Abstract
Objective
Radiosurgery may be contraindicated for lesions adjacent to the optic pathways because of the substantial risk of visual complication. Multisession radiosurgery has been tried as a compromise between single session radiosurgery and fractionated radiotherapy. The purpose of this study is to evaluate the outcomes of multisession gamma knife radiosurgery (GKRS) in 22 patients with perioptic lesions of benign pathology.
Methods
In all 22 cases, the lesions were within 1 mm of the optic apparatus and were therefore not considered suitable for single session radiosurgery. Radiation was delivered in 3 to 4 fractions with a median cumulated marginal dose of 20 Gy (range, 15-20 Gy).
Results
During a mean follow-up of 29 months (range, 14-44 months), tumor control was achieved in 21 patients. Visual function improved in 7 patients, remained unchanged in 14 patients, and deteriorated in 1 patient with tumor progression. No other complication was observed.
Conclusion
This preliminary result supports the idea that multisession GKRS may be an effective and safe alternative for treatment in perioptic lesions that are unsuitable for single session radiosurgery.
Keywords: Multisession radiosurgery, Gamma knife, Visual complication
INTRODUCTION
The traditional definition of radiosurgery is a therapeutic modality characterized by stereotactically focused irradiation in a single session6). Recently, it has become a commonly used technique for various intracranial lesions. But the close proximity of the anterior visual pathways such as the optic nerves and optic chiasm makes challenging in radiosurgical treatment of perioptic lesions. It is well known that the normal optic apparatus is one of the most vulnerable structures to radiation injury, and the maximal dose to the optic apparatus should not exceed 8 Gy in single dose irradiation. Therefore, in usual practice, radiosurgery is not recommended for lesions located within 2 mm of the anterior visual pathways8). Recent trials of multisession radiosurgery for perioptic lesions have been conducted in an attempt to achieve tumor control rates equal to single session radiosurgery while maintaining the risk of optic neuropathy as low as in fractionated radiotherapy1). Gamma knife has been used exclusively for single session radiosurgery, and there is no report of multisession gamma knife radiosurgery (GKRS) for perioptic lesions. In this report, we report, in detail, the visual outcome of 22 patients treated with multisession GKRS for perioptic lesions. This is the first report of gamma knife for this purpose.
MATERIALS AND METHODS
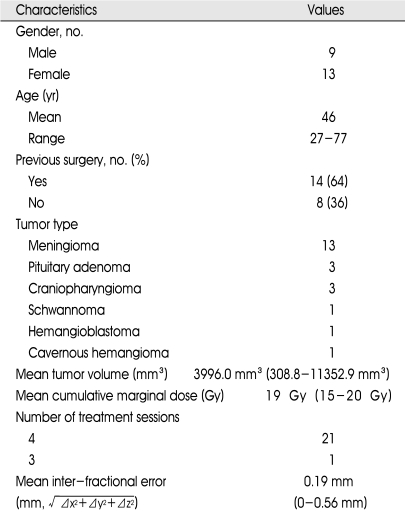
Twenty-two patients with perioptic lesions were treated with multisession GKRS. All patients met the usual recommended selection criteria for radiosurgery (i.e. small to moderate tumor size, benign pathology, previous surgery or coexisting morbidity precluding surgery), except tumor location. All patients in this study had tumors near the optic apparatus, within 1 mm distance. The subject group was composed of 13 women and 9 men, with a mean age of 46 years (range, 27-77 years) (Table 1). Open microsurgical resection had been done in 13 patients, and biopsy for pathologic confirmation had been done in 1 patient. In 8 patients, their presumptive diagnoses were established based on MRI findings (i.e. 6 meningiomas, 1 pituitary adenoma, and 1 cavernous hemangioma) without surgical resection or biopsy. Therefore, combined pathological and radiological findings led to diagnoses of 13 meningiomas, 3 pituitary adenomas including a case of GH (growth hormone)-secreting adenoma, 3 craniopharyngiomas, 1 schwannoma, 1 hemangioblastoma, and 1 cavernous hemangioma.
Table 1.
Characteristics of 22 patients in this series
All patients were evaluated by clinical examination and imaging studies before radiosurgery. Baseline evaluation of visual acuity and field testing with Snellen chart and kinetic quantitative perimetry were done, and 16 (73%) of the 22 patients were found to have visual disturbance. Serum hormone levels were also measured in patients with pituitary adenomas. All pituitary hormone levels were found to be normal, with the exception of elevated GH in the patient with a GH-secreting adenoma.
Radiosurgery was performed with the usual technique using Leksell Gamma Knife, except multisession irradiation. On the morning of the first day of multisession GKRS, Leksell stereotactic frame fixation was performed for planning radiosurgery. The dose planning method was similar to usual single-session radiosurgery, except for the prescribed dose, which was adjusted considering the dose to the optic apparatus and number of fractions. The decision of total dose, dose per session, and number of fractions was based on the previously widespread knowledge of optic nerve tolerance to single-session radiosurgery and experiences treating other cranial nerves including anterior optic pathways with multisession radiosurgery1,8). In any given session, we tried not to exceed 8 Gy of radiation to any portion of the anterior visual pathway. Radiation was delivered in 3 to 4 sessions, with an average target volume of 4130.9 mm3 (range, 396.5 mm3-16400 mm3), using a cumulative median marginal dose of 20 Gy (range, 15.0-20.0 Gy). Prescription dose was usually 50% (range, 46%-50%). Twenty-one patients were treated in 4 sessions, and one patient was treated in 3 sessions. Individual sessions were separated by 12-hour intervals in the 4-session cases and 24-hour intervals in the 3-session case. Dexamethasone (5 mg per 6 hours) was administered during the whole sessions. Through the treatment, the patients stayed in hospital for 3 to 4 days. The accuracy of stereotaxy was checked before the last session with MRI, and the mean inter-fractional displacement error was found to be 0.19 mm (range, 0-0.56 mm) (Table 1).
Formal visual tests with Snellen chart and kinetic quantitative perimetry were performed in 16 patients, with physical examination for visual function in all patients.
RESULTS
Mean follow-up period was 29 months (range, 14-44 months). The tumor volume decreased in 17 (77%) patients and remained stable in 4 (18%) patients at the last follow-up MRI scan (Table 2). Therefore, control of tumor growth was achieved in 21 (96%) patients. In 7 patients with available digital tumor volumetry data, the tumor volume decreased from a mean of 4694.0 mm3 at the time of GKRS to 3857.2 mm3 at last follow-up. One patient with craniopharyngioma experienced progressive tumor growth with visual deterioration and underwent two additional open surgical resections.
Table 2.
Treatment results in 22 patients
GKRS : gamma knife radiosurgery
Seven of 12 patients with markedly impaired visual function before treatment improved in visual acuity and/or visual field defect (a representative case is shown in Fig. 1), and 5 patients remained stable. There was no change of visual function in 9 (41%) patients in whom the lesions did not significantly affect visual function before treatment. Therefore, preservation of visual function was achieved in 21 patients (96%). One patient experienced deterioration of visual acuity and visual field due to tumor growth (Table 2).
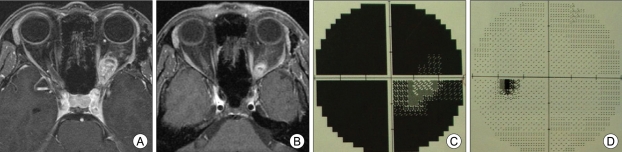
Fig. 1.
A 35-year-old female patient with left intraorbital schwannoma underwent multisession gamma knife radiosurgery (GKRS) (20 Gy in 4 fractions). Magnetic resonance images before (A) and 10 months after GKRS (B) show decrease in tumor size. Visual acuity improved from 0.1 to 1.0. Visual field examination shows large field defect before treatment (C) and only a small blind spot 10 months after treatment (D).
Elevated GH level in a patient with McCune-Albright syndrome (combination of GH-secreting pituitary adenoma, fibrous dysplasia, and multinodular thyroid goiter) was normalized at 23 months after multisession GKRS, accompanied by a decrease in tumor volume (Fig. 2). There was no permanent complication or morbidity related to GKRS.
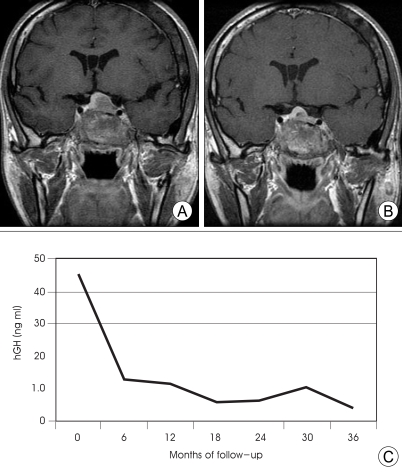
Fig. 2.
A 39-year-old female patient with McCune-Albright syndrome (pituitary adenoma, fibrous dysplasia, and multinodular thyroid goiter) underwent multisession gamma knife radiosurgery (GKRS) (20 Gy in 4 fractions). Magnetic resonance images before (A) and 23 months after GKRS (B) reveal tumor shrinkage. The patient's growth hormone (GH) levels were followed over the course of treatment. The graph (C) shows continually decreasing levels, finally approaching normal range without medical treatment.
DISCUSSION
Common strategies for managing benign tumors in close proximity to the optic nerves and optic chiasm include microsurgery, radiotherapy, or a combination of microsurgery and subsequent irradiation. One of the most important considerations in the treatment of perioptic lesions is effective disease control with preservation of visual function. Open microsurgical resection with decompression of the optic pathways is currently considered the first line treatment option in most cases. Frequently, surgical treatment may not be safe or effective, in spite of recent advances in imaging modalities and operative techniques. Additionally, some patients are not suitable for surgical treatment for other reasons (i.e. old age, poor general medical condition, fear of surgery, etc.).
The common second option for patients not eligible for surgery or those with incomplete surgical resection is conventional fractionated radiation therapy. Though fractionated radiation therapy may be safer than radical surgery, radiation therapy also has some intrinsic limitations. Even with sophisticated dose planning methods, the adjacent normal structures like the optic nerves and optic chiasm cannot escape from the irradiation margin due to set-up inaccuracy and may be exposed beyond the tolerance dose3). Dose per fraction and total dose should be compromised to fall between the risk of long-term complications and probability of tumor control. Radio-surgery has the advantage of better spatial accuracy compared to fractionated radiotherapy, and the biological equivalent dose of high single-dose radiosurgery may permissibly exceed that of the total dose given by conventional fractionated radiotherapy. As a result, tumor control after fractionated radiotherapy, as measured by tumor shrinkage, has been reported in 67%-100% of patients, figures which are not as good as those seen in radiosurgery (80%-98% tumor control)2). Functional normalization after fractionated radiotherapy in patients with pituitary adenomas is not as rapid as in patients undergoing radiosurgery5).
Traditional single-session radiosurgery can not always be recommended in perioptic lesions because it may be difficult to deliver an effective dose to the lesion while maintaining a dose to the optic apparatus that is low enough to guarantee safety. When the distance between the tumor and the antrior visual pathways is less than 2 or 3 mm, the optic apparatus easily receives more than 10 Gy in single session radiosurgery, and it is usual that radiosurgery is not recommended1).
Multisession radiosurgery aims at integrating the advantages of conventional fractionation radiotherapy (i.e. increased therapeutic index resulting from the different response to fractionated irradiation between normal tissue and tumor) and single-session radiosurgery (i.e. excellent accuracy and conformality). Adler et al.1) suggested that high accuracy irradiation may take advantage of "volume effect" (i.e. irradiation tolerance inversely proportional to the length of irradiated nerve), which permits larger doses to be delivered each session, without increasing the rate of complications. The larger dose per session results in a higher biological equivalent dose. A total dose of 20 Gy in 4 fractions can be converted into 140 Gy of biological equivalent dose with assumption of α/β=2 and 36 Gy with α/β=10. Though optimal dose and number of fractions are not certain because we do not know exact α/β ratio in real situation, greater tumor control without increasing accumulated dose to optic apparatus compared to the previous standard radiation therapy can be expected with this approach.
In this study, we evaluated tumor control and preservation of visual function during a follow-up period exceeding 2 years, and the outcome was favorable in the majority of the patients. Only one patients experienced visual deterioration from progression of the primary tumor. It is widely known that a small number of patients experience the improvement of their impaired visual function after radiosurgery, but in this study we observed high rate of visual function improvement. Considering that the visual symptoms of radiation injury usually occur within the first 2 years after treatment4), our results suggest that the procedure is safe, although there is a small chance of delayed visual deterioration seen in much longer follow-up periods.
Gamma knife has traditionally been used for the single session irradiation procedure because of the inconvenience of stereotactic frame fixation. In our series, multisession GKRS over three days was tolerable to all patients. In addition to relative safety and intermediate-term efficacy in the management of perioptic lesions, the multisession GKRS allows for a shortened treatment period and resultant cost-benefit. The treatment period shortens from 5-6 weeks with conventional fractionation radiotherapy to a half week with multisession GKRS. There are other systems that enable multisession radiosurgery, and discomfort with the stereotactic frame fixation may be a disadvantage of gamma knife treatment. Conversely, advantages of gamma knife treatment compared to other instruments include minimal interfractional displacement error and higher cumulative energy delivered to the target with the same marginal dose due to usual dose prescription at 50% isodose instead of 80% commonly used in radiosurgery with linear accelerator.
This preliminary study is not a prospective controlled trial, and it is too early to generalize these results. Also, comparison of outcomes with low dose single session radiosurgery is needed. More comparative studies with longer follow-up periods are needed to address the optimal dose per fraction, number of fractions, and final conclusion about the usefulness of this strategy. Construction of ideal model that determines the prescription dose with relation to dose per fraction, irradiation interval, and alpha-beta values of normal and tumor tissues, is another task to settle. More analysis about biological equivalent dose and accompanied adverse effects of accumulated shuttle dose of gamma knife with fractionation should be carried out. Despite these remaining uncertainties, multisession GKRS deserves to be considered as a effective method to increase the probability of tumor control and concomitant preservation of visual function.
CONCLUSION
In conclusion, multisession GKRS is a tolerable procedure with highly reliable precision and effectiveness and a low risk of visual deterioration for benign perioptic lesions. Multisession GKRS also has the advantage of shorter treatment time and lower cost. Long-term prospective studies with a large number of patients, comparing standard fractionation radiotherapy and low dose single session radiosurgery, are warranted to completely validate the procedure.
References
- 1.Adler JR, Jr, Gibbs IC, Puataweepong P, Chang SD. Visual field preservation after multisession cyberknife radiosurgery for perioptic lesions. Neurosurgery. 2006;59:244–254. doi: 10.1227/01.NEU.0000223512.09115.3E. discussion 244-254. [DOI] [PubMed] [Google Scholar]
- 2.Andrews DW, Suarez O, Goldman HW, Downes MB, Bednarz G, Corn BW, et al. Stereotactic radiosurgery and fractionated stereotactic radiotherapy for the treatment of acoustic schwannomas: comparative observations of 125 patients treated at one institution. Int J Radiat Oncol Biol Phys. 2001;50:1265–1278. doi: 10.1016/s0360-3016(01)01559-0. [DOI] [PubMed] [Google Scholar]
- 3.Cantore WA. Neural orbital tumors. Curr Opin Ophthalmol. 2000;11:367–371. doi: 10.1097/00055735-200010000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Danesh-Meyer HV. Radiation-induced optic neuropathy. J Clin Neurosci. 2008;15:95–100. doi: 10.1016/j.jocn.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Kong DS, Lee JI, Lim do H, Kim KW, Shin HJ, Nam DH, et al. The efficacy of fractionated radiotherapy and stereotactic radiosurgery for pituitary adenomas: long-term results of 125 consecutive patients treated in a single institution. Cancer. 2007;110:854–860. doi: 10.1002/cncr.22860. [DOI] [PubMed] [Google Scholar]
- 6.Lunsford LD, Witt TC, Kondziolka D, Flickinger JC. Stereotactic radiosurgery of anterior skull base tumors. Clin Neurosurg. 1995;42:99–118. [PubMed] [Google Scholar]
- 7.Movsas B, Movsas TZ, Steinberg SM, Okunieff P. Long-term visual changes following pituitary irradiation. Int J Radiat Oncol Biol Phys. 1995;33:599–605. doi: 10.1016/0360-3016(95)00221-J. [DOI] [PubMed] [Google Scholar]
- 8.Tishler RB, Loeffler JS, Lunsford LD, Duma C, Alexander E, 3rd, Kooy HM, et al. Tolerance of cranial nerves of the cavernous sinus to radiosurgery. Int J Radiat Oncol Biol Phys. 1993;27:215–221. doi: 10.1016/0360-3016(93)90230-s. [DOI] [PubMed] [Google Scholar]