Abstract
Guidelines around the world require children to provide assent for their participation in most research studies. Yet, little further guidance is provided on how review committees should implement this requirement, including which children are capable of providing assent and when the requirement for assent may be waived on the grounds that the research offers participating children the potential for important clinical benefit. The present paper argues that the assent requirement is supported by the importance of allowing children who are capable to make their own decisions. This suggests children are capable of assent when they become able to understand the research in question. While development varies across individual children, existing data suggest most children develop this ability by approximately age 14. Until instruments are developed to assess the assent capacity of individual children, this age should be used as the threshold for assent. In addition, the importance of protecting children from harm suggests that the sustained dissent of all children, including those who are unable to provide assent, should be respected. While the assent requirement may be waived when research participation offers the potential for important medical benefit that is unavailable outside the research context, analysis suggests that children's sustained dissent should be respected in all cases.
Keywords: paediatric research, assent
Recent initiatives are expected to dramatically increase the number of children who participate in clinical research.1 Data that show that almost half of all drugs provided to children in Europe involve either off label or unlicensed use have led to calls for more research on medications in children,2,3,4 and the European Forum for Good Clinical Practice has called for legislation in Europe to promote research with children.5 In 2004, the UK health minister announced a new initiative to encourage the development of medications for children, and the UK government intends to spend 100 million pounds on new research with children.6 In the US, the National Institutes of Health (NIH) now requires the inclusion of children in a broad range of research,7 while the Food and Drug Administration (FDA) offers 6 months additional marketing exclusivity to firms that submit data pertaining to the use of tested agents in paediatric populations.1i
Most national and international guidelines allow children to be enrolled in research only when their parents or legal guardians give their permission. Many guidelines, including the Council for International Organisations of Medical Science (CIOMS) guidelines,9 and guidelines from South Africa10 and the US,11 also require the assent, defined as “positive agreement,” of children who are capable of providing it. The Ugandan guidelines—for example, allow children to be enrolled in research that does not offer a prospect of direct benefit only when: “Adequate provisions have been made for the solicitation of the children's assent”.12 Similarly, the Indian Council on Medical Research (ICMR) guidelines state that: “the assent of the child should be obtained to the extent of the child's capabilities”.13 Other guidelines mandate that researchers must respect the dissent of paediatric research subjects. According to the Guidelines for the Ethical Conduct of Biomedical Research Involving Human Subjects in Kenya, when the “child refuses to participate in the research, that refusal must be respected unless there's no other medical alternative from which the child could benefit”.14
The assent requirement is one of the principal requirements for paediatric research, and often the only requirement to specify when children should have a say in whether they participate in clinical research.15 Failure to require children's assent when appropriate represents a failure to respect paediatric research participants,16,17 while failure to waive the assent requirement when appropriate may block parents' decisions to enrol their children in potentially beneficial research.18,19 Despite the importance of the assent requirement, little has been written on how investigators and review committees should implement the requirement in practice. The present paper attempts to address this gap by considering the ethical rationale for the assent requirement and, on that basis, considering how the assent requirement should be implemented in practice.
Ethical rationale
Investigators and review committees who attempt to implement the assent requirement must determine at what age children become capable of providing assent. Unfortunately, research ethics guidelines provide little guidance in this regard. The US federal regulations—for example, specify only that the determination of which children are capable of assent should be based on the children's “ages, maturity, and psychological state” (Department of Health and Human Services,11 45 CFR 46.608a). This guidance leaves many questions unanswered. Which aspects of children's age, maturity, and psychological state should investigators take into account when determining whether they are capable of assent? Is the ability to nod one's head sufficient? Must children understand certain aspects of their research participation, and, if so, which ones?
Many commentators seem to assume that the requirement to obtain children's assent is implied by the need to respect paediatric research participants. Yet, the principle of respect alone merely instructs investigators to treat research participants as they ought to be treated. Hence, it implies only that investigators should solicit children's assent at the ages at which it is appropriate to do so.
One might argue that investigators should be required to obtain children's assent to help teach them to become autonomous. Effectively teaching children to become responsible and autonomous adults involves asking them to make decisions they are capable of making. For this reason, parents typically begin with fairly minor decisions, and proceed, as children mature, to more important decisions. For instance, parents often begin by allowing younger children to help decide what to wear, or how to decorate their bedrooms.
Asking children to decide whether to enrol in research before they can understand is ill suited to teaching them to make good decisions. For instance, a child who can understand the risks or the potential benefits of research, but not both, is unlikely to make a good decision. This problem arises when children are able to understand some aspects of their research participation, but not others. To address this problem, children's assent should not be required until they are able to understand the research in question sufficiently. What constitutes sufficient understanding in this regard?
Setting an age threshold
Some commentators point out that children are able to understand some aspects of their research participation by age seven. These commentators often conclude that children's assent should be required, at least in the context of research that does not offer them a compensating potential for direct benefit, from age seven onwards.20,21,22
This rationale for the assent requirement is obviously in need of further justification. Most children can understand at least some aspects of their research participation much earlier than age seven, and most children do not understand other aspects of research until well after age seven. Hence, this position, if it is to offer an argument for a specific age threshold, must be supplemented with some account of the aspects of research participation that are relevant to the assent requirement.
Some commentators defend the age seven threshold by appeal to the centuries old “Rule of Sevens”, which “has stood at least ever since the time of Edward the Third” (1327–1377) to determine an age threshold for assent.23,24 The Rule of Sevens states, roughly, that children under age seven do not have the capacity necessary to make their own decisions; children from seven to fourteen years of age are presumed not to have this capacity until proven otherwise in individual cases, and children over age 14 are presumed to have capacity to make their own decisions and lead their own lives, unless proven otherwise.
One might argue that this practice does not gain much ethical weight simply in virtue of its rather impressive age. Instead, one needs to explain why these thresholds are relevant to paediatric research. In addition, it is worth noting that the claim that children are capable of assenting to research participation by age seven is not supported by the Rule of Sevens. Strict application of the Rule of Sevens would suggest that children between the ages of seven and fourteen should be regarded as not capable of making their own decisions, including the decision whether to participate in research, unless proven otherwise. Thus, in the absence of some argument about the specific aspects of research participation that are essential to assent, it seems that children's assent should be required at the point they are able to understand the research and make a prospective decision regarding enrolment.
The ability to decide
Some commentators argue that the assent requirement is implied not by some general principle of respect, but specifically by the principle of respect for persons. The principle of respect for persons mandates respect for individuals' ability to make their own autonomous decisions. To make an autonomous decision whether to enrol in research, potential subjects must be able to understand the study in question and their own medical and personal situations, and make a voluntary decision whether to participate on this basis. To understand the study in question, potential subjects must understand the so called “elements of informed consent”: the study's purpose, risks, potential benefits, requirements, procedures, and alternatives.25 In addition, potential subjects must appreciate how the elements of informed consent pertain to their own circumstances. Recognition that the capacity to make research decisions requires individuals to understand and appreciate all the elements of informed consent suggests one way to identify the age at which children develop this capacity, namely by identifying the age at which children come to understand and appreciate the “final”—that is to say, last to develop—element of informed consent.
Children first understand concrete facts about the world, and later come to understand more abstract facts. This suggests that the age at which children understand and appreciate the more abstract elements of informed consent provides an approximation of the age at which they can make their own research decisions. Perhaps the most abstract element of informed consent is the purpose of research. Research that does not offer a compensating potential for clinical benefit (henceforth “non‐beneficial research”)—the research for which children's assent is typically required—is intended to develop generalisable knowledge that might help future patients.
Importantly, understanding and appreciating the purpose of non‐beneficial research does not require that potential subjects are motivated to help others. An individual can understand fully that a particular study is intended to help others, but not care to help them in that way. Analogously, the capacity to decide whether to enrol in a phase III cancer treatment trial requires potential subjects to understand and appreciate that participation might improve their disease. It does not require that they are motivated to improve their disease.
Empirical studies of altruism tend to focus on helping behaviour, often labelled “pro‐social” behaviour, independent of why individuals behave in this way. Because these studies do not assess individuals' motivations for acting, they provide only limited evidence regarding when children develop the concept of altruism. The fact that children behave in a helping way at this or that age does not establish that they possess the concept of altruism at that age; it depends on why they act in this way.
Given the inherent difficulties in assessing the motivations underlying individual behaviour, it is not surprising that very few studies have assessed why children help others at various ages. Given this paucity of data, any recommendations must be tentative, subject to revision in light of future data. With this caveat in mind, the existing data suggest most children understand that there are moral reasons to help others, independent of the possibility of reward or punishment, at approximately age 14.26 That is, they begin to understand that there are moral reasons to help others, even when doing so is not required, and may place burdens on them. These data suggest most children do not develop the capacity to appreciate that the potential to help others provides a reason to enrol in non‐beneficial research until approximately 14 years of age. Therefore, to the extent the assent requirement is based on the principle of respect for autonomy, current data suggest the age threshold for assent should be fixed at 14 years of age.
Of course, this is only a general average. Some children develop the ability to make their own decisions at an earlier age and some not until a later age.27 This raises the question of whether investigators should assume a general threshold for all children, or whether investigators should tailor their practices to the abilities of specific children. To a large extent, answering this question will depend upon the development of instruments to assess children's ability to assent. The development of valid and practically feasible instruments would allow investigators to assess the abilities of individual children. What approach should be adopted until such instruments are developed?
Dissent
The importance of not harming individuals applies to individuals of all ages, whether they are autonomous or not. This principle implies that children should not be required to participate in non‐beneficial research that is more than minimally distressing. In many cases, children will not know whether research participation will be distressing until they experience it. Thus, requiring children to make a prospective decision whether to enrol does not offer an effective mechanism to protect them from harm, particularly since children may be reluctant to go back on agreements made with doctors. Children also may find it positively distressing to be asked to make decisions about research they cannot understand. Hence, protecting children from harm does not seem to support, and may well conflict in some cases, with the requirement to ask children to decide whether to enrol in non‐beneficial research before they are able to understand the research in question.
In contrast, once children are enrolled in research, they will be in a very good position to assess whether it is causing them distress. And because most children who experience distress will communicate this verbally or through body movements, protection of children from harm supports adoption of a dissent requirement to supplement existing assent requirements: the dissent of all children should be respected in the context of non‐beneficial research.28,29,30 The Tanzania guidelines for human subjects research—for example, stipulate that researchers “must recognize when a child is very upset by a procedure and accept that as genuine dissent from their being involved”.
Implementation of the assent requirement
The US federal regulations offer the clearest account of how the assent requirement should be implemented. Under the US regulations, the assent requirement may be waived for research that qualifies for a waiver of informed consent and also when research offers a “prospect of direct benefit that is important to the health or wellbeing of the children and is available only in the context of the research”.
Most research studies that offer a prospect of direct benefit that is important to the health or wellbeing of children, including drug trials, pose greater than minimal risk. However, some studies may offer a prospect of direct benefit that is available only in the research context and yet pose no greater than minimal risk. A study of a new method of massage for recurrent abdominal pain (RAP) of psychogenic origin may—for example—pose minimal risk and satisfy the conditions for waiver of assent. Specifically, this study may be deemed to offer a prospect of direct benefit in the form of the relief of symptoms that is important to the wellbeing of the child. In addition, access to this method of massage may be limited to the research setting, at least during the time that the method is being assessed. When these conditions are satisfied, the US regulations allow the review committee to waive the assent of children who are capable of providing assent.
The US federal regulations allow review committees to approve paediatric research in the category of prospect of direct benefit only when it satisfies three additional conditions (Department of Health and Human Service,11 45 CFR 46.405). Thus, to implement the assent requirement correctly, review committees must determine the relationship between studies that can be approved as offering a prospect of direct benefit and those for which assent may be waived. Specifically, do all studies that qualify for approval as offering a prospect of direct benefit also qualify for waiver of assent? Or does only some subset of these studies qualify for waiver of the assent requirement? This determination is complicated by the fact that the conditions for waiving assent and approving prospect of direct benefit research are similar, but not identical.
The first condition for approving research that offers a prospect of direct benefit refers to the benefits of “an intervention or procedure” or the benefits of “a monitoring procedure”. The regulations' focus on individual interventions or procedures suggesting review committees should assess whether each intervention or procedure included in the research offers a prospect of direct benefit. For studies that involve multiple interventions, review committees should not assess whether the study as a whole qualifies as offering a prospect of direct benefit. Instead, they should assess each intervention individually.31
The requirement to assess individual interventions implies that the decision review committees make with respect to approving one arm of a trial with multiple arms should not influence whether they approve the other arms of the trial. Some placebo controlled trials—for example, include one arm that provides an experimental treatment, and a second placebo arm. Review committees might approve the treatment arm as posing greater than minimal risk, but offering a prospect of direct benefit. This judgment, however, should not influence whether the institutional review board (IRB) approves the placebo arm of the trial. Whether the placebo arm can be approved depends on the risks and potential benefits of receiving the placebo, and the risks of not receiving other medications while on the placebo arm.
The first condition in the US regulations for waiving children's assent refers to “the [emphasis added] intervention or procedure involved in the research”. Although not entirely clear, this requirement suggests review committees should not make a separate determination regarding waiver of assent for each arm or intervention involved in a study. Rather, review committees should decide whether to waive children's assent for the entire study based on whether the intervention being assessed meets the three conditions for waiver of assent. Whatever determination the IRB makes for the intervention arm with respect to requiring or waiving children's assent applies to the entire study.
On this interpretation, when implementing the assent requirement, review committees do not need to assess whether each intervention of a multi‐arm study qualifies for approval as offering a prospect of direct benefit. Instead, they should assess whether at least one of the interventions or procedures offers a prospect of direct benefit. In the case of placebo controlled trials—for example, review committees should assess whether the intervention being tested offers a prospect of direct benefit. If so, the entire study may qualify for waiver of the assent requirement, provided this intervention meets the other two conditions for waiving children's assent.
The conclusion that review committees should require or waive children's assent for all aspects of a given study makes sense for most research protocols. Specifically, it would make little sense for review committees to waive the assent requirement for research enrolment, but then require children's assent for procedures that are required for participation in a given study. This approach would have the effect of requiring children's assent for the protocol as a whole. Children who decline to undergo a required procedure would not be able to participate in the study. In contrast, some studies include additional optional procedures, such as the storage of samples or an additional research scan. When research studies include optional procedures, review committees should assess whether to require children's assent for those procedures independent of the assessment for the required procedures. The fact that a study offers a prospect of direct benefit and qualifies for waiver of assent does not imply that review committees should waive the assent requirement for the optional procedures included in the study. Although not explicitly addressed in the US regulations, it seems that children's assent should be required for optional procedures unless they offer a prospect of direct benefit that is important to the health or wellbeing of the children and is available only in the research context.
The third condition in the US regulations for approving research as offering a prospect of direct benefit stipulates that the relation of the potential direct benefits to the risks must be “at least as favourable” as the available alternatives. This condition can be satisfied when the risk/benefit profile of the experimental intervention is at least as favourable as the clinical alternatives—for instance, when there exists clinical equipoise between the experimental and clinically available interventions. In contrast, the third condition for waiving assent stipulates that the prospect of direct benefit offered by the intervention must be “available only in the context of the research”.
One might assume that this condition requires evidence that the experimental intervention is better than the clinically available alternatives. The regulations do not stipulate, however, that the risk/benefit ratio of the experimental intervention must be more favourable than the clinical alternatives, only that the benefit offered by the experimental intervention must not be available outside of research. Therefore, the experimental intervention must be at least as good, and different, not necessarily better, than the clinical alternative.
Research studies typically focus on new interventions that may be better than the existing standard of care. Studies on new interventions that qualify for approval as offering a prospect of direct benefit are likely to also offer a prospect of direct benefit that is unavailable outside the research context. A randomised controlled trial of the standard of care alone versus the standard of care plus an experimental, add on treatment would—for example—offer a prospect of direct benefit that is unlikely to be available outside the research context—namely, access to the new experimental treatment. Hence, most interventions that satisfy the third condition for approval as research that offers a prospect of direct benefit also should satisfy the third condition for waiver of the assent requirement.
There are, however, at least two exceptions. First, research studies sometimes are limited to assessing the care individuals receive in the community. A clinical trial—for example, might assess the comparative efficacy of two treatments used in the community, for which there are insufficient comparative efficacy data. These interventions likely offer a prospect of direct benefit that “justifies” their risks, thus satisfying the second condition for approval as research that offers a prospect of direct benefit. Moreover, because these treatments are the same as those offered in the community, they would offer a risk/benefit ratio that is “at least as favourable” as the available alternatives, satisfying the third condition as well. Conversely, because the prospect of direct benefit available in these studies also is available outside research, studies limited to assessing clinically available interventions would not qualify for waiver of children's assent. Similarly, some trials assess a new use for a widely available intervention, which may be available to research participants outside of the context of research, on an “off label” basis.
Two step decision procedure
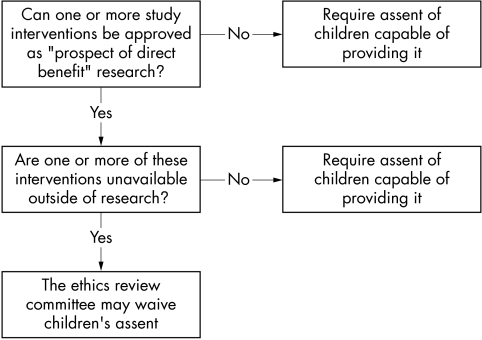
The present analysis of the US regulations suggests a relatively straightforward two step decision procedure (fig 1) that ethics review committees can use to determine when to waive the assent requirement. First, the review committee should determine whether the intervention being tested offers paediatric participants a “prospect of direct benefit”. For studies that involve several interventions, review committees should make this assessment for each intervention. If no interventions in the study qualify for approval as research offering a prospect of direct benefit, the assent of all children who are capable of providing it should be required.
Figure 1 Decision pathway for implementing assent requirement.
If one or more of the interventions in the study qualifies for approval as research that offers a prospect of direct benefit, the review committee should assess whether one or more of these interventions typically is unavailable to children outside of the research context. If so, the review committee may waive the requirement to obtain children's assent. If all these interventions are available outside of research, the investigators should be required to obtain the assent of children who are capable of providing it.
Assent and dissent
The assent requirement is the only requirement in the US federal regulations to address children's participation in the decision making process regarding their involvement in clinical research. When review committees waive the assent requirement, whether in the context of minimal risk research or research that poses more than minimal risk, investigators are under no regulatory obligation to respect children's decisions, including their dissent to research participation. Similarly, investigators have no regulatory obligation to respect the dissent of children deemed incapable of providing assent, even in the context of research that offers no prospect of direct benefit.
As we have seen, this is a mistake. Respecting children's dissent offers a way to protect them from harm. Expressions of dissent provide evidence that a child is experiencing distress or pain. Thus, failure to respect a child's dissent not only precludes the child from being part of the decision making process, it also represents a failure to stop research that may be causing the child distress or pain. This suggests the dissent of all children should be respected, even when the children are deemed unable to provide assent or the research qualifies for waiver of children's assent.
Because most children who experience distress will communicate this verbally or through bodily movements, respect for children's dissent implies that research participation should be stopped at the first sign of distress (Medical Research Council,30 p 320).29 Some verbal or behavioural objections may not reflect actual distress, whereas others may reflect only temporary distress. Rather than require dissenting individuals to be withdrawn automatically, review committees and investigators could adopt a policy of stop, assess, and address. Research procedures should be stopped, and evaluation provided, whenever a subject objects or physically resists. Reassurance, a short pause, or minor modification may be sufficient to eliminate the distress. Individuals who express sustained dissent should be withdrawn in the context of non‐beneficial research.
The US National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research argued that when a “research protocol includes an intervention from which the subjects might derive significant benefit to their health or welfare, and that intervention is available only in a research context, the objection of a small child may be overridden” (National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research,22 p 16). On this approach, children's dissent may be overridden when the investigators are conducting a randomised trial comparing standard of care alone to the standard of care plus an additional agent, provided the additional agent offers the prospect of important health benefit and is unavailable outside the research context. One might support this approach on the grounds that children's medical interests justify overriding their dissent in the context of research that offers a prospect of direct benefit. If the benefit is important, and the distress temporary, the children will be better off if the investigators override their dissent.
Because sustained dissent provides evidence of distress or pain, children's medical interests can justify overriding their dissent only when the intervention has been shown to be more effective than the interventions available in the community. If there is clinical equipoise between existing care and the experimental treatment, children's medical interests will not justify forcing them to participate in research, as opposed to receiving the standard of care available in the community. This suggests children's medical interests can justify overriding their dissent in rare cases only. In addition, although children's medical interests are an important consideration, they are not the only relevant consideration.
Forcing children to undergo medical procedures against their will can be extremely distressing to clinicians, and presents at least the appearance that children are being forced to suffer for the benefit of others. In the context of clinical care, forced medical care can be justified on the grounds that it is for the child's own interests. In the context of research, there remains, even in the context of research that offers the potential for important medical benefit, the concern that the child is being forced to undergo a particular intervention for the good of society. This concern suggests a child's sustained dissent should be binding in all cases, including research that offers a prospect of direct benefit. In the rare cases where removal from the research would preclude the child from obtaining care known to be more effective than what is available in the clinical setting, arrangements could be considered for supplying the drug outside of the research context.
Conclusion
Analysis suggests that children become capable of assent when they are capable of understanding the research in question and making a prospective decision whether to participate. There is a paucity of empirical literature on child development in this regard, and any recommendations must be tentative, awaiting future research. The current research suggests that children become capable of assent at approximately 14 years of age. Consideration of the existing US regulations provides a simple two step process for determining when children's assent may be waived on the grounds that the research offers them a prospect of direct benefit. Analysis also suggests that, in addition to assent, investigators should be required to respect the sustained dissent of all children.
Acknowledgements
Thanks to Ben Wilfond, Jehanna Peerzada, and participants at the American Society of Clinical Oncology (ASCO) and Clinical Oncology Group (COG) meeting on ethical issues in research with children, for their helpful comments.
Abbreviations
CIOMS - Council for International Organisations of Medical Science
FDA - Food and Drug Administration
Footnotes
iSection 111 of Title I of the Food and Drug Administration Modernization Act 1997, signed into law by President Clinton on 21 November 1997, created the Federal Food, Drug and Cosmetic Act.
The opinions expressed are the author's own. They do not represent any position or policy of the National Institutes of Health, Public Health Service, or Department of Health and Human Services.
References
- 1.Caldwell P H Y, Murphy S B, Butow P N.et al Clinical trials in children. Lancet 2004364803–811. [DOI] [PubMed] [Google Scholar]
- 2.Conroy S, Choonara I, Impicciatore P.et al Survey of unlicensed and off label drug us in paediatric wards in European countries. BMJ 200032079–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smyth R L. Research with children. BMJ 20013221377–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choonara I. Clinical trials of medicines in children. BMJ 20003211093–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medicines in children The EFGCP News. 2002.
- 6.Paediatric research should take centre stage [editorial] Lancet. 2004;364:732. doi: 10.1016/S0140-6736(04)16946-8. [DOI] [PubMed] [Google Scholar]
- 7.NIH policy and guidelines on the inclusion of children as participants in research involving human subjects March 6, 1998. http://www.grants1.nih.gov/grants/guide/notice‐files/not98‐024.html (accessed 23 Dec 2005)
- 8. Federal Food, Drug and Cosmetic Act (USC 355a)
- 9.Council for International Organisations of Medical Sciences International ethical guidelines for biomedical research involving human subjects. Geneva: CIOMS, 1968 [PubMed]
- 10. http://www.mrc.ac.za/ethics/values.htm (accessed 23 Dec 2005)
- 11.Department of Health and Human Services Protection of human subjects research. Washington: US Government Printing Office, 1991, 45 CFR 46.116
- 12.Uganda National Council of Science, Technology (UNCST) Guidelines for the conduct of health research involving human subjects in Uganda. Kampala, Uganda: UNCST, 1998
- 13. http://icmr.nic.in/ethical.pdf (accessed 23 Dec 2005)
- 14.National Council for Science and Technology Guidelines for the ethical conduct of biomedical research involving human subjects in Kenya. Nairobi, Kenya: NCST, 2004
- 15.Kodish E. 2003. Informed consent for pediatric research: is it really possible, J Pediatrics 20034289–90. [DOI] [PubMed] [Google Scholar]
- 16.Committee on Bioethics, American Academy of Pediatrics Informed consent, parental permission, and assent in pediatric practice. Pediatrics 199595314–317. [PubMed] [Google Scholar]
- 17.Rossi W C, Reynolds W, Nelson R M. Child assent and parental permission in pediatric research. Theor Med Bioeth 200324131–148. [DOI] [PubMed] [Google Scholar]
- 18.Leikin S. Minors' assent, consent, or dissent to medical research. IRB 1993151–7. [PubMed] [Google Scholar]
- 19.Broome M E, Kodish E, Geller G.et al Children in research: new perspectives and practices for informed consent. IRB 200325(suppl)20–3S. [PubMed] [Google Scholar]
- 20.Nicholson R H. ed. Medical research with children: ethics, law and practice. Oxford: Oxford University Press, 1986, ch 7
- 21.American Academy of Pediatrics Informed consent, parental permission, and assent in pediatric patients. Pediatrics 199595314–317. [PubMed] [Google Scholar]
- 22.The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research Research involving children: report and recommendations. Recommendation 7. Washington, DC: NCPHSBBR, 1977: RDHEW Pub no (OS) 77– 0004
- 23.Blackstone's commentaries on the laws of England [book IV, ch 2]. http, www.lonang.com/exlibris/blackstone/bla‐402.htm#fn1u.s (accessed 23 Dec 2005)
- 24. Caldwell and Caldwell vBechtol Supreme Court of Tennessee 724 SW2d1987
- 25.Berg J W, Appelbaum P S, Lidz C W.et al Informed consent: legal theory and clinical practice. Oxford: Oxford University Press, 200112
- 26.Wendler D, Shah S. Should children decide whether they are enrolled in non‐beneficial research? Am J Bioeth 200331–7. [DOI] [PubMed] [Google Scholar]
- 27.Bartholomew W G. Informed consent, parental permission, and assent in pediatric practice. Pediatrics 199596981–982. [PubMed] [Google Scholar]
- 28.Ackerman T F. Fooling ourselves with child autonomy and assent in non‐therapeutic clinical research. Clin Res 197927345–348. [PubMed] [Google Scholar]
- 29.Ackerman T F. Moral duties of parents and non‐therapeutic clinical research procedures in children. Bioethics Q 1980294–111. [DOI] [PubMed] [Google Scholar]
- 30.Medical Research Council The ethical conduct of research in children. In: Brody BA, ed. The ethics of biomedical research: an international perspective. New York: Oxford University Press, 1998320–321.
- 31.Miller F, Wendler D, Wilfond B. When do the federal regulations allow placebo controlled trials in children? J Pediatr 2003142102–107. [DOI] [PubMed] [Google Scholar]