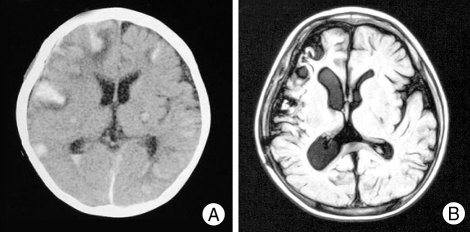
After anterior temporal lobectomy, unexpected bilateral multiple Intracerebral hemorrhages (ICHs) are shown in routine postoperative computed tomography scan (A). The patient tolerated well with conservative care and there were no definite neurological symptoms. ICHs are totally resolved in magnetic resonance imaging taken 3 months later (B).
Other minor complications were 2 cases of subdural hygroma, 2 cases of intracranial infection or meningitis, 2 cases of superficial wound infection, 1 case of wound dehiscence, and 1 case of throat pain after turning on the VNS stimulator.
Almost all complications above mentioned are common complications, which can develop after any other neurosurgical procedures. Here, the authors will present other eccentric experiences; first we have two cases of 'unexplainable brain swelling and/or global infarction'. The first case was a 13-month-old boy who died from immediate postoperative bilateral global infarction after multiple lobectomy for cortical dysplasia in the right temporoparietooccipital. He died at 9th postoperative day (Fig. 3). The second case was a 9-year-old girl who was diagnosed as Rassmussen's encephalitis. She underwent first stage of functional hemispherectomy in right hemisphere, and was recovered to preoperative state after the surgery. On the 10th postoperative day, she dropped into status epilepticus with unexplainable diffuse brain swelling in contralateral untouched hemisphere. Thereafter, the patient deteriorated slowly and was fixed to vegetative state. Long-term follow-up MRI revealed bilateral diffuse hemispheric atrophic changes. Next, there were three cases of so-called 'failure to keep postoperative airway'.One case was reversible without sequelae but other two cases fell into global hypoxic brain injury. The first case of global hypoxic injury was an 8-month-old girl who underwent peri-insular hemispherotomy(PIH) for Sturge-Weber syndrome involving the left hemisphere. She has been dropped into vegetative state because of global hypoxic brain injury from suffocation that accidentally occurred due to the dislodging of the endotracheal tube during immediate postoperative transportation of the patient from OR to ICU. The second case was a 17-year-old male of temporal lobe epilepsy. He died of hypoxic brain damage from hypoventilation induced by deep sedation, which was administered to relieve prolonged severe head-ache after the left anterior temporal lobectomy (ATL).
Fig. 3.
Preoperative magnetic resonance imaging reveals cortical dysplasia in right temporoparietooccipital region (A). The patient underwent multi-lobectomy, and postoperative computed tomography (CT) reveals bilateral global infarctions (B). Ninth postoperative day CT shows severe aggravation of infarction (C) and the patient died the day after this CT.
Irrelevant to surgery itself, we have experienced 2 additional mortality cases. The first case was a 5 year-old boy who was suffering from viral encephalitis and resultant intractable status epilepticus for 4 days. The clinical state was very poor and desperate surgery was done, frontal lobectomy and additional nearby topectomy. He died on the fifth postoperative day because of aggravation of premorbid encephalitis. This case was not included in rational indication for epilepsy surgery and we tried only amelioration of seizure attacks in hopeless clinical status, so we used expression so-called 'desperate' surgery. The second case was an 8-year-old girl having Lennox-Gastaut syndrome whose seizure was well controlled for more than 2 years after implantation of VNS system. One day she died unexpectedly during sleep so this case can be classified as SUDEP (Sudden Unexpected Death in Epilepsy). These 2 cases were not directly related to the surgery, and therefore we did not count as complications.
DISCUSSION
As previously described, complication rate of our series is 16.7% in total series (30/179), 9.8% in diagnostic procedures (4/41), and 18.8% in therapeutic procedures (26/138). Moreover, our series has two mortality cases and two severely disabled results.
Behrens et al.2) reported complication frequencies after analysis of 708 procedures (279 diagnostic and 429 therapeutic). The rate of surgical complications in diagnostic procedures was 2.9%, and all were transient complications. Among 429 therapeutic procedures, 36 surgical complications occurred (8.4%). The rate of permanent neurological complications was 0.7%. They had no mortality case. In their series, complications were subdivided into transient and permanent complications that approximately correspond to the definitions of minor and major complications in this study. Rydenhag et al.13) performed 654 surgical procedures. These procedures were performed at six different centers in Sweden. Of these, 205 were invasive electrode procedures, and 449 were therapeutic procedures. After invasive electrode procedures, only minor complications were reported (6.3%). For all 449 therapeutic procedures (including reoperations), minor complications were reported in 8.9% and major complications in 3.1%. Only one major complication was reported in a patient under the age of 35. In their study, they emphasized that risk is related to age. In patients younger than 35, the risk for a major complication after invasive subdural strip electrode investigation and epilepsy surgery was low. There was one mortality case in this report. Inoue et al.6) reported a 5.1% (8 cases) rate of serious neurological deficits (5 patients with hemiparesis and 3 with hemianopsia) after 157 surgical procedures.
As compared with these reports, our result was poorer in respect of rate of minor complications and was comparable in respect of rate of major complications. Although rate of major complications was comparable, our results have relatively high frequency of mortality and severe morbidity. We believe that the causes of these generally poorer results as follows. First, our series had relatively large proportion of young pediatric patients who required wide resective surgeries. In this series, wide resective or disconnective surgeries such as hemispherectomy (yielded 6 complications) and other lobectomy (yielded 8 complications) are the main source of complications including 4 major complications. In contrast, surgeries necessitating small resection or no cortical manipulation such as local topectomy, callosotomy, and implantation of VNS device produced only small numbers of minor complications. Second, inattentive immediate and delayed postoperative care. The 'inattentive postoperative care 'indicates mainly patient's airway problem. The endotracheal tubes using in small baby usually has no cuff, so these tubes are easily movable and vulnerable to dislodge out from proper airway despite heavy anchoring around mouth using tapes. We routinely transferred the patients from operation room to intensive care unit in unrecovered state from anesthesia with Ambu bagging via CT room for immediate postoperative CT scan. After accident, we changed our routine sequence of immediate postoperative care in young children as follows; (1) try full recovery at the end of surgery and extubate in operation room, (2) no immediate postoperative CT because this is an only just customary procedure, (3) close observation in ICU and take CT scan if worsening of condition is evident. As previously described, another airway problem was a result of inadvertent use of intravenous sedatives to control postoperative headache. Unusually prolonged severe headache after routine ATL made medical staffs indifferent to patient's complaints and many analgesics were prescribed without proper supervision. Finally, a potent intravenous injection was administered and no close observation was paid for his respiration. Significant respiratory suppression developed and progressed to respiratory arrest. When this accident has noted, the patient's condition was already irreversible. 3) We had two cases of unfortunate phenomena developed by irresistible force such as immediate postoperative global infarction and unexplainable delayed postoperative brain swelling. We did search references about these phenomena but could find only one article having a similar morbidity case. Shimizu and Maehara described briefly a case of 3-year-old boy who developed severe bilateral brain swelling 6 days after hemispherectomy and became severely disabled14). They did not comment about the cause of this unusual phenomenon. We also could not presume the causes of our experiences of immediate postoperative global infarction and delayed diffuse brain swelling just yet. In our case of immediate postoperative infarction, there were no events warning the catastrophic result during the surgery.
In diagnostic procedures, we realized that the surgeons should always keep in mind the subgrid infarction can develop at any time after subdural electrodes insertion. In particular, a large grid can't accommodate the round convexity of hemispherical surface, and it can compress brain cortex itself and cortical vessels with mass effect16). We think it is wise to use multiple small grids instead of single large grid to reduce compression by kinky large grid when cover wide surface of hemisphere.
Generally, intraoperative hypovolemia may be one of the main factors for surgical morbidity or mortality in small babies. In our series, 7 infants have undergone heavy surgical procedures such as hemispherectomy or multi-lobectomy. These procedures could be performed without any morbidity in terms of volume depletion by virtue of sophisticated anesthesiologic technique.
CONCLUSION
Our results indicate that epilepsy surgery may be associated with substantial rate of morbidity and even mortality. Although the occurrence rate of minor complication was higher than other reports, the practical contents of these complications are acceptable and even negligible. But, several major complications made our results definitely poorer compared to previous other reports.
When performing an operation the more wide surgery, the more meticulous technique to handling pia mater and small cortical vessels is needed to prevent complications such as hydrocephalus, infarction of nearby cortex, and intracranial hemorrhage. Moreover, we have learned that proper postoperative management is as important as intraoperative surgical techniques.
In conclusion, complications of epilepsy surgery procedure can occur at any circumstance like all other neurological surgeries, so the surgeon should always keep these in mind to make correct decision and to give proper information and/or advice to patients and their families.
References
- 1.Beckung E, Uvebrant P, Hedström A, Rydenhag B. The effects of epilepsy surgery on the sensorimotor function of children. Dev Med Child Neurol. 1994;36:893–901. doi: 10.1111/j.1469-8749.1994.tb11780.x. [DOI] [PubMed] [Google Scholar]
- 2.Behrens E, Schramm J, Zentner J, König R. Surgical and neurological complications in a series of 708 epilepsy surgery procedures. Neurosurgery. 1997;41:1–9. doi: 10.1097/00006123-199707000-00004. discussion 9-10. [DOI] [PubMed] [Google Scholar]
- 3.Engel JJ, Van Ness PC, Rasmussen TB, Ojemann LM. Outcome with respect to epileptic seizures. In: Engel JJ, editor. Surgical Treatment of the Epilepsies. ed 2. New York: Raven Press; 1993. pp. 609–622. [Google Scholar]
- 4.Hoffman HJ. Benefits of early surgery in Sturge-Weber syndrome. In: Tuxhorn I, Holthausen H, Boenigk H, editors. Pediatric Epilepsy Syndromes and Their Surgical Treatment. London: John Libbey; 1997. pp. 364–370. [Google Scholar]
- 5.Hughes TS, Abou-Khalil B, Lavin P, Fakhoury T, Blumenkopf B, Donahue SP. Visual field defects after temporal lobe resection : a prospective quantitative analysis. Neurology. 1999;53:167–172. doi: 10.1212/wnl.53.1.167. [DOI] [PubMed] [Google Scholar]
- 6.Inoue Y, Mihara T, Seino M. Timing of epilepsy surgery : Its relevance for psychosocial rehabilitation. In: Tuxhorn I, Holthausen H, Boenigk H, editors. Pediatric Epilepsy Syndromes and Their Surgical Treatment. London: John Libbey; 1997. pp. 76–84. [Google Scholar]
- 7.International League. A global survey on epilepsy surgery, 1980-1990 : a report by the commission on neurosurgery of epilepsy, the International League Against Epilepsy. Epilepsia. 1997;38:249–255. doi: 10.1111/j.1528-1157.1997.tb01105.x. [DOI] [PubMed] [Google Scholar]
- 8.Jensen I, Seedorff HH. Temporal lobe epilepsy and neuro-ophthalmology. Ophthalmological findings in 74 temporal lobe resected patients. Acta Ophthalmol (Copenh) 1976;54:827–841. doi: 10.1111/j.1755-3768.1976.tb01803.x. [DOI] [PubMed] [Google Scholar]
- 9.Malmgren K, Sullivan M, Edstedt G, Kullberg G, Kumlien E. Health-related quality of life after epilepsy surgery : a Swedish multicenter study. Epilepsia. 1997;38:830–838. doi: 10.1111/j.1528-1157.1997.tb01471.x. [DOI] [PubMed] [Google Scholar]
- 10.Manji H, Plant GT. Epilepsy surgery, visual fields, and driving : A study of the visual field criteria for driving in patients after temporal lobe epilepsy surgery with a comparison of Goldmann and Esterman perimetry. J Neurol Neurosurg Psychiatry. 2000;68:80–82. doi: 10.1136/jnnp.68.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marino R, Jr, Rasmussen T. Visual field changes after temporal lobectomy in man. Neurology. 1968;18:825–835. doi: 10.1212/wnl.18.9.825. [DOI] [PubMed] [Google Scholar]
- 12.Palmini A, Costa da Costa J, Andermann F, Dubeau F, Gambardella A, Kim H-I, et al. Surgical results in epilepsy patients with localized cortical dysplastic lesions. In: Tuxhorn I, Holthausen H, Boenigk H, editors. Pediatric Epilepsy Syndromes and Their Surgical Treatment. London: John Libbey; 1997. p. 216.p. 224. [Google Scholar]
- 13.Rydenhag B, Silander HC. Complications of epilepsy surgery after 654 procedures in Sweden, September 1990-1995 : a multicenter study based on the Swedish National Epilepsy Surgery Register. Neurosurgery. 2001;49:51–56. doi: 10.1097/00006123-200107000-00007. discussion 56-57. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu H, Maehara T. Modification of peri-insular hemispherectomy and surgical results. Neurosurgery. 2000;47:367–372. doi: 10.1097/00006123-200008000-00018. discussion 372-373. [DOI] [PubMed] [Google Scholar]
- 15.Silander H, Blom S, Malmgren K, Ros I, Uvebrant P. Surgical treatment for epilepsy : a retrospective Swedish multicenter study. Acta Neurol Scand. 1997;95:321–330. doi: 10.1111/j.1600-0404.1997.tb00219.x. [DOI] [PubMed] [Google Scholar]
- 16.Son EI. Complications and its managements of the epilepsy surgery. J Korean Neurosurg Soc. 1997;26:1772–1779. [Google Scholar]
- 17.Tecoma ES, Laxer KD, Barbaro NM, Plant GT. Frequency and characteristics of visual field deficits after surgery for mesial temporal sclerosis. Neurology. 1993;43:1235–1238. doi: 10.1212/wnl.43.6.1235. [DOI] [PubMed] [Google Scholar]
- 18.Van Buren JM. Complications of surgical procedures in the diagnosis and treatment of epilepsy. In: Engel JJ, editor. Surgical Treatment of the Epilepsies. New York: Raven Press; 1987. pp. 465–475. [Google Scholar]
- 19.Vickrey BG, Hays RD, Hermann BP, Bladin PF, Batzel LW. Outcomes with respect to quality of life. In: Engel JJ, editor. Surgical Treatment of the Epilepsies. ed 2. New York: Raven Press; 1993. pp. 623–636. [Google Scholar]