Abstract
In this double-blind, placebo-controlled trial, bupropion (BUPRO, 300 mg/day) was compared to placebo (PBO) for concurrent treatment of opioid and tobacco addiction in 40 opioid-dependent smokers stabilized on buprenorphine (BUPRE, 24 mg/day). Participants received contingent, monetary reinforcement for abstinence from smoking, illicit opioids, and cocaine. Significant differences in treatment retention were observed (BUPRE+BUPRO, 58%; BUPRE+PBO, 90%). BUPRO treatment was not more effective than placebo for abstinence from tobacco, opioids, or cocaine in BUPRE stabilized patients. These preliminary findings do not support the efficacy of BUPRO, in combination with BUPRE, for concurrent treatment of opioid and tobacco addiction.
Keywords: opioid dependence, smoking cessation, bupropion, buprenorphine
Despite declining rates of smoking in the general population, rates of cigarette smoking continue to be elevated among those dependent on opioids, with estimates ranging from 71% to 98%.1, 2 Converging lines of evidence support the complex interaction between nicotine and the endogenous opioid system3, with opioids increasing cigarette smoking4–8 and nicotine and tobacco increasing opioid use9, and levels of the endogenous opioid, β-endorphin.10–13 Given the synergistic relationship between nicotine and opioids, the disproportionate rate of smoking in opioid users is not surprising.
Only recently have researchers begun to evaluate smoking cessation treatments for opioid-dependent individuals.8, 14–17 Interventions have involved behavioral treatments (e.g., supportive counseling, motivational interviewing, and contingency management [CM]) or behavioral treatment in conjunction with pharmacotherapy (i.e., nicotine patch, nicotine gum, or bupropion [BUPRO]). In general, these trials have shown modest or null findings in terms of cessation rates in this challenging population of smokers.
No placebo controlled trials of smoking cessation pharmacotherapies have been conducted in opioid dependent patients, although behavioral interventions have been compared to each other.14, 16 Further, all smoking cessation trials in opioid dependent smokers have involved concurrent methadone maintenance treatment (MMT). In recent years, a new treatment option for opioid dependent patients has emerged in the form of buprenorphine (BUPRE) maintenance treatment (BMT). Although similar in efficacy to methadone, buprenorphine (when combined with naloxone) has reduced potential for diversion.18, 19 To date, no smoking cessation trials in BMT patients have been reported in the literature. The widening use of BMT and its agonistic effects on smoking warrant careful study in the context of a clinical trial.4
The purpose of this preliminary study was to evaluate the safety, tolerability, and efficacy of bupropion in opioid and nicotine dependent participants maintained on buprenorphine, using a randomized, placebo-controlled design. We selected bupropion since it has been well tolerated in MMT patients, both as a smoking cessation intervention15, and as an intervention for cocaine and opioid dependence.20–23 We chose to provide contingent monetary reinforcement for abstinence from smoking, illicit opioids, and cocaine, as the robust nature of contingency management procedures are an essential therapeutic platform to show pharmacotherapy effects in opioid and cocaine using patients.16, 24
METHODS
Sample and Recruitment
Forty treatment-seeking male and female opioid- and nicotine-dependent smokers were recruited to take part in an outpatient clinical trial at the Opiate Treatment Research Program, at the Veteran’s Affairs (VA) Connecticut Healthcare System in West Haven, CT. Responding to advertisements in the VA facility, participants were informed that they would be stabilized on buprenorphine and would receive either bupropion or placebo. They were instructed that the goal was to abstain from illicit opioid, cocaine, and tobacco use, and that monetary reinforcement would be provided for abstinence.
Eligible participants: (a) were age 18–65; (b) were currently opiate and nicotine dependent25; (c) had smoked ≥ 10 cigarettes/day for the past year; (d) were seeking treatment for opiate and tobacco use; (e) had stable physical and psychiatric health; (f) had no current diagnosis of alcohol and other drug dependence or abuse (other than opioids, nicotine, and cocaine); (g) were not using prescription psychoactive drugs; and (h) for women, were not pregnant, planning pregnancy, or lactating. Eligibility was determined by a research psychiatrist based on physical, psychiatric and laboratory examination. Baseline measures included urine toxicology, the Structured Clinical Interview for DSM-IV (SCID)26, the Addiction Severity Index (ASI)27, and the Fagerstrom Test of Nicotine Dependence (FTND).28 All participants provided written informed consent to participate in the current study, which was approved by the Yale and VA Human Studies Committees.
Design and Procedure
The study was a randomized, double-blind, placebo-controlled trial conducted during from August 2004 to August 2005. Participants were randomized to either bupropion (BUPRE+BUPRO, n = 20 [19 analyzed]) and placebo (BUPRE+PBO, n = 20) treatment. An urn randomization procedure was used to ensure balanced distribution on race and sex.29 One enrolled participant (BUPRE+BUPRO) quit smoking prior to starting the trial, and was not included in the analyses. The study involved a 1-week dose run-up of buprenorphine and bupropion (or placebo), followed by a 10-week treatment period. Each participant was given a quit date beginning approximately one week after the bupropion treatment was started. Participants received counseling (1 hour/week) that emphasized motivational enhancement and skills training for relapse prevention and contingency management to help them to quit tobacco, illicit opioids, and cocaine. Each participant received US$5.00 for being abstinent from tobacco (CO result <10 ppm), illicit opiates (<200 ng/mL for opioids), and cocaine use (<300 ng/mL for benzoylecognine) for a maximum of US$15.00 per week (US$150.00 total possible). Urine assays were completed by the West Haven VA toxicology laboratory using an Olympus AU 640 Emit system. Participants were only discharged from the study for missing 3 consecutive days of medication or 3 consecutive counseling sessions.
Medications
Buprenorphine
Buprenorphine was administered as a sublingual tablet containing buprenorphine and naloxone in a ratio of 4:1. On day one of the trial, each participant received 4 mg, with 8 mg increases each day until reaching 24 mg on day 4. All weekday doses were directly observed. Take-home doses were also are given for self-administration twice-a-day on Saturdays and Sundays. All participants were stabilized on 24 mg/day. Depending on individual needs, at the end of the 10-week trial, participants received a 2–4 week buprenorphine detoxification.
Bupropion
Sustained-release bupropion tablets or placebo were administered twice daily: once while attending the clinic for buprenorphine administration, and one to take home. Take-home doses were also are given for self-administration twice-a-day on Saturdays and Sundays. Bupropion pills were over-encapsulated to match placebo pills. Participants initially received 150 mg/day of bupropion for 3 days, after which the dose was increased to 150 mg twice daily for the duration of the trial. At the end of the 10-week clinical trial, all participants were tapered off bupropion (or placebo) over a seven-day period.
Outcome Variables
Safety and tolerability were assessed, respectively, according to adverse events and treatment retention (weeks in study). The primary outcome measure was combined abstinence from smoking, illicit opioids, and cocaine. Smoking abstinence was based on expired carbon monoxide (CO < 10 ppm), obtained three times weekly (Monday, Wednesday, Friday).30 All abstinence data reflect point-prevalence estimates at each observation time. We also obtained urine samples when carbon monoxide samples were taken to establish illicit opioid and cocaine use. Nicotine withdrawal31 and opioid withdrawal were assessed.32
Statistical Analysis
All analyses were conducted with the Statistical Analysis System Version 9.1.3.33 The current sample size of 40 was based on available resources, and the need to first determine if the combination of buprenorphine and bupropion was safe and tolerable. An additional goal was to characterize treatment effect sizes to inform subsequent power analyses for a larger trial, if warranted. Unless otherwise stated, values of p<.05 were considered statistically significant, based on two-tailed tests. Treatment retention was evaluated using Kaplan-Meier survival analysis. Abstinence from each of the three target substances, tobacco, cocaine, and opioid, together and individually, was analyzed using repeated-measures logistic regression. Abstinence data were analyzed under an intention-to-treat policy where missing observations were coded as positive for substance use. Cigarettes/day, carbon monoxide levels, and withdrawal were evaluated with repeated measures ANCOVA. In each repeated measures model, the effects of medication, time, and their interaction were assessed. Substance use was summarized into 5, biweekly periods (i.e., biweeks) while all other continuous outcomes were evaluated at the visit or weekly level. The value of the dependent measure at the initial visit (when no medication had been administered) was used as covariate. Exceptions included cocaine and opiate use, in which self-reported use in the 30 days preceding treatment was employed as the covariate.27 Type I error rate was controlled using a Tukey adjustment.
RESULTS
Demographic Characteristics
Means and frequencies are presented for the entire sample since comparisons by treatment group revealed no statistically significant differences. The sample (N = 39) was comprised primarily of men (85%), mean age 34.2 (SD = 11.2). The majority of participants were white (82%), while the balance was African-American (8%) or other (10%). Most participants had at least high school education (51%), and were employed (72%). Baseline smoking characteristics were as follows: mean smoking rate, 23.8 cigarettes/day (SD = 10.7); CO level 14.6 ppm (SD = 8.3), and FTND score, 5.2 (SD = 2.6). All participants met DSM-IV lifetime criteria for opioid dependence, with 18.1 days (SD = 14.5) use in the 30 days preceding treatment. A 25% minority of participants also met DSM-IV lifetime criteria for cocaine dependence, with 1.1 days (SD =2.0) use in the 30 days preceding treatment.
Adverse Events
Fewer BUPRE+PBO participants were administratively discharged for 3 consecutive missed-medication appointments (n = 2) than BUPRE+BUPRO participants (n = 5). Of the 4 remaining non-completers in the BUPRE+BUPRO group, 1 moved during the study and 3 were related to adverse events. The 3 adverse events included pancreatitis (n = 1), agitation (n = 1) and “disliked medication” (n = 1).
Retention
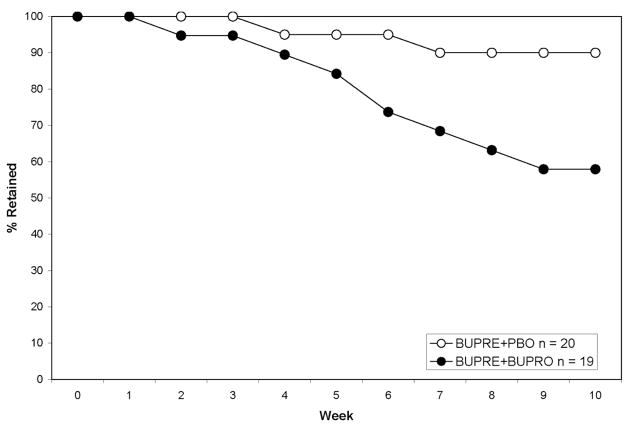
Survival analysis indicated that those treated in the BUPRE+BUPRO condition were retained at a lower rate (58%) than those in the BUPRE+PBO (90%), during the 10-week treatment period, Log Rank Statistic = 5.09, d.f. = 1, p = .0241 (see Figure 1).
Figure 1.
Retention rates by medication group in the 10-week treatment period. Fewer participants in the BUPRE+BUPRO condition were retained at a lower rate (58%) than those in the BUPRE+PBO (90%), p = .0241.
Substance Use Outcomes
Combined Abstinence
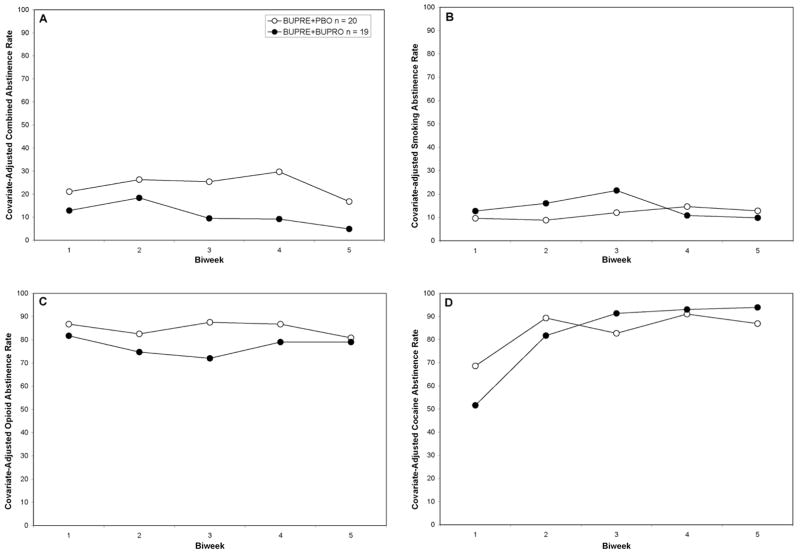
Combined abstinence rates for smoking, opioid use, and cocaine use during the 10-week treatment phase did not differ, by medication, χ2(1) = 2.76, p = .10, biweek, χ2(4) = 7.14, p = .13, or their interaction, χ2(4) = 2.87, p = .58 (see Figure 2, Panel B). Overall combined abstinence rates were BUPRE+PBO (23.5%) and BUPRE+BUPRO (10.1%).
Figure 2.
Panel A. Combined abstinence rates in the 10-week treatment period, adjusted for baseline CO-level as well as days of illicit opioid and cocaine use in the 30-days preceding enrollment.
Panel B. Smoking abstinence rates in the 10-week treatment period, adjusted for baseline CO-level.
Panel C. Illicit opioid abstinence rates in the 10-week treatment period, adjusted for days of illicit opioid use in the 30-days preceding enrollment.
Panel D. Cocaine abstinence rates in the 10-week treatment period, adjusted for days of cocaine use in the 30-days preceding enrollment. Abstinence rates differed as function of biweek, p<.001 and medication × biweek. p = .0259.
Cigarette Smoking
Abstinence rates during the 10-week treatment phase did not differ, by medication, χ2(1) = .28, p = .50, biweek, χ2(4) = 1.53, p = .82, or their interaction, χ2(4) = 3.31, p = .51 (see Figure 2, Panel B). Overall smoking abstinence rates were BUPRE+PBO (11.4%) and BUPRE+BUPRO (13.7%). A sensitivity analysis was undertaken defining abstinence as CO less than 8 and 3 ppm, respectively.30, 34 Although the relative superiority of BUPRE+BUPRO to BUPRE+PBO persisted, no statistically reliable differences were detected.
Opioid Use
Although some evidence for a medication effect was observed, no effects were statistically significant, χ2(1) = 0.70, p =. 40, biweek, χ2(4) = 2.82, p = .59 or their interaction were seen χ2(4) = 6.91, p = .14 (see Figure 2, Panel C). Overall illicit opioid abstinence rates were BUPRE+PBO (85.0%) and BUPRE+BUPRO (77.5%).
Cocaine Use
Cocaine use tended to decline in both groups over time, biweek, χ2(4) = 25.0, p <.0001 (see Figure 2, Panel D). However, the trends in reduction differed slightly by medication group, medication × biweek, χ2(4) = 11.1, p = .0259. No main effect of medication was seen, χ2(1) = .06, p = .8046. Overall cocaine abstinence rates were BUPRE+PBO (85.0%) and BUPRE+BUPRO (86.3%).
Other Outcomes
CO Reduction
Carbon monoxide levels did not change as function of treatment, time, or their interaction. Overall, carbon monoxide levels were BUPRE+PBO (M = 14.4, SE = .68) and BUPRE+BUPRO (M = 12.9, SE = .71).
Smoking Reduction
Cigarettes per day tended to decline somewhat during the treatment period, week, F(9,277) = 9.09, p <.0001, but no effects of medication, or medication × week. Daily smoking rates were BUPRE+PBO (M = 14.2, SE = 1.4) and BUPRE+BUPRO (M = 15.6, SE = 1.5).
Nicotine and Opioid Withdrawal
Nicotine withdrawal tended to increase in week 1 of treatment, before declining to pre-quit levels, week, F(9,291) = 4.84, p <.0001, but no effects of medication, or their interaction. Opioid withdrawal also tended to peak in week 1 of smoking cessation, after which it rapidly declined, week, F(9,293) = 10.2, p <.0001.
DISCUSSION
In this study, we conducted a 10-week, randomized, placebo-controlled trial of bupropion in combination with buprenorphine for concurrent treatment of tobacco, opioid, and cocaine addiction in opioid addicted cigarette smokers. Participants were provided monetary reinforcement contingent on providing biochemical samples negative for carbon monoxide, opioids, and cocaine. A key observation in this study was that those treated with bupropion were significantly less likely to complete treatment. In addition, bupropion was not more effective than placebo for abstinence from tobacco, opioids, or cocaine in buprenorphine stabilized patients.
We observed that those taking bupropion and buprenorphine were significantly less likely to complete the 10-week course of treatment (58%) vs. (90%). In non-opioid dependent samples of smokers, discontinuation rates in bupropion treated smokers have been between 6–12%.35 Richter and coworkers15, evaluated bupropion as a smoking cessation treatment in methadone-maintained patients. Although no placebo control group was included, bupropion was well tolerated, with just 4% of their sample discontinuing treatment due side effects. Two placebo-controlled trials of bupropion in MMT opioid dependent patients, where treatment was targeted primarily at cocaine abstinence, found no significant differences between bupropion and placebo-treated participants in terms of treatment completion20, 22 While the nature of the adverse events that precipitated discontinuation were not clearly related to bupropion, this level of differential attrition would not be clinically acceptable.
Few randomized, controlled trials of smoking cessation interventions in opioid dependent smokers have been published8, 16, 36, all in MMT patients. In the current study, overall abstinence rates fell between those seen in these earlier studies, BMT+PBO (11.4%) and BMT+BUP (13.7%). In the two studies using pharmacotherapy (i.e., nicotine patch with no placebo control), one found a significant effect of contingency management16, while a more recent study found no effect of a tailored intervention including a motivational intervention, skills counseling, and relapse prevention.36 In the former trial, 7-day, CO-confirmed abstinence rates averaged nearly 25% in CM-treated participants in the 12-week treatment period, while overall abstinence rates in the second study did not exceed 7%. Effective smoking cessation treatments for opioid dependent smokers remain elusive.
Unlike these earlier reports, we targeted not only smoking for abstinence, but also illicit opioid and cocaine use. We elected to promote cessation of all three substances since cessation of tobacco alone might have been undermined by the use of opioids or cocaine. A recent study by Peirce et al.37 demonstrated that contingent reinforcement of stimulant and alcohol abstinence in MMT patients increased abstinence from these drugs of abuse as well as illicit opioids. The issue of timing of smoking cessation in dually addicted smokers remains open to consideration.38 Attempting to quit smoking while still using drugs of abuse, that cause behavioral disinhibition or which kindle the desire for nicotine, is likely impractical. The benefits of simultaneously quitting tobacco and opioids (as well as other illicit drugs) versus delaying treatment for smoking cessation after opioid addiction is stabilized needs to be further examined
This preliminary study had several limitations. First, this preliminary study was designed to assess safety and tolerability, as well as effect sizes for design of future studies, if warranted, and thus had limited statistical power. Second, we only evaluated participants newly started and stabilized on buprenorphine, and thus the question of bupropion’s efficacy remains unassessed in those receiving buprenorphine maintenance therapy. Third, only one dose of bupropion was used and it is possible that lower doses of bupropion may have had cessation efficacy with reduced side effects c.f., 39. Forth, the CM intervention was relatively simple and use of escalating or intermittent schedules of reinforcement, with larger magnitudes, might have produced statistically and clinically significant effects. Finally, participants were not followed-up after treatment, and thus the durability of treatment effects, if any, was not assessed.
In summary, the current study was a preliminary effort to evaluate the safety, tolerability, and efficacy of bupropion, in combination with buprenorphine, for concurrent treatment of opioid and tobacco addiction. While the current findings do not support this treatment, only a larger trial with adequate statistical power could definitively exclude this combination therapy. However, safety and tolerability issues raised here do not support such a study. There continues to be lack of effective, evidence-based smoking cessation treatments in opioid dependent individuals.
Acknowledgments
This research was supported by National Institute on Drug Abuse (NIDA) grant P50-DA18197 and the VA New England MIRECC. Reckitt Benckiser provided an unrestricted grant of buprenorphine for this study. Individual authors were supported by individual career development awards: K01-DA-019446 (MM), K05-DA0454 (TRK), and K12-00167 (MS). We thank the participants for taking part in this study.
References
- 1.Kalman D, Morissette SB, George TP. Co-morbidity of smoking in patients with psychiatric and substance use disorders. Am J Addict. 2005;14(2):106–123. doi: 10.1080/10550490590924728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCool RM, Richter KP, Choi WS. Benefits of and barriers to providing smoking treatment in methadone clinics: findings from a national study. Am J Addict. 2005;14(4):358–366. doi: 10.1080/10550490591003693. [DOI] [PubMed] [Google Scholar]
- 3.Pomerleau OF. Endogenous opioids and smoking: a review of progress and problems. Psychoneuroendocrinology. 1998;23(2):115–130. doi: 10.1016/s0306-4530(97)00074-7. [DOI] [PubMed] [Google Scholar]
- 4.Mello NK, Lukas SE, Mendelson JH. Buprenorphine effects on cigarette smoking. Psychopharmacology (Berl) 1985;86(4):417–425. doi: 10.1007/BF00427902. [DOI] [PubMed] [Google Scholar]
- 5.Mello NK, Mendelson JH, Sellers ML, Kuehnle JC. Effects of heroin self-administration on cigarette smoking. Psychopharmacology (Berl) 1980;67(1):45–52. doi: 10.1007/BF00427594. [DOI] [PubMed] [Google Scholar]
- 6.Richter KP, Hamilton AK, Hall S, Catley D, Cox LS, Grobe J. Patterns of smoking and methadone dose in drug treatment patients. Exp Clin Psychopharmacol. 2007;15(2):144–153. doi: 10.1037/1064-1297.15.2.144. [DOI] [PubMed] [Google Scholar]
- 7.Schmitz JM, Grabowski J, Rhoades H. The effects of high and low doses of methadone on cigarette smoking. Drug Alcohol Depend. 1994;34(3):237–242. doi: 10.1016/0376-8716(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 8.Story J, Stark MJ. Treating cigarette smoking in methadone maintenance clients. J Psychoactive Drugs. 1991;23(2):203–215. doi: 10.1080/02791072.1991.10472237. [DOI] [PubMed] [Google Scholar]
- 9.Spiga R, Schmitz J, Day J., 2nd Effects of nicotine on methadone self-administration in humans. Drug Alcohol Depend. 1998;50(2):157–165. doi: 10.1016/s0376-8716(98)00020-9. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert DG, Meliska CJ, Williams CL, Jensen RA. Subjective correlates of cigarette-smoking-induced elevations of peripheral beta-endorphin and cortisol. Psychopharmacology (Berl) 1992;106(2):275–281. doi: 10.1007/BF02801984. [DOI] [PubMed] [Google Scholar]
- 11.Meliska CJ, Gilbert DG. Hormonal and subjective effects of smoking the first five cigarettes of the day: a comparison in males and females. Pharmacol Biochem Behav. 1991;40(2):229–235. doi: 10.1016/0091-3057(91)90544-c. [DOI] [PubMed] [Google Scholar]
- 12.Pomerleau OF, Fertig JB, Seyler LE, Jaffe J. Neuroendocrine reactivity to nicotine in smokers. Psychopharmacology (Berl) 1983;81(1):61–67. doi: 10.1007/BF00439275. [DOI] [PubMed] [Google Scholar]
- 13.Seyler LE, Jr, Pomerleau OF, Fertig JB, Hunt D, Parker K. Pituitary hormone response to cigarette smoking. Pharmacol Biochem Behav. 1986;24(1):159–162. doi: 10.1016/0091-3057(86)90062-6. [DOI] [PubMed] [Google Scholar]
- 14.Stein MD, Weinstock MC, Herman DS, Anderson BJ, Anthony JL, Niaura R. A smoking cessation intervention for the methadone-maintained. Addiction. 2006;101(4):599–607. doi: 10.1111/j.1360-0443.2006.01406.x. [DOI] [PubMed] [Google Scholar]
- 15.Richter KP, McCool RM, Catley D, Hall M, Ahluwalia JS. Dual pharmacotherapy and motivational interviewing for tobacco dependence among drug treatment patients. J Addict Dis. 2005;24(4):79–90. doi: 10.1300/j069v24n04_06. [DOI] [PubMed] [Google Scholar]
- 16.Shoptaw S, Rotheram-Fuller E, Yang X, et al. Smoking cessation in methadone maintenance. Addiction. 2002;97(10):1317–1328. 1325. doi: 10.1046/j.1360-0443.2002.00221.x. [DOI] [PubMed] [Google Scholar]
- 17.Shoptaw S, Jarvik ME, Ling W, Rawson RA. Contingency management for tobacco smoking in methadone-maintained opiate addicts. Addict Behav. 1996;21(3):409–412. doi: 10.1016/0306-4603(95)00066-6. [DOI] [PubMed] [Google Scholar]
- 18.Boothby LA, Doering PL. Buprenorphine for the treatment of opioid dependence. Am J Health Syst Pharm. 2007;64(3):266–272. doi: 10.2146/ajhp060403. [DOI] [PubMed] [Google Scholar]
- 19.Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2003;(2):CD002207. doi: 10.1002/14651858.CD002207. [DOI] [PubMed] [Google Scholar]
- 20.Margolin A, Kosten TR, Avants SK, et al. A multicenter trial of bupropion for cocaine dependence in methadone-maintained patients. Drug Alcohol Depend. 1995;40(2):125–131. doi: 10.1016/0376-8716(95)01198-6. [DOI] [PubMed] [Google Scholar]
- 21.Margolin A, Kosten T, Petrakis I, Avants SK, Kosten T. Bupropion reduces cocaine abuse in methadone-maintained patients. Arch Gen Psychiatry. 1991;48(1):87. doi: 10.1001/archpsyc.1991.01810250089015. [DOI] [PubMed] [Google Scholar]
- 22.Poling J, Oliveto A, Petry N, et al. Six-month trial of bupropion with contingency management for cocaine dependence in a methadone-maintained population. Arch Gen Psychiatry. 2006;63(2):219–228. doi: 10.1001/archpsyc.63.2.219. [DOI] [PubMed] [Google Scholar]
- 23.Poling J, Pruzinsky R, Kosten TR, et al. Clinical efficacy of citalopram alone or augmented with bupropion in methadone-stabilized patients. Am J Addict. 2007;16(3):187–194. doi: 10.1080/10550490701375640. [DOI] [PubMed] [Google Scholar]
- 24.Carroll KM, Kosten TR, Rounsaville BJ. Choosing a behavioral therapy platform for pharmacotherapy of substance users. Drug Alcohol Depend. 2004;75(2):123–134. doi: 10.1016/j.drugalcdep.2004.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 26.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID -I/P, Version 2.0) Biometric Research Department; NY: 1995. [Google Scholar]
- 27.McLellan AT, Kushner H, Metzger D, et al. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9(3):199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 28.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 29.Aickin M. A program for balancing the allocation of subjects to treatment in a clinical trial. Comput Biomed Res. 1982;15(6):519–524. doi: 10.1016/0010-4809(82)90014-3. [DOI] [PubMed] [Google Scholar]
- 30.Benowitz NL, Jacob P, Ahijevych K, et al. Biochemical verification of tobacco use and cessation. Nicotine and Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 31.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43(3):289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 32.Bradley BP, Gossop M, Phillips GT, Legarda JJ. The development of an opiate withdrawal scale (OWS) Br J Addict. 1987;82(10):1139–1142. doi: 10.1111/j.1360-0443.1987.tb03294.x. [DOI] [PubMed] [Google Scholar]
- 33.The SAS System for Windows [computer program]. Version 9.13. Cary, NC: SAS Institute Inc.; 2006. [Google Scholar]
- 34.Javors MA, Hatch JP, Lamb RJ. Cut-off levels for breath carbon monoxide as a marker for cigarette smoking. Addiction. 2005;100(2):159–167. doi: 10.1111/j.1360-0443.2004.00957.x. [DOI] [PubMed] [Google Scholar]
- 35.Aubin HJ. Tolerability and safety of sustained-release bupropion in the management of smoking cessation. Drugs. 2002;62(Suppl 2):45–52. doi: 10.2165/00003495-200262002-00005. [DOI] [PubMed] [Google Scholar]
- 36.Stein MD, Anderson BJ, Niaura R. Nicotine replacement therapy - Patterns of use after a quit attempt among methadone-maintained smokers. Journal of General Internal Medicine. 2006;21(7):753–757. doi: 10.1111/j.1525-1497.2006.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peirce JM, Petry NM, Stitzer ML, et al. Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment: a National Drug Abuse Treatment Clinical Trials Network study. Arch Gen Psychiatry. 2006;63(2):201–208. doi: 10.1001/archpsyc.63.2.201. [DOI] [PubMed] [Google Scholar]
- 38.Richter KP. Good and bad times for treating cigarette smoking in drug treatment. Journal of Psychoactive Drugs. 2006;38(3):311–315. doi: 10.1080/02791072.2006.10399857. [DOI] [PubMed] [Google Scholar]
- 39.Hurt RD, Sachs DP, Glover ED, et al. A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med. 1997;337(17):1195–1202. doi: 10.1056/NEJM199710233371703. [DOI] [PubMed] [Google Scholar]