Abstract
Objective
The authors present their experiences with stereotactic multiplanar reformatted (MPR) computed tomography (CT)-guided catheter placement for thrombolysis of spontaneous intracerebral hematoma (sICH) and their clinical results.
Methods
In 23 patients with sICH, MPR CT-guided catheter placement was used to select the trajectory and target point of hematoma drainage. This group was comprised of 11 men and 12 women, and the mean age was 57.5 years (range, 31-79 years). The patients' initial Glasgow Coma Scale scores ranged from 7 to 15 with a median of 11. The volume of the hematoma ranged from 24 mL to 86 mL (mean 44.5 mL). A trajectory along the main axis of the hematoma was considered to be optimal for thrombolytic therapy. The trajectory was calculated from the point of entry through the target point of the hematoma using reformatted images.
Results
The hematoma catheter was left in place for a median duration of 48.9 hours (range 34 to 62 hours). In an average of two days, the average residual hematoma volume was 6.2 mL (range 1.4 mL to 10.2 mL) and was reduced by an average of 84.7% (range 71.6% to 96.3%). The residual hematoma at postoperative seven days was less than 5 mL in all patients. There was no treatment-related death during hospitalization.
Conclusion
The present study indicates that stereotactic MPR CT-guided catheter placement for thrombolysis is an accurate and safe procedure. We suggest that this procedure for stereotactic removal of sICH should be considered for the optimization of the trajectory selection in the future.
Keywords: Intracerebral hematoma, Stereotactic aspiration, Surgical treatment, Thrombolysis
INTRODUCTION
Intracerebral hematoma (ICH) is associated with the highest mortality rate of all forms of stroke. The role of stereotactic surgery in the treatment of ICH has gained importance over the past decade. Clot removal is beneficial because it reduces the volume of the hematoma and may therefore also lowers intracranial pressure, reduces the chance of edema formation and improves perfusion in the affected hemisphere4,12,14,18). However, the main disadvantage of this technique is slowness in the reduction of hematoma volume, taking many days in some cases, as observed in patients with large hematomas15). Among the strategies for stereotactic removal of an ICH, it is important to remove as much of the blood clot as possible. To remove as quickly blood clot as possible, it is important that the catheter be placed accurately along the predetermined trajectory into the target point of the hematoma. In the past our experiences, the residual hematoma after conventional computed tomography (CT)-guided surgery using only axial CT images was mostly observed at the posterior and inferior portion of the hematoma. In conventional CT-guided methods, an optimal catheter insertion is difficult because the aiming trajectory is calculated from the point of entry through the target point of the hematoma using only axial images of the hematoma. The authors present experiences with stereotactic MPR CT-guided catheter placement for thrombolysis of spontaneous ICH and their clinical results.
MATERIALS AND METHODS
Patient population
Between January 2005 and December 2006, 46 patients with spontaneous supratentorial ICH underwent CT-guided stereotactic aspiration surgery. This group did not include patients who underwent frameless stereotactic surgery, patents with subcortically extending massive ICH, patients with signs of advanced transtentorial herniation, and patients with subacute or chronic hematomas. All patients had arterial hypertension previously or at admission with normal coagulation parameters. ICH localization and volume estimation were performed with 4- (Lightspeed, General Electronic Medical Systems, USA) and 64-(Brilliance, Philips Medical Systems, USA) channel CT scanners. They had an ICH volume exceeding 20 mL. For patients younger than 60 years of age, CT angiography was performed to rule out the presence of underlying vascular anomalies and revealed no vascular anomaly. Surgery had gone possible within 24 hours after ICH onset.
Twenty-three of the 46 patients treated by stereotactic aspiration surgery underwent conventional CT-guided catheter placement using only axial images of the hematoma. MPR CT-guided catheter placement was performed to select the trajectory for hematoma drainage in another 23 of the 46 patients. This group treated by MPR CT-guided catheter placement was comprised of 11 men and 12 women, and the mean age was 57.5 years (range, 31-79 years). The patients' initial Glasgow Coma Scale scores ranged from 7 to 15 with a median of 11. The demographic data and the findings of the initial CT scans of these 23 patients are described in detail in Table 1. The ICH volume was estimated using a validated practical rule (ABC/2, where A=biggest diameter, B=diameter at 90 degrees from A, C=number of slices; the presence of intraventricular hemorrhage was not included in the equation) and ranged from 24 mL to 86 mL (mean 44.5 mL)13). All patients were followed up for a minimum of 30 days. The outcome of the patients was assessed 30 days post-hemorrhage using the Glasgow Outcome Scale (GOS)9).
Table 1.
Summary of 23 patients who underwent stereotactic multiplanar reformatted computed tomography-guided catheter placement and thrombolysis of spontaneous intracerebral hematoma
GCS : Glasgow Coma Scale, Rt : right, Lt : left, Put : putamen, Tha : thalamus, Fro : frontal, Par : parietal, GOS : Glasgow Outcome Scale, GR : good recovery, MD : moderately disabled (independent), SD : severely disabled (dependent on others), VS : vegetative state, D : dead
Operative technique
The catheter was placed with the aid of a Cosman Roberts-Wells head frame (Radionics, Inc). The operations were performed under general anesthesia. An ipsilateral frontal standard burr hole location (3 cm lateral to midline and just anterior to the coronal suture) was generally used for the treatment of the hematoma. A silicone catheter (Youshin Medical; length 35 cm, 1.6/3.0 mm inner/outer diameter) with small openings at its proximal end over a length of 1.5 cm was used. The picture archiving and communication system (PACS) automatically generated MPR images, regardless of CT scanner type10). The MPR images include coronal and sagittal reformatted images that are generated in axial thin-section source images. The aiming trajectory was calculated from the point of entry through the target point of the hematoma using reformatted images (axial, sagittal, and coronal plane) such as the Cartesian coordinates x, y, and z (Fig. 1, 2). The catheter was trajected along the main axis of the hematoma and the small openings of the catheter were placed in the center of the hematoma. A catheter was inserted to the precalculated depth and target point, followed by careful manual hema-toma aspiration and irrigation with a small volume of normal saline. The catheter was anchored to the skin, and connected to a closed external drainage system.
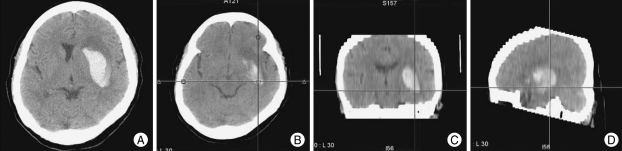
Fig. 1.
This 65-year-old hemiplegic patient with a left basal ganglia hematoma (A) underwent stereotactic multiplanar reformatted computed tomography-guided catheter placement for thrombolysis and drainage. We were able to select the optimal trajectory for the catheter along the main axis of the hematoma using reformatted axial (B), sagittal (C) and coronal (D) images.
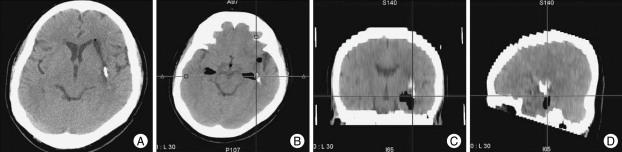
Fig. 2.
After the 2-day drainage period, the hematoma was almost completely removed (A). Axial (B), sagittal (C) and coronal (D) reformatted images show the catheter, which is placed along the main axis of the hematoma, as planned preoperatively.
Immediately after surgery, the postoperative hematoma volume and catheter position were investigated by CT. All catheters were placed exactly at the precalculated target point of the hematoma. Urokinase (8,000 IU/2 mL saline) was administered through the catheter, subsequently flushed with 2 mL of saline and then clamped. After 30 minutes the catheter was unsealed and the liquefied hematoma was drained naturally. The evacuated volume was recorded after every procedure. This evacuation and urokinase injection procedure were performed several times at 6- or 8-hour intervals until the hematoma was completely drained. The catheter was removed when repeated CT scan revealed less than 10 mL of the ICH volume after surgery.
RESULTS
ICH volumes ranged from 24 to 86 mL with a mean of 44.5 mL. The hematoma catheter was left in place for a median duration of 48.9 hours (range 34 to 62 hours). In an average of two days after surgery, the mean residual hematoma volume was 6.2 mL (range 1.4 mL to 10.2 mL) and the ICH volume was reduced by an average of 84.7% (range 71.6% to 96.3%). The residual hematoma at postoperative seven days was less than 5 mL in all patients. The mean number of urokinase instillations was 6.2 times (range 3 to 9 times) and the mean dose of urokinase instillations was 49,600 IU (range 24,000 IU to 72,000 IU). There were 5 subcortically extended ganglionic ICHs and 18 pure ganglionic ICHs. The selection of an appropriate trajectory and the burr hole site were not restricted by the stereotactic device in most of the patients. But, in 3 subcortically extended ganglionic hemorrhages, a burr hole location was modified to place the tip of catheter in the center of hematoma. The modified burr hole site was located 3 cm lateral to midline and about 1 cm posterior to the coronal suture.
There were no surgical complications except for 2 minor bleedings around the hematoma catheter, which had resolved on follow-up CT scan. There were no treatment-related deaths during hospitalization. Within the 30 days follow-up period, 8 patients were discharged, but they remained to be dependent because of residual hemiparesis (GOS=SD). Twelve patients suffered a mild neurological deficits (GOS=MD). Three patients achieved good recovery (GOS=GR).
DISCUSSION
Optimal treatment of ICH remains a complex and controversial issue5,7). Clinical presentation, age, and the size and localization of the hematoma influence the decision for conservative or surgical treatment11). Surgical treatment can be accomplished by several methods, including microsurgical removal after craniotomy with or without ultrasonic guidance, endoscopic hematoma evacuation or the use of stereotactically placed catheters for subsequent fibrinolytic therapy with urokinase, or tPA. Craniotomy has been the standard approach for ICH removal. However, the major disadvantage of an extensive surgical approach is that it may lead to further brain damage, particularly in patients with deep hematomas. Most of the randomized controlled trials have failed to demonstrate a superiority of craniotomy over medical therapy for ICH evacuation3,16). Minimally invasive surgical techniques may substantially decrease the volume of a hematoma while avoiding the morbidity associated with major craniotomy procedures, especially in elderly and debilitated patients1). Several experimental studies have shown that the infusion of urokinase promotes clot lysis and resorption without producing neurotoxicity, histopathological alterations, or recurrent bleeding6,17).
The application of stereotactic surgery and minimally invasive therapies to cerebrovascular surgery has led investigators to utilize these techniques to reduce the hematoma volume in the treatment of ICH. The volume of ICH has been consistently shown to be a powerful predictor of poor outcome regardless of clot location, age, or neurological condition5,7). The rationale for ICH evacuation is that the reduction of clot volume may improve neurological recovery and clinical outcome. The removal of a focal mass effect may improve perfusion of compromised brain parenchyma and prevent intracranial hypertension. It may also enhance the clearance of blood breakdown products, thereby preventing secondary brain edema and other potential sources of neurotoxicity.
Catheter placement along the main axis in the center of the hematoma was considered to be optimal for effective clot lysis19,21). Hondo described the use of a silicone catheter that was inserted into the center of the hematoma after three-dimensional CT images or biplane CT images were taken to determine the coordinates of the target point8). Unfortunately, detailed information on how often the adequate selection of the target point allowed optimal catheter positioning was not provided in the already published series on the use of the stereotactic CT-guided method for sICH. Catheter placement is difficult with the conventional CT-guided method because the aiming trajectory is calculated from the point of entry through the main axis of the hematoma using only axial CT images of the hematoma. The selection of the trajectory is based on the experience and spatial orientation capabilities of the neurosurgeon. In the past our experiences, the residual hematoma after conventional CT-guided surgery using only axial CT images was mostly observed at the posterior and inferior portion of the hematoma. Therefore, the target point of the hematoma was generally determined to the posterior and inferior portion of the hematoma. If the entry point should be fixed before the target point is selected, the selection of an appropriate trajectory would sometimes be restricted and the catheter would be placed the outside of the hematoma. The selections of the target point and the trajectory are initially determined and then the proper entry point can be selected. In 3 cases of the extended subcortical lobar hemorrhage, a burr hole location was modified to place a catheter in the center of the hematoma. Catheter placement along the main axis was successful in all cases.
An advantage of this technique is that MPR images are automatically generated, regardless of CT scanner type. And, the selection of the trajectory is not based on the experience and spatial orientation capabilities of the operator but the objective data from MPR CT images. So, catheter placement along the main axis in the center of the hematoma could be achieved with high accuracy and safety using MPR CT-guided methods.
Surgical treatment of ICH using various stereotactic techniques, including fibrinolytic instillation, has evolved over the years, but many investigators have reported aspiration rates ranging from 30% to 80% on average over the first few days2,8). Compared with data from the literature, the results obtained using this MPR CT-guided method were highly satisfactory for clot removal. Rhode et al. reported that the development of delayed perifocal edema was intensified by fibrinolytic therapy, which was probably related to the function of tPA as a mediator of edema formation after thrombin release and ischemia20). Substantial decompression of the hematoma cavity by the mass effect from the surrounding edema would have been anticipated in all of the patients in this study, and there were no instances of clinical deterioration due to edema associated with residual hematoma. Early removal of the hematoma allowed the patients to be discharged from the hospital faster and provided a greater chance of neurological recovery. This method appears to be effective at decreasing ICH volume and it is not associated with significant management morbidity. Further applications of this method are being evaluated to clarify whether it provides better neurological and overall recovery than standard craniotomy or conventional stereotactic surgery.
CONCLUSION
The present study indicates that stereotactic MPR CT-guided catheter placement is an accurate and safe procedure for thrombolysis. In comparison with the conventional CT-guided method, the MPR CT-guided method offers objective selection of the optimal trajectory and the appropriate target point for catheter placement. We suggest that this procedure for stereotactic removal of sICH should be considered for the optimization of trajectory selection in the future.
References
- 1.Auer LM, Deinsberger W, Niederkorn K, Gell G, Kleinert R, Schneider G, et al. Endoscopic surgery versus medical treatment for spontaneous intracerebral hematoma : a randomized study. J Neurosurg. 1989;70:530–535. doi: 10.3171/jns.1989.70.4.0530. [DOI] [PubMed] [Google Scholar]
- 2.Backlund EO, Von Holst H. Controlled subtotal evacuation of intracerebral haematomas by streotactic technique. Surg Neurol. 1978;9:99–101. [PubMed] [Google Scholar]
- 3.Batjer HH, Reisch JS, Allen BC, Plaizier LJ, Su CJ. Failure of surgery to improve outcome in hypertensive putaminal hemorrhage : a prospective randomized trial. Arch Neurol. 1990;47:1103–1106. doi: 10.1001/archneur.1990.00530100071015. [DOI] [PubMed] [Google Scholar]
- 4.Chambers IR, Banister K, Mendelow AD. Intracranial pressure within a developing intracerebral haemorrhage. Br J Neurosurg. 2001;15:140–141. doi: 10.1080/02688690120036847. [DOI] [PubMed] [Google Scholar]
- 5.Fayad PB, Awad IA. Surgery for intracerebral hemorrhage. Neurology. 1998;51:S69–S73. doi: 10.1212/wnl.51.3_suppl_3.s69. [DOI] [PubMed] [Google Scholar]
- 6.Findlay JM, Weir BK, Steinke D, Tanabe T, Gordon P, Grace M. Effect of intrathecal thrombolytic therapy on subarachnoid clot and chronic vasospasm in a primate model of SAH. J Neurosurg. 1988;69:723–735. doi: 10.3171/jns.1988.69.5.0723. [DOI] [PubMed] [Google Scholar]
- 7.Hankey GJ, Hon C. Surgery for primary intracerebral hemorrhage : is it safe and effective? A systematic review of cases and randomized trials. Stroke. 1997;28:2126–2132. doi: 10.1161/01.str.28.11.2126. [DOI] [PubMed] [Google Scholar]
- 8.Hondo H, Uno M, Sasaki K, Ebisudani D, Shichijo F, Toth Z, et al. Computed tomography controlled aspiration surgery for hypertensive intracerebral hemorrhage : Experience of more than 400 cases. Stereotact Funct Neurosurg. 1990;54-55:432–437. doi: 10.1159/000100248. [DOI] [PubMed] [Google Scholar]
- 9.Jennett B, Bond M. Assessment of outcome after severe brain damage : A practical scale. Lancet I. 1975:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 10.Jeong DK, Lee KH, Kim BH, Kim KJ, Kim YH, Bajpai V, et al. On-the-fly generation of multiplanar reformation images independent of CT scanner type. J Digit Imaging. doi: 10.1007/s10278-007-9032-9. Epub ahead of print, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaneko M, Tanaka K, Shimada T, Sato K, Uemura K. Long-term evaluation of ultra-early operation for hypertensive intracerebral hemorrhage in 100 cases. J Neurosurg. 1983;58:838–842. doi: 10.3171/jns.1983.58.6.0838. [DOI] [PubMed] [Google Scholar]
- 12.Kingman TA, Mendelow AD, Graham DI, Teasdale GM. Experimental intracerebral mass : description of model, intracranial pressure changes and neuropathology. J Neuropathol Exp Neurol. 1988;47:128–137. doi: 10.1097/00005072-198803000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–1305. doi: 10.1161/01.str.27.8.1304. [DOI] [PubMed] [Google Scholar]
- 14.Lopez Valdes E, Hernandez Lain A, Calandre L, Grau M, Cabello A, Gomez Escalonilla C. Time window for clinical effectiveness of mass evacuation in a rat balloon model mimicking an intraparenchymatous hematoma. J Neurol Sci. 2000;174:40–46. doi: 10.1016/s0022-510x(99)00288-9. [DOI] [PubMed] [Google Scholar]
- 15.Marquardt G, Wolff R, Sager A, Hartung A, Lorenz R. Manual stereotactic aspiration of spontaneous deep-seated intracerebral haematomas in non-comatose patients. Br J Neurosurg. 2001;15:126–131. doi: 10.1080/02688690120036810. [DOI] [PubMed] [Google Scholar]
- 16.Morgenstern LB, Frankowski RF, Shedden P, Pasteur W, Grotta JC. Surgical treatment of intracerebral hemorrhage (STICH) : a single-cineter, randomized clinical trial. Neurology. 1998;51:1359–1363. doi: 10.1212/wnl.51.5.1359. [DOI] [PubMed] [Google Scholar]
- 17.Narayan RK, Narayan TM, Katz DA, Kornblith PL, Murano G. Lysis of intracranial hematomas with urokinase in a rabbit model. J Neurosurg. 1985;62:580–586. doi: 10.3171/jns.1985.62.4.0580. [DOI] [PubMed] [Google Scholar]
- 18.Nehls DG, Mendelow AD, Graham DI, Sinar EJ, Teasdale GM. Experimental intracerebral hemorrhage : progression of hemodynamic changes after production of a spontaneous mass lesion. Neurosurgery. 1988;23:439–444. doi: 10.1227/00006123-198810000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Niizuma H, Otsuki T, Johkura H, Nakazato N, Suzuki J. CT-guided stereotactic aspiration of intracerebral hematomas : results of a hematoma-lysis method using urokinase. Appl Neurophysiol. 1985;48:427–430. doi: 10.1159/000101172. [DOI] [PubMed] [Google Scholar]
- 20.Rohde V, Rohed I, Thiex R, Ince A, Dückers G, Gröschel K, et al. Fibrinolysis therapy achieved with tissue plasminogen activator and aspiration of the liquefied clot after experimental intracerebral hemorrhage : rapid reduction in hematoma volume bur intensification of delayed edema formation. J Neurosurg. 2002;97:954–962. doi: 10.3171/jns.2002.97.4.0954. [DOI] [PubMed] [Google Scholar]
- 21.Schaller C, Rohde V, Meyer B, Hassler W. Stereotactic puncture and lysis of spontaneous hemorrhage using recombinant tissue plasminogen activator. Neurosurgery. 1995;36:328–335. doi: 10.1227/00006123-199502000-00012. [DOI] [PubMed] [Google Scholar]