Abstract
Objective
In Moyamoya disease, the primary goal of treatment is to improve collateral circulation through angiogenesis. In the present study, we obtained and sub-cultured bone marrow stromal cells (BMSCs) from rats without a cell-mediated immune response. Then, we injected the labeled BMSCs directly into adjacent temporal muscle during encephalomyosynangiosis (EMS). Three weeks after BMSC transplantation, we examined the survival of the cells and the extent of neovascularization.
Methods
We divided 20 rats into a BMSC transplantation group (n=12) and a control group (n=8). Seven days after the induction of chronic cerebral ischemia, an EMS operation was performed, and labeled BMSCs (1×1066/100 µL) were injected in the temporal muscle for the transplantation group, while an equivalent amount of culture solution was injected for the control group. Three weeks after the transplantation, temporal muscle and brain tissue were collected for histological examination and western blot analysis.
Results
The capillary/muscle ratio in the temporal muscle was increased in the BMSC transplantation group compared to the control group, showing a greater increase of angiogenesis (p<0.05). In the brain tissue, angiogenesis was not significantly different between the two groups. The injected BMSCs in the temporal muscle were vascular endothelial growth factor (VEGF)-positive by immunofluorescence staining. In both temporal muscle and brain tissue, the expression of VEGF by western blot analysis was not much different between the two groups.
Conclusion
During EMS in a chronic cerebral ischemia rat model, the injection of BMSCs resulted in accelerated angiogenesis in the temporal muscle compared to the control group.
Keywords: Bone marrow stromal cells, Chronic cerebral ischemia, Angiogenesis, Vascular endothelial growth factor
INTRODUCTION
For the last decades, there has been an intensive study of methods for using stem cells in treating acute cerebral infarction, with some cases of clinical application3). However, to achieve a therapeutic effect from the use of stem cells in a brain that has been damaged extensively, as in acute cerebral infarction, transplanted cells need to replace nerve cells and establish adequate synapses to existing nerve cells or create a favorable environment for the recovery of existing damaged nerve cells and its connection. However, when stem cells are transplanted in cerebral infarction patients, it is still hard to measure the therapeutic effect, and even while some patients may show improvement in their symptoms, the mechanism is not clearly understood.
However, in patients with Moyamoya disease, which is a chronic cerebral ischemic state, the brain damage can be prevented through improving blood flow by applying various kinds of direct or indirect revascularization. For indirect revascularization, providing the dura mater, vessels, and muscles, among others, to contact the surface of the brain, induces neovascularization. Since the risk of this operation is relatively lower than with direct anastomosis, it is usually performed in childhood patients where the vessel to be anastomosed is too small, or the general condition is poor. However, indirect anastomosis is slower in improving blood flow than direct anastomosis, and the effectiveness in angiogenesis depends on the patient. VEGF is known to play an important regulatory role in angiogenesis5,19). In addition, recent progress in stem cell research has led to laboratory and clinical applications involving stem cell transplantation that can accelerate angiogenesis in ischemic heart disease2,10,20,21) and ischemic limb diseases11,12). This approach is based on the phenomenon of stem cells increasing the expression of VEGF when tissue is in an ischemic state.
Based on the results of previous research, we conducted this study to see whether the intramuscular injection of stem cells accelerates angiogenesis in an EMS operation, which is a kind of indirect revascularization to treat chronic cerebral ischemia in patients with Moyamoya disease.
MATERIALS AND METHODS
All procedures were approved by the Institution Animal Care and Use Committee of our hospital. Male Fisher rats weighing between 180-200 g were chosen for this study and anesthetized with ketamine (50 mg/kg) and xylazine (2.5 mg/kg) intraperitoneally.
The harvest and culture of BMSCs
After anesthetization and sterilization with alcohol and povidine, fresh bone marrow was harvested aseptically from femurs and tibias of Fisher rats. Using a 21 gauge needle connected to a 5 mL syringe in the bone shaft, bone marrow was flushed out with PBS (phosphate buffered saline). Subsequent procedures were the same as the previous one24). After 5 subcultures for 5 passages, the number of cells to be transplanted was adjusted using a cytometer. For each animal, a total of 100 µL and 1×106 cells were prepared for transplantation.
Chronic cerebral ischemia rat model
Twenty male Fisher rats were either assigned to the BMSC transplantation group (A) (n=12) or the control group (B) (n=8). They were immobilized in the supine position, both sides of the neck region were shaved and disinfected, and then a vertical incision was made along the center. The common carotid arteries on both sides were exposed, and a ligation was performed with 3-0 silk. After the operation, there was a 7-day recovery.
BMSCs labeling
For the follow-up observation of injected cells, we labeled the BMSCs obtained from the Fisher rats using PKH26 red fluorescent cell linker kit (PKH26; Sigma, St. Louis, MO). We put 2×107 BMSCs in a 15 mL cone-shaped tube, washed them with 10 mL PBS, centrifuged them (200×g, 15 minutes), and obtained 25 µL of precipitate. To the precipitate, we added 1 mL of Diluent C (Sigma) and mixed well, and then we added 4×10-6 M of PKH26 staining reagent at room temperature for 5 minutes to obtain a final volume of 2 mL of 2×107 BMSCs. In order to stop the staining reaction, we added the same amount of 1% bovine serum albumin (BSA) and diluted it with the same amount of 10% FBS DMEM. Then, the precipitate was obtained through centrifugation (200×g, 10 minutes, 25℃) and washing 3 times with PBS. After the donor cells were labeled and before tran-splantation was confirmed using a microscope, the BMSCs (1×106/100 µL per individual) were injected directly into 3-4 sites of temporal muscle during the EMS operation.
EMS operation
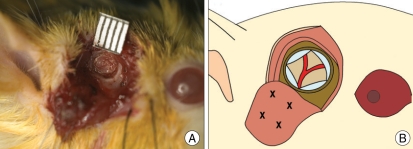
Seven days after the operation to create Moyamoya rat models, intraperitoneal anesthesia was performed using the same method as for the BMSC transplantation group (A) and the control group (B). A vertical incision was made on the right-side temporal region and the temporal muscle was reflected like a ∩-shape from the skull. A piece of skull around 4 mm in diameter was removed from the temporoparietal region using an electric drill (Fig. 1). The dura mater was removed carefully using micro forceps without damaging the brain, and labeled BMSCs (1×106 cells/100 µL) were slowly injected into animals from the BMSC transplantation group (A) and an equivalent amount of culture solution was slowly injected into animals from the control group (B) at 3 to 4 sites of temporal muscle using a Hemilton syringe. Then the temporal muscle and the skin were sutured.
Fig. 1.
A : The encephalomyosynangiosis operation is performed in the right temporo-parietal area after 7 days of chronic cerebral ischemia. We perform a 4-mm diameter-sized craniectomy. B : Schematic drawings showing the injection sites (X).
Analysis of capillary density
Three weeks after the EMS operation, intraperitoneal anesthesia was induced by the same methods as previously described, and the chest wall was incised so that an 18-gauge needle could be inserted through the left ventricle and positioned at the origin of the aorta, followed by perfusion of physiological normal saline through the needle. Immediately after the start of perfusion, the right atrium was incised so that venous blood and the injected physiolo-gical normal saline could drain out through the incision. After about 200-300 mL of physiological normal saline was injected, 300-400 mL of 4% formalin and India ink at a ratio of 1 : 1 was perfused in order to fix the brain and temporal muscle tissue. After the fixation, the scalp was removed and a slice of right-side temporal muscle was removed. Then, starting from the foramen magnum, we removed the skull using a rongeur, and separated the brain by cutting the cerebral nerves at the skull base. Based on the site from which the skull was removed, the part around -3.0 to -7.0 mm from the bregma according to the rat brain atlas was cut along the coronal plane17). Tissues were fixed in 4% formalin for over 8 hours and then submerged completely in 20% sucrose. The frozen temporal muscle was sliced at a thickness of 15 µm and at intervals of 1 mm, with 3 slices for each individual. Using an optical microscope (Nikon ECLIPSE TE300, Tokyo, Japan) and a magnification of ×200, we counted the capillaries marked with India ink at three sites per sample, and the results were recorded as the capillary/muscle ratio for around 180 muscle cells.
Immunohistological staining and follow-up of BMSCs
For immunological staining and follow-up observation of BMSCs in the BMSC transplantation group (n=10) and the control group (n=6), tissue slices were obtained through frozen thin section by the same method as above 3 weeks after the EMS operation, and the slices were dried at room temperature for 1 hour, dipped in PBS for a short time, and fixed with acetone for 5 minutes. The slices were dried again at room temperature for 30 minutes, washed twice with PBS, and incubated with 5% normal goat serum (Sigma) at room temperature for 30 minutes. The 5% normal goat serum was shaken off, and antibody against VEGF (rabbit polyclonal anti-VEGF Ab, Santa Cruz Biotechnology, Santa Cruz, CA) was diluted to 1 : 100 and incubated at room temperature for 2 hours. After washing the slices thoroughly with PBS, a FITC-conjugated secondary antibody (goat anti-rabbit secondary IgG-FITC, Santa Cruz Biotechnology) was diluted at 1 : 100 and reacted at room temperature for 1 hour. Again, the slices were washed thoroughly with PBS, the nucleus was stained with diamino-2-phenylindole 2 HCL (DAPI; sigma) for contrast staining and each sliced was analyzed under the microscope.
Western blot analysis of VEGF
Three weeks after the EMS operation, anesthesia was performed using the same methods as previously described in the BMSC transplantation group (n=2) and the control group (n=2), and then each rat's head was removed. For each group, the right-side temporal muscle was sliced in sections 4×4 mm, centering on the part from which the skull was removed. In addition, we obtained the right half of the brain about 5 mm length along the coronal plane centering on the part from which the skull was removed. The brain was broken into pieces using a crusher, and the cells were homogenized in a RIPA buffer solution (1% Noidet P-40, 100 mM NACL, 20 mM Tris-HCL, 10 mM Naf, 1 mM sodium orthovanadate, 30 nM Na-glycerophosphate, and protein inhibitor mixture). The protein solution was centrifuged twice (10,000×g, 15 minutes, 4℃), and a western blot was performed using the supernatant. For protein quantification, a Bradford protein assay was performed using a microplate reader (Model 680; Bio-Rad laboratories, Hercules, CA) based on BSA as the standard. Each protein solution containing 50 µg of protein was electrophoresed on a 10% sodium dodecyl sulfatepolyacrylamide gel (SDS-PAGE). Then, the proteins were transferred to polyvinylidene fluoride (PVDF) film and blocked in 5% skim milk for 1 hour. The blots were then incubated with an antibody against β-actin (1 : 1000 rabbit polyclonal anti β-actin Ab, Santa Cruz Biotechnology) and an antibody against VEGF (1 : 400 rabbit polyclonal anti-VEGF Ab, Santa Cruz Biotechnology) at room temperature for 2 hours. We then incubated the blots with 1 : 5000 of the respective Horse-radish peroxidase (HRP)-conjugated secondary antibodies (goat anti-rabbit secondary Ab, Santa Cruz Biotechnology) at room temperature for 1 hour, and the signal for each antibody was detected using an ECL kit (Amersham Pharmacia Biotech, Piscataway, NJ) and exposure to X-ray film.
Statistical analysis
For statistical analysis of the difference in the capillary/muscle ratios between the two groups, we performed the Mann-Whitney test, which is a non-parametric test, using SPSS 12.0. A p<0.05 was considered statistically significant.
RESULTS
Angiogenesis
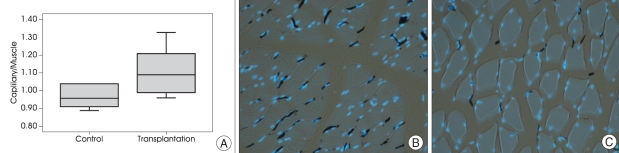
The EMS operation was performed a week after the induction of Moyamoya rat models, and the capillary/muscle ratio in the BMSC transplantation group and the control group was examined 3 weeks after the EMS operation. The capillary/muscle ratio was significantly higher in the BMSC transplantation group (1.11±0.12) than in the control group (0.97±0.63) (Mann-Whitney test, p=0.011) (Fig. 2).
Fig. 2.
A : The injection of BMSCs into the temporal muscle increase angiogenesis. Chronic cerebral ischemia is induced by bilateral common carotid artery ligation, and BMSCs are injected into the temporal muscle 7 days after the induction. The capillary/muscle ratio is increased in the BMSCs transplantation group compared to the control group (p=0.011). B and C : Photomicroscopic examination findings of the temporal muscles stained with India ink and DAPI also shows increased angiogenesis in the BMSC transplantation group. (B) The temporal muscle of the BMSCs transplantation group shows a more prominent capillary/muscle ratio than the (C) control group. The nuclear DNA is stained with DAPI (blue dot), and the capillary is stained with India ink (black dot)(Magnification ×200). DAPI : diamino-2-phenylindole 2 HCL, BMSCs : bone marrow stromal cells.
Follow-up of injected BMSCs
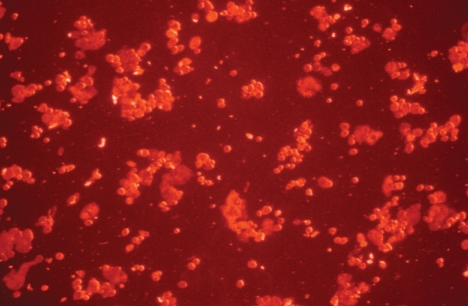
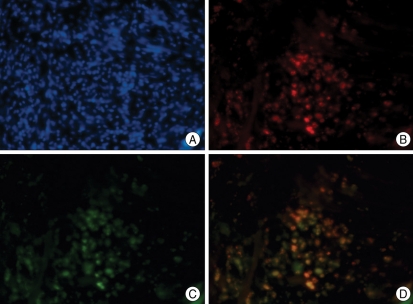
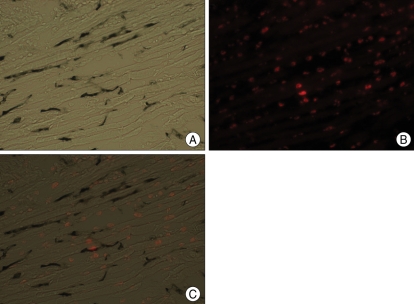
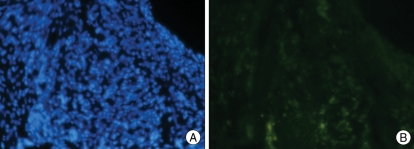
We confirmed that most BMSCs were stained with PKH26 before injection (Fig. 3). We then injected the PKH26-labeled BMSCs into the temporal muscle, and after 3 weeks we observed PHK26-positive cells using a microscope. In the temporal muscle tissue of the BMSC transplantation group, we observed PKH26-positive cells showing red fluorescence among DAPI-stained nuclei, and some of the PKH26-positive cells were VEGF-positive (Fig. 4). The relative positions of the PKH26-positive cells with respect to capillaries stained with India ink suggested that the injected cells are not directly forming vessel walls, but were instead mainly distributed around the vessels (Fig. 5). There were no PKH26-positive cells in the temporal muscle tissue of the control group, and only a few cells were VEGF-positive (Fig. 6). In the brain tissues from the BMSC transplantation group and control group, there were no PKH26-positive cells, and only a few cells were VEGF-positive.
Fig. 3.
Identification of PKH26-labeled bone marrow stromal cells before injection into the temporal muscle (Magnification ×200)
Fig. 4.
A : Double-labeled microphotographs for PKH26/VEGF at temporal muscle in the BMSC transplantation group. B : Nuclear DNA is stained with DAPI (blue dot) for counter staining. C : BMSCs labeled with PKH26 are visualized abundantly in the ipsilateral temporal muscle in the BMSC transplantation group. PKH26 is added to the BMSCs as a reliable marker for the identification of transplanted cells. D : Multiple VEGF-positive cells are found in the ipsilateral temporal muscle of the BMSC transplantation group. Merged image from the same view as B and C. Multiple VEGF-positive cells are co-localized with PKH26 positive cells (Magnification ×200). VEGF : vascular endothelial growth factor, DAPI : diamino-2-phenylindole 2 HCL, BMSCs : bone marrow stromal cells.
Fig. 5.
The localization of transplanted cells in the temporal muscle at the chronic cerebral ischemic rat during encephalomyosynangiosis operation. A : Fluorescence-labeled BMSCs injected into the temporal muscle at 7 days after induction of ischemia are examined by microscopy at 3 weeks after the BMSCs injection. B : Without the fluorescence filter, the black dots represent vessels stained by India ink among the muscle fibers. C : With the red fluorescence filter, multiple red fluorescence-labeled cells are observed. Merged image from the same view as A and B. Fluorescence-labeled cells are usually detected around the vessels, but not directly incorporated into the vessels themselves (magnification ×200). BMSCs : bone marrow stromal cells.
Fig. 6.
Double-labeled microphotographs for PKH26/VEGF at temporal muscle in the control group. A : Nuclear DNA is stained with DAPI (blue dot) for counter staining. B : Some VEGF-positive cells are found in the temporal muscle of the control group (Magnification ×200) VEGF : vascular endothelial growth factor, DAPI : diamino-2-phenylindole 2 HCL.
Expression of VEGF
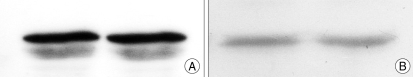
According to the results of the western blot analysis using samples obtained 3 weeks after the EMS operation, the brain tissues of the BMSC transplantation group and the control group did not show a difference in the VEGF expression. In addition, there was no significant difference in the expression of VEGF in the temporal muscle between the BMSC transpl-antation group and the control group (Fig. 7).
Fig. 7.
Western blot demonstrating VEGF protein expression in the ipsilateral temporal muscle (A) and in the brain (B) after the encephalomyosynangiosis operation. A single band around 45 Kd is detected with rabbit polyclonal antibody against VEGF in the brain, and a double band is detected around 40 Kd in muscle. Both muscles and brain tissue show no definite difference in levels of expression. VEGF : vascular endothelial growth factor.
DISCUSSION
Stem cells are found in a variety of tissues. Since the discovery that neurons are differentiated from adult BMSCs in humans and rats, there has been remarkable progress in research about the use of BMSCs for various neurological diseases23). While clinical successes have been limited, Bang and his colleagues have reported that they have achieved an effect using bone marrow cells for the clinical treatment of acute cerebral infarction3). Theoretically, however, to achieve a therapeutic effect with stem cells in central nervous system diseases such as cerebral infarction, the transplanted cells would need to replace nerve cells and have adequate synapses to existing nerve cells.
In contrast to other central nervous system diseases that require synapses to complex nerve cells, ischemic heart diseases and ischemic limb circulatory disorders can be treated by increasing angiogenesis at ischemic sites. Kim et al. reported that Buerger's disease patients injected with umbilical cord blood-derived stem cells in the proximal and distal muscles and the subcutaneous tissue of their limbs, showed improvement in their symptoms and an increase in angiogenesis11). More active research has been done on myocardial infarction patients to show that BMSC transplantation can improve myocardial perfusion and systolic function and such improvements are probably caused by myocardial regeneration and angiogenesis related to stem cells2,10,20,21). From a therapeutic point of view, Moyamoya disease is similar to ischemic heart disease, as well as ischemic limb circulatory disorders. Moyamoya disease does not require neuron replacement and connections, but only angiogenesis.
Embryologically, angiogenesis is mediated by angioblasts during the fetal period, but in adulthood, it is mediated by bone-marrow-derived endothelial progenitor cells1). Unlike angiogenesis in the fetal period, angiogenesis in adults involves external factors such as genetic changes related to hypoxia or the presence of a tumor, and the expression of VEGF plays the most important regulatory role5,19). If brain tissue falls suddenly into an ischemic state, the secretion of VEGF in macrophages increases within several hours and maintains a high level in nerve cells, glia cells, and other cells for around 10 days8,13,15). If brain tissue falls into a chronic cerebral ischemic state, the expression of VEGF begins to increase at 24 hours and reaches the peak in around a week, and then decreases slowly for around 3 weeks and returns to the normal level in around 90 days6). According to Zentilin et al., even if brain tissue is not in an ischemic state, the injection of VEGF into tissue or the increase in the expression of VEGF increases the migration of cells from the bone marrow and accelerates angiogenesis25).
According to Grunewald and his colleague, recruited bone-marrow-derived circulating cells (RBCCs), which are bone-marrow-derived monocytes that differ in nature from endothelial progenitor cells, are involved in angiogenesis5). It was reported that RBCCs recruited by VEGF localize to vessels induced by stromal cell-derived factor 1 (SDF-1) secreted from myofibroblasts around the vessels and proliferate vascular endothelial cells. In addition, it was reported that, unlike endothelial progenitor cells, monocytes recruited by VEGF from the bone marrow are not directly conjugated in angiogenesis, but rather stay around existing cells and help their proliferation5,25).
In our experiment, the results of histological examination in the third week from the injection of the BMSCs into the temporal muscle showed an increase in angiogenesis in the temporal muscle compared to that in the control group. The injected cells were distributed mainly in the temporal muscle, and rather than directly forming vessel walls, they existed around the vessel walls. Some of the injected cells were VEGF-positive according to immunofluorescence staining. The injected BMSCs were not directly conjugated in angiogenesis, such as with the bone-marrow-derived monocytes recruited by VEGF, but they provide a favorable environment for angiogenesis through the paracrine effect, like VEGF secretion around vessels5,25). In our experiment, angiogenesis increased but there was no significant difference found in VEGF expression by western blot. This suggests that the expression of VEGF is already elevated in brain tissue that is in a chronic ischemic state and in the temporal muscle on which EMS was performed. Thus, 3 weeks after the injection of BMSCs, the expression of VEGF might not be much different from that in the control group. However, there were many VEGF-positive cells by immunofluorescence staining, and some of them were tagged with PKH26, suggesting that the effect of the injected BMSCs lasted for over 3 weeks. Also, in addition to the direct effect of VEGF in angiogenesis caused by the injection of BMSCs, there may be other factors such as SDF-1, which is secreted from the myofibroblasts or vascular endothelial cells, or metalloproteinase-9, which is secreted from bone marrow cells9).
The acceleration of angiogenesis in the corresponding temporal muscle was confirmed, but no notable difference was observed in the brain tissue. This is probably because the effect of enhanced angiogenesis had not reached the brain tissue within the 3-week period following the EMS operation and the injection of BMSCs. Thus, longer observation is considered necessary in future research. A large number of BMSCs may be required to facilitate angiogenesis in the brain. Simultaneous transplantation of BMSCs at adjacent areas of the brain might be beneficial. Therefore, dosing strategies and identification of transplantation sites need further development. Moreover, cerebral blood flow studies including single-photon emission computerized tomography (SPECT) and functional recovery of the brain following BMSC transplantation also warrant future research.
The expression of VEGF in ischemic tissue is accelerated by the direct injection of VEGF protein7), gene therapy using vehicle such as plasmid and virus14,22), cell therapy using endothelial progenitor cells or BMSCs2,3,8,10,12,20,21), or combined therapy using two or more of the above therapies. In the method of direct protein injection, however, because protein has a short half-life, it should be injected at a relatively high concentration several times, and this may cause side effects such as edema and hypotension18). In addition, gene therapy using a vehicle is not safe for direct application to patients. However, with respect to cell therapy using the patient's own bone marrow, and particularly for Moyamoya disease patients, treatment can be safe and effective by transplanting autologous BMSCs into the corresponding muscle during EMS operation.
Unlike other central nervous system diseases, Moyamoya disease is similar to ischemic heart diseases and ischemic limb circulatory disorders in terms of therapy because cerebral blood flow is improved by increasing angiogenesis through collateral circulation in brain tissue that is in a chronic cerebral ischemic state. Accordingly, Moyamoya disease is a good first candidate to test in research on cell therapy treatment of central nervous system diseases.
CONCLUSION
The present study obtained and subcultured BMSCs cells from rats of the same species without cell-mediated immune responses and injected the BMSCs directly into the temporal muscle of chronic cerebral ischemia model rats during an EMS operation. Three weeks after the injection, angiogenesis in temporal muscle was accelerated and histology revealed that some of the injected BMSCs were VEGF-positive. Further study is necessary to determine the effective number of cells injected, the pattern of temporal change in VEGF, and the pattern of angiogenesis in the brain tissue.
Acknowledgements
The authors wish to acknowledge the financial support of the Catholic Institute of Cell therapy Basic Science Programs Foundation made in the program year of 2006.
References
- 1.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 2.Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCAREAMI) Circulation. 2002;106:3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 3.Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 4.Chu K, Park KI, Lee ST, Jung KH, Ko SY, Kang L, et al. Combined treatment of vascular endothelial growth factor and human neural stem cells in experimental focal cerebral ischemia. Neurosci Res. 2005;53:384–390. doi: 10.1016/j.neures.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, et al. VEGF-induced adult neovascularization : recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 6.Hai J, Li ST, Lin Q, Pan QG, Gao F, Ding MX. Vascular endothelial growth factor expression and angiogenesis induced by chronic cerebral hypoperfusion in rat brain. Neurosurgery. 2003;53:963–970. doi: 10.1227/01.neu.0000083594.10117.7a. [DOI] [PubMed] [Google Scholar]
- 7.Harrigan MR, Ennis SR, Masada T, Keep RF. Intraventricular infusion of vascular endothelial growth factor promotes cerebral angiogenesis with minimal brain edema. Neurosurgery. 2002;50:589–598. doi: 10.1097/00006123-200203000-00030. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi T, Abe K, Suzuki H, Itoyama Y. Rapid induction of vascular endothelial growth factor gene expression after transient middle cerebral artery occlusion in rats. Stroke. 1997;28:2039–2044. doi: 10.1161/01.str.28.10.2039. [DOI] [PubMed] [Google Scholar]
- 9.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang HJ, Kim HS, Zhang SY, Park KW, Cho HJ, Koo BK, et al. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction : the MAGIC cell randomised clinical trial. Lancet. 2004;363:751–756. doi: 10.1016/S0140-6736(04)15689-4. [DOI] [PubMed] [Google Scholar]
- 11.Kim SW, Han H, Chae GT, Lee SH, Bo S, Yoon JH, et al. Successful stem cell therapy using umbilical cord blood-derived multipotent stem cells for Buerger's disease and ischemic limb disease animal model. Stem Cells. 2006;24:1620–1626. doi: 10.1634/stemcells.2005-0365. [DOI] [PubMed] [Google Scholar]
- 12.Kirana S, Stratmann B, Lammers D, Negrean M, Stirban A, Minartz P, et al. Wound therapy with autologous bone marrow stem cells in diabetic patients with ischaemia-induced tissue ulcers affecting the lower limbs. Int J Clin Pract. 2007;61:690–692. doi: 10.1111/j.1742-1241.2007.01303.x. [DOI] [PubMed] [Google Scholar]
- 13.Kovacs Z, Ikezaki K, Samoto K, Inamura T, Fukui M. VEGF and flt. Expression time kinetics in rat brain infarct. Stroke. 1996;27:1865–1872. doi: 10.1161/01.str.27.10.1865. [DOI] [PubMed] [Google Scholar]
- 14.Kusaka N, Sugiu K, Tokunaga K, Katsumata A, Nishida A, Namba K, et al. Enhanced brain angiogenesis in chronic cerebral hypoperfusion after administration of plasmid human vascular endothelial growth factor in combination with indirect vasoreconstructive surgery. J Neurosurg. 2005;103:882–890. doi: 10.3171/jns.2005.103.5.0882. [DOI] [PubMed] [Google Scholar]
- 15.Marti HJ, Bernaudin M, Bellail A, Schoch H, Euler M, Petit E, et al. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol. 2000;156:965–976. doi: 10.1016/S0002-9440(10)64964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miki Y, Nonoguchi N, Ikeda N, Coffin RS, Kuroiwa T, Miyatake S. Vascular endothelial growth factor gene-transferred bone marrow stromal cells engineered with a herpes simplex virus type 1 vector can improve neurological deficits and reduce infarction volume in rat brain ischemia. Neurosurgery. 2007;61:586–594. doi: 10.1227/01.NEU.0000290907.30814.42. [DOI] [PubMed] [Google Scholar]
- 17.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th ed. UK: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- 18.Renault MA, Losordo DW. Therapeutic myocardial angiogenesis. Microvasc Res. 2007;74:159–171. doi: 10.1016/j.mvr.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–851. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 20.Strauer BE, Brehm M, Zeus T, Kostering M, Hernandez A, Sorg RV, et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106:1913–1918. doi: 10.1161/01.cir.0000034046.87607.1c. [DOI] [PubMed] [Google Scholar]
- 21.Tse HF, Kwong YL, Chan JK, Lo G, Ho CL, Lau CP. Angiogenesis in ischaemic myocardium by intramyocardial autologous bone marrow mononuclear cell implantation. Lancet. 2003;361:47–49. doi: 10.1016/S0140-6736(03)12111-3. [DOI] [PubMed] [Google Scholar]
- 22.Wang YQ, Guo X, Qiu MH, Feng XY, Sun FY. VEGF overexpression enhances striatal neurogenesis in brain of adult rat after a transient middle cerebral artery occlusion. J Neurosci Res. 2007;85:73–82. doi: 10.1002/jnr.21091. [DOI] [PubMed] [Google Scholar]
- 23.Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J neuroscience Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 24.Yeu IS, Lee HJ, Yi JS, Yang JH, Lee IW, Lee HK. The survival and migration pattern of the bone marrow stromal cells after intracerebral transplantation in rats. J Korean Neurosurg Soc. 2004;36:400–404. [Google Scholar]
- 25.Zentilin L, Tafuro S, Zacchigna S, Arsic N, Pattarini L, Sinigaglia M, et al. Bone marrow mononuclear cells are recruited to the sites of VEGF-induced neovascularization but are not incorporated into the newly formed vessels. Blood. 2006;107:3546–3554. doi: 10.1182/blood-2005-08-3215. [DOI] [PubMed] [Google Scholar]
- 26.Zhu W, Mao Y, Zhao Y, Zhou LF, Wang Y, Zhu JH, et al. Transplantation of vascular endothelial growth factor-transfected neural stem cells into the rat brain provides neuroprotection after transient focal cerebral ischemia. Neurosurgery. 2005;57:325–333. doi: 10.1227/01.neu.0000166682.50272.bc. [DOI] [PubMed] [Google Scholar]