Abstract
Objective
The standard treatment strategy of intracranial aneurysms includes either endovascular coiling or microsurgical clipping. In certain situations such as in giant or dissecting aneurysms, bypass surgery followed by proximal occlusion or trapping of parent artery is required.
Methods
The authors assessed the result of extracranial-intracranial (EC-IC) bypass surgery in the treatment of complex intracranial aneurysms in one institute between 2003 and 2007 retrospectively to propose its role as treatment modality. The outcomes of 15 patients with complex aneurysms treated during the last 5 years were reviewed. Six male and 9 female patients, aged 14 to 76 years, presented with symptoms related to hemorrhage in 6 cases, transient ischemic attack (TIA) in 2 unruptured cases, and permanent infarction in one, and compressive symptoms in 3 cases. Aneurysms were mainly in the internal carotid artery (ICA) in 11 cases, middle cerebral artery (MCA) in 2, posterior cerebral artery (PCA) in one and posterior inferior cerebellar artery (PICA) in one case.
Results
The types of aneurysms were 8 cases of large to giant size aneurysms, 5 cases of ICA blood blister-like aneurysms, one dissecting aneurysm, and one pseudoaneurysm related to trauma. High-flow bypass surgery was done in 6 cases with radial artery graft (RAG) in five and saphenous vein graft (SVG) in one. Low-flow bypass was done in nine cases using superficial temporal artery (STA) in eight and occipital artery (OA) in one case. Parent artery occlusion was performed with clipping in 9 patients, with coiling in 4, and with balloon plus coil in 1. Direct aneurysm clip was done in one case. The follow up period ranged from 2 to 48 months (mean 15.0 months). There was no mortality case. The long-term clinical outcome measured by Glasgow outcome scale (GOS) showed good or excellent outcome in 13/15. The overall surgery related morbidity was 20% (3/15) including 2 emergency bypass surgeries due to unexpected parent artery occlusion during direct clipping procedure. The short-term postoperative bypass graft patency rates were 100% but the long-term bypass patency rates were 86.7% (13/15). Nonetheless, there was no bypass surgery related morbidity due to occlusion of the graft.
Conclusion
Revascularization technique is a pivotal armament in managing complex aneurysms and scrupulous prior planning is essential to successful outcomes.
Keywords: Cerebral aneurysm, Extracranial-intracranial bypass, Outcomes
INTRODUCTION
The current treatment strategy of intracranial aneurysms includes either endovascular coiling or microsurgical clipping. Stent or balloon assisted endovascular procedure is getting its favor with improving techniques and evolving technology but microsurgical management is still useful for management of complex intracranial aneurysms1,2). However, in certain situations, neither the endovascular nor micro-surgical clip may be applicable in complex aneurysm cases. Such situations include extreme ends of aneurysm size, location, wall thickness due to calcification or atheroscle-rotic plaques, intramural thrombosis, dissecting aneurysm, and incorporation of perforators or major artery from the aneurysmal neck or sac13,16). In these situations, vascular reconstruction followed by occlusion of parent artery is required.
EC-IC bypass technique was first directed its usage in the treatment of ischemic stroke. Although discouraged by the international randomized trial for preventing ischemic stroke, its technique has made advancement in the use of different graft materials and in alignment with improving protective measures for the temporary occlusion period8). With advancement in the cardiovascular bypass field, intracranial vascular reconstruction has marched its progression as well. Change was made from venous graft to arterial graft after the problem of arterial spasm was resolved using pressure distension technique and use of calcium channel blocker21). Use of mild hypothermia, mannitol and drugs lowering brain metabolism prevented the assault to brain during temporary occlusion. Through many dedicated surgeons' efforts, the technique is now widely accepted as a valuable mean to treat aneurysm or skull base tumors7,9, 11-13,17-19,22).
In this article, complex aneurysm cases treated with EC-IC bypass tech-nique are presented and the bypass graft patency rate and overall clinical outcomes are analyzed to identify the risk factors for poor outcome.
MATERIALS AND METHODS
Patient selection
From 2003 to 2007, 15 cases of revascularization were performed by a single neurovascular surgeon (JSA) for the treatment of complex cerebral aneurysms at our center. Table 1 represents a summary of patients, the type of bypass and graft used. Table 2 shows graft patency rate, outcomes of patients and complications occurred. There were thirteen elective cases and two emergency bypass attempts where prolonged ischemia occurred by unexpected parent artery occlusion during direct clipping attempts of blood blister-like aneurysms.
Table 1.
Summary of 15 revascularization cases for aneurysm treatment
ICA : internal carotid artery, MCA : middle cerebral artery, PCA : posterior cerebral artery, PICA : posterior inferior cerebellar artery, STA : superficial temporal artery, OA : occipital artery
Table 2.
Surgical outcomes and complications
*Emergency bypass surgery after intraoperative ischemia had occurred. GOS : Glasgow Outcome Scale
The study included 9 female and 6 male patients. Their age ranged from 14 to 76 years (mean 48.7 years). Follow-up periods ranged from 2 to 48 months (mean 15 months). The majority of aneurysm was located in the ICA, 11 cases. Two cases were in the MCA and one each in PCA and in PICA. Symptoms related to the mass effect were noted in three cases. Six cases were presented as subarachnoid hemorrhage (SAH). Among unruptured cases, 2 presented as TIA and one with infarction. There was one recurrent aneurysm case where endovascular coiling was done in other institute 22 months prior due to SAH.
Preoperative evaluation
Preoperatively, all patients were evaluated for cardiopulmonary function for general anesthesia. Preoperative workup includes conventional angiography and magnetic resonance (MR) or computed tomographic (CT) angiography. Patients with aneurysms in the ICA underwent balloon test occlusion or compression study followed by single photon emssision computed tomography (SPECT) for evaluation of perfusion in case of parent artery occlusion. Low-flow bypass was used when the occlusion or compression test does not evoke any neurological symptoms but maintenance of perfusion via circle of Willis is not adequately achieved in SPECT. Neurologic symptoms during the occlusion or compression test mandate high-flow bypass surgery. When high-flow bypass was indicated, Allen test was performed to confirm the patency of the ulnar artery preoperatively for radial artery graft and radial artery angiography was done during preoperative angiography1).
Follow-up evaluation
Angiography was followed within one week postoperatively in all cases to evaluate the graft patency and occlusion of aneurysms. MR or CT angiography was used to follow up in the outpatient clinic. Glasgow outcome scale (GOS) was used to evaluate clinical outcome.
Surgical procedure
For bypass surgery, the patient should have central venous line and arterial line monitoring during the procedure. A prophylactic antibiotic was administered intravenously one hour before the incision and continued for 72 hours postoperatively. Ventriculostomy was used in one patient with SAH to relieve acute hydrocephalus. Intraoperative neurophysiological monitorings were adopted including electroencephalogram, somatosensory evoked potentials and motor evoked potential.
The radial artery or saphenous vein was harvested using Harmonic scalpel® (Johnson & Johnson) just before the anastomosis and alignment was marked with marking dye. When the artery was harvested, intraluminal blood was cleared with heparinized saline using a small blunt needle and caution should be taken not to injure the endothelium. Pressure distension technique was used to expand the vessel and to prevent postoperative vasospasm16).
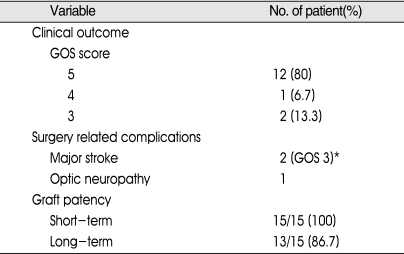
Pterional craniotomy with additional cranial base approach depend on the aneurysm location was performed for anterior circulation aneurysms. The recipient arterial site, usually M2 for high-flow bypass and M4 for low-flow bypass, was selected and exposed after sylvian dissection (Fig. 1). Surgical techniques were well described in the article by Martin15).
Fig. 1.
Superficial temporal artery-middle cerebral artery bypass is done on M4 segment of temporal operculum for low-flow bypass.
During the temporary occlusion period, pentobarbital was used as a protective measure to reduce brain metabolism. The anastomotic time was maintained less than 45 minutes as recommended by Mohit et al.16). Antiplatelet agent was used postoperatively only after patient fully recovered from general anesthesia. RAG harvest site was checked for first 24 hours for possible hand ischemia and hematoma formation.
Aneurysm was managed as a staged operation ensuring the adequate circulation from bypassed graft except on the 2 unplanned cases. Endovascular coil occlusion of parent artery was done in 4 cases and balloon occlusion was used in one case. Parent artery occlusion with clipping was done in 9 and aneurysm clipping was done in one case.
RESULTS
Table 2 summarizes the outcomes of the series. The overall clinical outcome was measured using GOS. Twelve cases were allocated to GOS 5 and 1 case to GOS 4 group, resulting in 86.7% good outcome. Fig. 2 shows a representative case of GOS 5 group where successful bypass surgery was performed with good long term patency of graft. One in GOS 4 group presented with thromboembolic thalamic infarction and motor weakness Grade IV preoperatively due to a large (2.3 cm) thrombosed P2 aneurysm and the bypass of STA-PCA was successfully performed with trapping of the aneurysm without further ischemia. The sequellae of preoperative infarction left the patient in GOS 4 group.
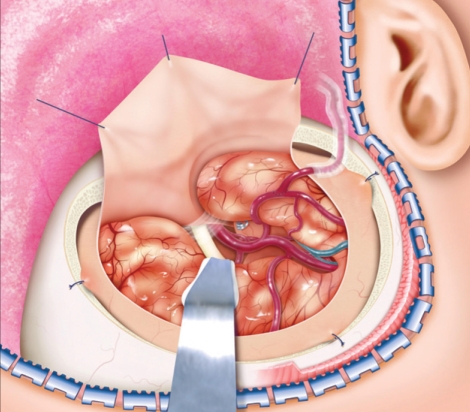
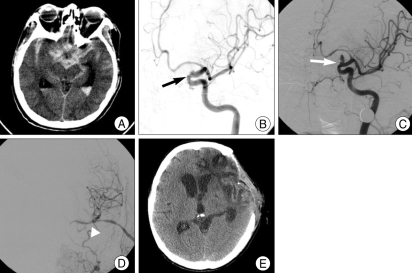
Fig. 2.
A case of 61-year-old female presented with headache and visual disturbance due to giant intracavernous aneurysm. A : Anteroposterior right carotid angiography shows intracavernous aneurysm involving the whole cavernous internal carotid artery segment measuring 2× 4 cm (black arrow). B : Postoperative angiogram shows patent radial artery graft anastomosis on M2 (white arrow) with complete occlusion of aneurysm and parent artery by balloon. C and D : Postoperative computed tomography scan shows resolution of preoperative mass effect of aneurysm.
Two cases in the GOS 3 group were cases with blood blister-like ICA aneurysms where direct clipping resulted in tearing of aneurysm neck and emergency bypass was performed after prolonged intraoperative ICA trapping. In the first case, a 36 year-old male patient, preoperative neurologic state was Hunt and Hess Grade II and postoperatively deterioration occurred due to MCA and ACA territory infarction in spite of bypass surgery (STA-M4 bypass). The other GOS 3 group case, a 56 year-old female patient, falls into the no improvement group since she presented as Hunt and Hess Grade IV preoperatively due to ruptured aneurysm (Fig. 3). Radial artery interposition graft was used since STA was lost during craniotomy procedure and postoperative patency was confirmed with angiography. However, postoperative brain swelling led to decompressive craniectomy procedure, sacrificing the graft.
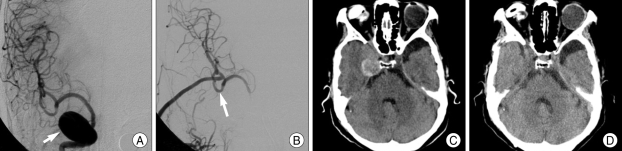
Fig. 3.
A 56-year-old female presented with altered consciousness. A : Precontrast computed tomography scan revealed Fisher grade IV subarachnoid hemorrhage. B : On postictal day 1, preoperative anteroposterior left internal carotid artery (ICA) angiography showed blood blister-like aneurysm (black arrow). C : On postictal day 4, angiography reveals enlarged size of aneurysm (white arrow). D : Emergent radial artery bypass (M2) with ICA trapping was done to salvage unexpected occlusion of ICA during direct clipping of aneurysm (arrow head). E : Decompressive craniectomy was done to relieve brain swelling due to postoperative infarction and resulted in occlusion of graft.
There was no mortality and the overall surgery-related morbidity was 20% (3/15). The GOS 3 group cases were previously mentioned where unexpected bypass surgery was done to salvage prolonged intraoperative ischemic insults. The last morbidity case was a 54 year-old female presented with subarachnoid hemorrhage due to right ICA blood blister-like aneurysm. Bypass of STA-M2 was prepared and done first and then direct trapping of ICA segment including blood blister-like aneurysm was done non-eventfully. Nevertheless, she was left with ipsilateral monocular blindness possibly due to unexplained microvascular compromise to optic nerve.
The short-term graft patency rate evaluated with CT angiography immediate postoperatively was 100%. The long-term graft patency rate was 86.7% (13/15) during the mean follow up period of 15 months (2 to 48 months). Among the occluded cases, one case was due to graft loss during craniectomy procedure as previously mentioned. The other case presented with sudden onset headache and preoperative angiography revealed giant aneurysm in the cavernous portion of right ICA occluding the right ICA. Right A1 was hypoplastic and right MCA was supplied by ECA collateral and pial leptomeningeal collateral. STA-M4 bypass with ICA occlusion was done and immediate postoperative angiography revealed reduced flow through STA but MCA filling was noted. Six month postoperative CT angiography showed graft occlusion with mild perfusion delay but the patient stayed stable without neurologic deficit. It seemed adequate collateral flow developed before graft occlusion.
DISCUSSION
Intracranial artery bypass technique was first introduced by Donaghy and Yasargil in 19677). After much refinements of the surgical technique by multiple authors, bypass technique started to establish its role in the management of unclippable complex aneurysm and cranial base tumors involving major cranial arteries11,12,14,18,20,24). Currently, endovascular treatment is gaining its favor with improving techniques and instrumentation using stent or balloon assisted coiling of wide neck aneurysm2,6). However, it still has its limitation of recanalization, instent stenosis and occlusion in managing aneurysm. Microsurgical clipping can be applied in managing complex aneurysm, such as reconstruction of parent vessel with multiple fenestrated clips, although limited by intramural thrombosis. Hence, the bypass surgery for management of complex and difficult intracranial aneurysm is accepted as a viable treatment method for aneurysm by many cerebrovascular surgeons9,20,21,26).
The main application is done where major artery or parent artery sacrifice is required for the treatment of aneurysm. As seen in the morbidity cases of this series, treatment of blood blister-like aneurysms in the ICA trunk often requires sacrifice of parent artery since it has tendency to tear due to fragile wall and aggressive clipping causes stenosis and occlusion of parent artery. All of the morbidity cases in this series fall into this type of aneurysm and careful preoperative planning for bypass surgery prior to direct management of aneurysm is necessary to prevent neurologic deficits since emergent attempt of bypass surgery often results in prolonged compromise of circulation.
Treatment of giant unruptured aneurysm carries high mortality and morbidity rate. Nakase et al. reported 4% mortality and 19% morbidity in surgical group. In bypass group, the mortality and morbidity rates are 0% and 20% respectively14). In our series, 8 cases of large to giant aneurysm were treated with bypass surgery without mortality and morbidity. Major hurdle of the bypass surgery is increased risk of cerebral infarction due to temporary occlusion even under brain protection measures. In this series, major morbidity occurred in the emergency cases where preoperative bypass planning was not done and cerebrovascular ischemia has already occurred during direct clipping attempt. Although heterogeneous group such in aneurysm size, location, with or without hemorrhage, and selection bias in the treatment modality, our series show overall mortality and morbidity rate of 0% (0/15) and 20% (3/15) and no graft patency related mortality and morbidity. Therefore, bypass technique can be a safe and effective treatment method in well preplanned complex aneurysm cases.
When a major artery such as ICA or vertebral artery sacrifice is required, whether to perform bypass surgery to all patients is still in debate. A selective approach is done based on the cerebrovascular flow dynamic study using neurologic monitoring, and transcranial Doppler flow monitoring, spectroscopy, positron emission tomography (PET) and xenon based study on preoperative balloon test occlusion. Universal approach uses bypass in all cases of parent artery occlusion even with good collateral based on the argument that these patients face higher risk of cerebrovascular stroke incidence13,22). In this series, selective approach is used using clinical and angiographic results with SPECT result after balloon test occlusion. Cases with enough collateral circulation not leaving neurological change on the test occlusion but with decreased perfusion on imaging study, low-flow bypass was adopted to compensate possible risk of ischemia. High-flow bypass was instilled in cases where parent artery occlusion was required and occlusion test revealed neurological deterioration and marked decrease in perfusion on SPECT. Comparison of long-term outcome and stroke incidence between the patients who underwent the sacrifice of major artery without bypass surgery and patients with bypass surgery is required to establish the benefits of bypass surgery further in this context.
Choice of revascularization conduit is depend on the required amount of perfusion after parent artery occlusion, size of recipient and donor artery and experience of surgeons16). For high-flow bypass, saphenous vein and radial artery grafts are considered. SVG technique was first adopted in the intracranial bypass technique as in the cardiovascular field. Its advantage is easy access in the lower extremities, longer lengths of harvestable conduit, and low risk of vasospasm. However, as longer follow up studies in coronary bypass study revealed its reduction in patency rate, RAG gained its favor in the cardiovascular bypass surgery. RAG use was first introduced by Carpentier et al. in 1973 but lost its stand due to early spasm causing graft occlusion and intimal hyperplasia as a cause of early graft failure4,5). Reduction of intimal hyperplasia with use of calcium channel blocker in the experimental setting allowed regained interest in RAG in the cardiovascular field10). As early vasospasm complication was solved with pressure distension technique, RAGs gained its favor in cerebrovascular field as well22). The most important reason for the preference for the RAGs is the patency rate. Although the data are from the coronary bypass results, the long-term patency rate of SVGs decreases to 60% in 11 years but 91.9% in 5 years for RAGs3,19). In this series, most high-flow bypass cases used RAG except in one case and there was no difference in patency rate yet.
Intraoperative measures for preventing ischemic events include burst suppression using barbiturates, propofol or fentanyl, and hypothermia24). Neurophysiological monitoring including electroencephalogram, somatosensory evoked potential, motor evoked potential, and brainstem evoked potential should be used to adjust the anesthetic techniques accordingly. On rare occasion, cranial nerve monitoring may be added if there is a risk of damage during exposure and treatment of aneurysms in the posterior fossa9,16-18, 24). To reduce temporary clipping time, anastomosis time depending solely on surgeons' skill and practice is the most important factor. In addition, excimer laser-assisted non occlusive anastomosis technique was designed and under continued development for improved reliability23,25,27).
Graft thrombosis in perioperative period could raise fatal outcome. Hence, pre- and postoperative use of antiplatelet agents, careful measurements of coagulation profile, and platelet aggregation profile may help to reduce thrombosis related problems. Intraoperative use of heparin may cause coagulopathy along with the use of mannitol and hypothermia. In this series, antiplatelet agent was used in postoperative period only and intravenous systemic heparin was not used. Delicate handling of vessels not to damage the endothelium prevented the thrombosis formation and limited use of heparin for flushing of graft and anastomosis site was adequate to prevent thrombosis in this series.
CONCLUSION
Cerebral revascularization may be required when sacrifice of major vessels is needed in surgical management of complex aneurysm. Each treatment procedure of aneurysm should evaluate risk and benefit related to the treatment and tailored approach suitable to each patient's circumstance should be considered. Careful and detailed preoperative planning is essential requirement to successful bypass surgery without complication. Incorporation of endovascular, microsurgical and bypass technique can greatly enhance the overall outcome in treatment of complex aneurysms when it occurred in team efforts. Hence, the arterial bypass technique can be a pivotal measure to treat complex aneurysm without neurological sequellae.
References
- 1.Allen EV. Thromboangiitis obliterans : methods of diagnosis of chronic occlusive arterial lesions distal to the wrist with illustrative cases. Am J Med Sci. 1929;178:237–244. [Google Scholar]
- 2.Benitex RP, Silva MT, Klem J, Veznedaroglu E, Rosenwasser RH. Endovascular occlusion of wide-necked aneurysms with a new intracranial microstent (Neuroform) and detachable coils. Neurosurgery. 2004;54:1359–1367. doi: 10.1227/01.neu.0000124484.87635.cd. discussion 1368. [DOI] [PubMed] [Google Scholar]
- 3.Bourassa MG, Fisher LD, Campeau L, Gillespie MJ, McConney M, Lesperance J. Long-term fate of bypass grafts : The Coronary Artery Surgery Study (CASS) and Montreal Heart Institute experiences. Circulation. 1985;72:V71–V78. [PubMed] [Google Scholar]
- 4.Carpentier A. Selection of coronary bypass : anatomic, physiological, and aniographic considerations of vein and mammary artery grafts. J Thorac Cadiovasc Surg. 1975;70:429–430. [PubMed] [Google Scholar]
- 5.Carpentier A, Guermonprez JL, Deloche A, Frechette C, DuBost C. The aorta-to coronary radial artery bypass graft : a technique avoiding pathological changes in grafts. Ann Thorac Surg. 1973;16:111–121. doi: 10.1016/s0003-4975(10)65825-0. [DOI] [PubMed] [Google Scholar]
- 6.Cekirge SH, Yavuz K, Geyik S, Saatci I. HyperForm balloonassisted endovascular neck bypass technique to perform balloon or stent-assisted treatment of cerebral aneurysms. AJNR Am J Neuroradiol. 2007;28:1388–1390. doi: 10.3174/ajnr.A0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donaghy R, Yasargil MG. Microvascular Surgery. St Louis: CV Mosby; 1967. [Google Scholar]
- 8.EC/IC Bypass Study Group. Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke : results of an international randomized trial. N Engl J Med. 1985;313:1191–1200. doi: 10.1056/NEJM198511073131904. [DOI] [PubMed] [Google Scholar]
- 9.Evans JJ, Sekhar LN, Rak R, Stimac D. Bypass grafting and revascularization in the management of posterior circulation aneurysms. Neurosurgery. 2004;55:1036–1049. doi: 10.1227/01.neu.0000140822.64362.c6. [DOI] [PubMed] [Google Scholar]
- 10.Guyotat J, Pelissou-Guyotat I, Lievre M, Chignier E. Inhibition of subintimal hyperplasia of autologous vein bypass grafts by nimodipine in rats : a placebo-controlled study. Neurosurgery. 1991;29:850–855. doi: 10.1097/00006123-199112000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Kang SD. Extracranial-intracranial bypass surgery : surgical technique and perioperative management. Kor J Cerebrovasc Dis. 2002;4:119–123. [Google Scholar]
- 12.Kim DS, Kim JK, Yoo DS, Huh PW, Cho KS, Kim MC. Current indication of extracranial-intracranial bypass surgery. Kor J Cerebrovasc Dis. 2002;4:99–103. [Google Scholar]
- 13.Larson JJ, Tew JM, Jr, Tomsick TA, van Loveren HR. Treatment of aneurysms of the internal carotid artery by intravascular balloon occlusion : long-term follow-up of 58 patients. Neurosurgery. 1995;36:26–30. discussion 30. [PubMed] [Google Scholar]
- 14.Lawton MT, Hamilton MG, Morcos JJ, Spetzler RF. Revascularization and aneurysm surgery : current techniques, indications, and outcome. Neurosurgery. 1996;38:83–92. doi: 10.1097/00006123-199601000-00020. discussion 92-94. [DOI] [PubMed] [Google Scholar]
- 15.Martin NA. Arterial bypass for the treatment of giant and fusiform intracranial aneurysms. Tech Neurosurgery. 1998;4:153–178. [Google Scholar]
- 16.Mohit AA, Sekhar LN, Natarajan SK, Brits GW, Ghodke B. High-flow bypass grafts in the management of complex intracranial aneurysms. Neurosurgery. 2007;60:ONS105–ONS122. doi: 10.1227/01.NEU.0000249243.25429.EE. discussion ONS122-123. [DOI] [PubMed] [Google Scholar]
- 17.Nakase H, Shin Y, Kanemoto Y, Ohnishi H, Morimoto T, Sakaki T. Long-term outcome of unruptured giant cerebral aneurysms. Neurol Med Chir (Tokyo) 2006;46:379–384. doi: 10.2176/nmc.46.379. discussion 384-386. [DOI] [PubMed] [Google Scholar]
- 18.Peerless SJ, Hampf CR. Extracranial to intracranial bypass in the treatment of aneurysms. Clin Neurosurg. 1985;32:114–154. [PubMed] [Google Scholar]
- 19.Possati G, Gaudino M, Alessandrini F, Luciani N, Glieca F, Trani C, et al. Midterm clinical and angiographic results of radial artery grafts used for myocardial revascularization. J Thorac Cardiovasc Surg. 1998;116:1015–1021. doi: 10.1016/S0022-5223(98)70054-6. [DOI] [PubMed] [Google Scholar]
- 20.Sekhar LN, Bucur SD, Bank WO, Wright DC. Venous and arterial bypass grafts for difficult tumors, aneurysms, and occlusive vascular lesions : evolution of surgical treatment and improved graft results. Neurosurgery. 1999;44:1207–1223. doi: 10.1097/00006123-199906000-00028. discussion 1223-1224. [DOI] [PubMed] [Google Scholar]
- 21.Sekhar LN, Duff JM, Kalavakonda C, Olding M. Cerebral revascularization using radial artery grafts for the treatment of complex intracranial aneurysms : techniques and outcomes for 17 patients. Neurosurgery. 2001;49:646–658. doi: 10.1097/00006123-200109000-00023. discussion 658-659. [DOI] [PubMed] [Google Scholar]
- 22.Sekhar LN, Patel SJ. Permanent occlusion of the internal carotid artery during skull-base and vascular surgery: is it really safe? AM J Otol. 1993;14:421–422. doi: 10.1097/00129492-199309000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Solomon RA. Principles of aneurysm surgery : cerebral ischemic protection, hypothermia and circulatory arrest. Clin Neurosurg. 1994;41:351–363. [PubMed] [Google Scholar]
- 24.Spetzler RF, Schuster H, Roski RA. Elective extracranial-intracranial arterial bypass in the treatment of inoperable giant aneurysms of internal carotid artery. J Neurosurg. 1980;53:22–27. doi: 10.3171/jns.1980.53.1.0022. [DOI] [PubMed] [Google Scholar]
- 25.Streefkerk HJ, Bremmer JP, Tulleken CA. The ELENA technique : high flow revascularization of the brain. Acta Neurochir. 2005;(Suppl 94):143–148. doi: 10.1007/3-211-27911-3_23. [DOI] [PubMed] [Google Scholar]
- 26.Sundt TM, Jr, Piepgras DG, Marah WR, Fode NC. Saphenous vein bypass grafts for giant aneurysms and intracranial occlusive disease. J Neurosurg. 1986;65:439–450. doi: 10.3171/jns.1986.65.4.0439. [DOI] [PubMed] [Google Scholar]
- 27.van Doormaal TP, van der Zwan A, Verweij BH, Langer DJ, Tulleken CA. Treatment of giant and large internal carotid artery aneurysms with a high-flow replacement bypass using the excimer laser-assisted nonocclusive anastomosis technique. Neurosurgery. 2006;59:ONS328–ONS334. doi: 10.1227/01.NEU.0000233971.08409.F0. discussion ONS334-335. [DOI] [PubMed] [Google Scholar]