Abstract
Group B Streptococcus (GBS) is a common cause of neonatal sepsis and meningitis. A major GBS virulence determinant is its sialic acid (Sia)-capped capsular polysaccharide (CPS). Recently, we discovered the presence and genetic basis of capsular Sia O-acetylation in GBS. We now characterize a GBS Sia O-acetylesterase that modulates the degree of GBS surface O-acetylation. The GBS Sia O-acetylesterase operates cooperatively with the GBS CMP-Sia synthetase, both part of a single polypeptide encoded by the neuA gene. NeuA de-O-acetylation of free 9-O-acetyl-N-acetylneuraminic acid (Neu5,9Ac2) was enhanced by CTP and Mg2+, the substrate and co-factor respectively of the N-terminal GBS CMP-Sia synthetase domain. In contrast, the homologous bi-functional NeuA esterase from E. coli K1 did not display cofactor dependence. Further analyses showed that in vitro, GBS NeuA can operate via two alternate enzymatic pathways: de-O-acetylation of Neu5,9Ac2, followed by CMP-activation of Neu5Ac; or, activation of Neu5,9Ac2, then de-O-acetylation of CMP-Neu5,9Ac2. Consistent with in vitro esterase assays, genetic deletion of GBS neuA led to accumulation of intracellular O-acetylated Sias, and over-expression of GBS NeuA reduced O-acetylation of Sias on the bacterial surface. Site-directed mutagenesis of conserved asparagine residue 301 abolished esterase activity, but preserved CMP-Sia synthetase activity, as evidenced by hyper-O-acetylation of CPS Sias on GBS expressing only the N301A NeuA allele. These studies demonstrate a novel mechanism regulating the extent of capsular Sia O-acetylation in intact bacteria, and provide a genetic strategy for manipulating GBS O-acetylation, in order to explore the role of this modification in GBS pathogenesis and immunogenicity.
N-acetylneuraminic acid (Neu5Ac) is the most common type of sialic acid (Sia) found in nature, and is present as an outer modification on the surfaces of all vertebrate cells, where it plays important roles in biology, evolution and disease (1, 2). A subset of pathogenic bacteria also express terminal Neu5Ac residues, (hereafter also referred to simply as Sia), using a biosynthetic pathway evolutionarily related to that of the vertebrate host. Sia biosynthesis in bacteria appears ancient (1); yet, only some bacteria endogenously express this carbohydrate structure (3). Bacterial Sia expression is related to several distinct virulence phenotypes, including complement evasion (4–7), intracellular survival (8), and biofilm formation (9). However, the precise mechanistic underpinnings of Sia-dependent bacterial virulence phenotypes remain largely uncharacterized.
Group B Streptococcus (GBS), or Streptococcus agalactiae, is the leading cause of neonatal sepsis and has become increasingly associated with invasive infections in elderly and immune-compromised individuals (10). The most extensively studied and widely appreciated virulence factor of GBS is its Sia-capped capsular polysaccharide (CPS). To date, the structures of nine antigenically distinct GBS CPS serotypes have been described, all of which express terminal α2–3-linked Neu5Ac (11). Animal models of infection confirm that GBS mutants in the Sia biosynthetic pathway are severely attenuated for virulence (12).
In addition to being a virulence determinant, the CPS is also an important target of both innate (lectin-mediated complement) and adaptive (IgG) host immune responses against the pathogen (13, 14). Recently, it was shown that bacterial Sias can facilitate interactions with certain host Sia-binding proteins called Siglecs (15, 16), a phenomenon that could potentially favor pathogen or host, depending on several variables. In short, the sialylated CPS of GBS represents both an important host-pathogen interface and a relevant model for studying Sia-dependent bacterial interactions with the immune system.
Recently we described the biochemical and genetic basis of capsular Sia O-acetylation in GBS (17, 18), a modification unrecognized during earlier GBS capsule studies. The O-acetyl modification of Sias and related molecules is not unique to GBS; rather, it is characteristic of many bacterial pathogens (17, 19–26) as well as many vertebrate cell types (27–32). Sia O-acetylation influences several Sia-dependent functions of vertebrate cells, including apoptotic regulation (33), susceptibility to the alternative pathway of complement, and binding to leukocyte-expressed Sia-binding proteins (34). Given the effects of this modification on vertebrate cell-cell interactions, we hypothesize that Sia O-acetylation on the bacterial surface is also likely to modulate interactions between GBS and host. A combined biochemical and genetic approach for manipulating Sia-O-acetyl regulation in GBS is necessary to address these and other pertinent questions about Sia-dependent virulence at the host pathogen interface.
Biosynthesis of sialylated structures requires activation of the Sia monomer by transfer of the α-phosphate of CTP to the anomeric oxygen of Neu5Ac (35). Synthesis of CMP-Neu5Ac is catalyzed by a cytidyltransferase (CMP-Sia synthetase). Some bacterial CMP-Sia synthetases have an additional C-terminal domain related to the GSDL family of hydrolases. In GBS and Escherichia coli K1, these two-domain CMP-Sia synthetases are encoded by the neuA gene, and were recently shown to possess esterase functionality in vitro (36–38). Initial experiments on the bi-functional enzymes employed pNP-acetate and (acetylated) platelet activating factor (PAF) as substrates of the esterase reaction (36, 37). More recently, work by Vimr et. al. suggests that in E. coli, the native substrate of the NeuA esterase domain is O-acetylated Sia (38). Based on these studies and our earlier observations, we hypothesized that GBS NeuA acts natively as a Sia-O-acetylesterase - an intracellular process that could mitigate overall O-acetylation of the sialylated capsule in vivo.
To test this hypothesis, we used a defined in vitro system to explore mechanistic aspects of Sia-O-acetyl removal, coupled with live bacterial models to study the biochemical phenotypes resulting from genetic deletion, over-expression, and site-directed enzymatic inactivation of the NeuA esterase in GBS. Taken together, these studies demonstrate that the bifunctional GBS NeuA can act through two different enzymatic pathways, and show for the first time that O-acetyl removal prior to capsular assembly is an important regulatory mechanism controlling the extent of capsular O-acetylation in intact bacteria.
EXPERIMENTAL PROCEDURES
Purification of recombinant NeuA enzymes
Full-length neuA homologs were cloned into pET15b or pET22b(+) vectors from GBS serotype V strain 2603V/R, N. meningitidis group B strain MC58, and E. coli K1 strain ATCC13027 as previously described. C-terminal hexahistidine-tagged proteins were expressed in IPTG-induced E. coli BL21 cells and purified by nickel-nitrilotriacetic acid-agarose chromatography (39). Enzymes were stored at 4 °C in Tris-HCl buffer (20 mM, pH 7.5) containing 10% glycerol. We observed some variability in overall GBS enzyme activity between enzyme batches over time. Findings were confirmed using at least two different batches of GBS enzyme.
Chemoenzymatic synthesis of Neu5,9Ac2 and CMP-Neu5,9Ac2
6-O-Acetyl ManNAc (ManN2,6Ac2) was prepared as previously described (40). Briefly, 200 mg ManNAc was dissolved in a mixture of CH3CN and DMSO (3:1, v/v, 6 ml). Trifluoroethyl acetate (1 ml) and Protease N (100 mg, Amano Enzyme Inc.) were added and the suspension was stirred vigorously at 45 °C for 2 days. Protein was then removed by filtration and the filtrate was concentrated on a rotary evaporator. The resulting residue was purified by flash column chromatography (EtOAc:MeOH = 5:1 v/v) using Silica gel 60 Å (40–63 μm, Sorbent technologies) to yield ManN2,6Ac2 (121 mg, 51% yield).
Neu5,9Ac2 was subsequently prepared in a Tris-HCl (100 mM pH 7.5) buffer containing MgCl2 (20 mM), 6-O-acetyl ManNAc (10 mM), sodium pyruvate (50 mM), and the recombinant Sia aldolase from E. coli K12 ATCC47076 (0.2 mg/ml) (39). The reaction mixture was incubated in an isotherm incubator at 37 °C with 140 rpm agitation for 2 h. The reaction was stopped by adding EtOH when TLC (EtOAc:MeOH:H2O:HOAc = 4:2:2:0.2, v/v) analysis indicated the completion of the reaction. The precipitates in the reaction mixture were removed by centrifugation. The supernatant was concentrated and purified by BioGel P-2 gel filtration chromatography to yield Neu5,9Ac2 (60%) as white solid after lyophilization. 1H NMR (300 MHz, D2O) δ 4.31 (dd, 1 H, J = 3.0, 12.0 Hz), 4.14 (dd, 1 H, J = 5.7. 11.4 Hz), 3.86–4.01 (m, 2 H), 3.67–3.70 (m, 1 H), 3.57 (d, 1 H, J = 6.6 Hz), 2.20 (dd, 1 H, J = 4.5, 12.9 Hz), 2.08 (s, 3 H), 2.02 (s, 3 H), 1.79 (t, 1 H, J = 12.9 Hz); 13C NMR (75 MHz, D2O) δ 176.62, 174.83, 174.53, 96.60, 70.21, 68.27, 67.91, 67.26, 66.34, 52.26, 39.40, 22.19, 20.35. N-Acetylmannosamine, EtOAc, and trifluoroethyl acetate were purchased form Sigma. CH3CN, DMSO, MeOH, HOAc, Tris, and MgCl2 were from Fisher. The presence of small quantities of Neu5,7Ac2 and Neu5,8Ac2 seen by HPLC (Fig. 1A) and likely by NMR (see left shoulder of 9-OAc peak in Fig 3A) probably reflect an equilibrium of O-acetyl migration along the exocyclic 3-carbon side-chain of N-acetylneuraminic acid (41).
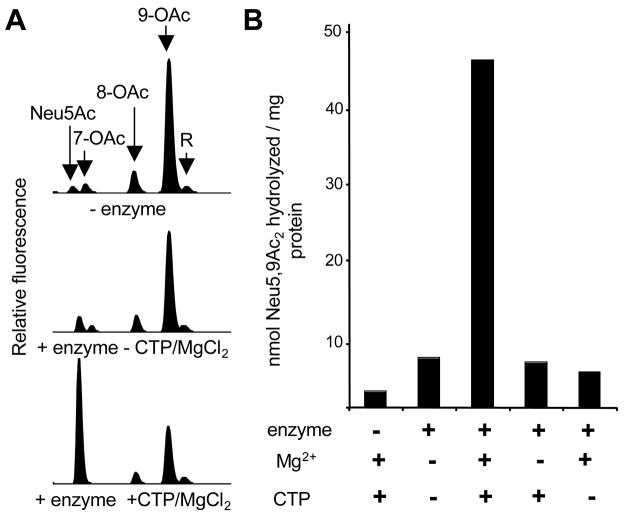
Figure 1. Purified GBS NeuA hydrolyzes 9-O-acetylated Neu5Ac in a CTP- and Mg2+-dependent manner.
His-tagged GBS NeuA was purified and 9-O-acetylated Neu5Ac (“Neu5,9Ac2” or “9-OAc”) was synthesized using a chemoenzymatic approach as described in Experimental Procedures. Enzyme assays were performed in 100 mM Tris pH 7.5 with 1μM enzyme and 8 μM substrate and allowed to proceed for 40 min followed by DMB derivatization and HPLC resolution of the 9-O-acetylated substrate “9-OAc,” and non-O-acetylated product “Neu5Ac”. “R” is a reagent peak of unknown identity. A. HPLC profiles of reactions performed in the presence or absence of enzyme or CMP-Sia synthetase co-factors (CTP and Mg2+) B. Quantitation of Sia-O-acetyl hydrolysis by integration of Neu5Ac peak. Optimal NeuA esterase activity requires the presence of both CTP (5 mM) and MgCl2 (20 mM).
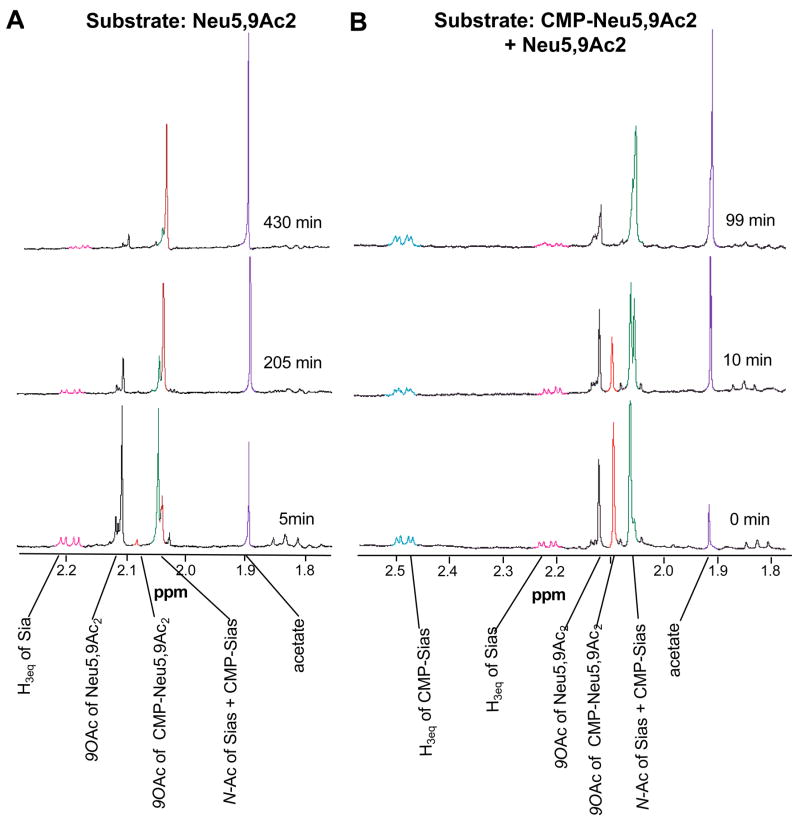
Figure 3. CMP-Neu5,9Ac2 is a direct substrate of the NeuA esterase and is de-O-acetylated more rapidly than free Neu5,9Ac2.
NeuA reactions were monitored by 600 MHz 1H NMR. Experiments were carried out at 37 °C in a total volume of 0.7 mL. Assays included 2.5 μM GBS NeuA with 2.5 mM substrate, CTP and MgCl2 in 20 mM deuterated phosphate buffer pH 7.5. About 5 min elapsed between the addition of the enzyme, and the acquisition of the first time point (defined as t = 0 min). NMR spectra were acquired every 2 minutes. Data shown are representative of the trends observed throughout the reaction. A. Neu5,9Ac2 alone was used as substrate. B. Enzymatically synthesized CMP-Neu5,9Ac2 was used as substrate, but contained a significant amount of free Neu5,9Ac2 allowing internal comparison.
CMP-Neu5,9Ac2 was synthesized from 6-O-acetyl ManNAc using the Sia aldolase and NeuA of N. meningitidis (the latter does not possess a C-terminal esterase domain) as reported (39) except that a Tris-HCl buffer of pH 7.5 was used instead of pH 8.5. The product CMP-Neu5,9Ac2 was stored as a lyophilized powder at −80 °C. This species was not completely stable and was broken down spontaneously during the lyophilization and storage to form some CMP and Neu5,9Ac2 (42). DMB derivatization with or without NaBH4 treatment, (as described below), showed that the CMP-Neu5,9Ac2 used in our assays contained approximately 50% Neu5,9Ac2.
Preparation of [9-O-acetyl-3H]Neu5,9Ac2
Radioactive substrate for NeuA esterase assays was prepared as previously described (43). Briefly, rat liver golgi vesicles were prepared by the method of Leelevathi, (44) and subsequently labeled with [acetyl-3H]acetyl-coenzymeA, of which 75–85% was incorporated into O-acetylated Neu5Ac (43). Sias were released by treatment with Arthrobacter urafaciens sialidase (AUS) and further purified using Dowex-1 and Dowex 3×4A ion exchange resins as previously described. Finally, any O-acetylation existing at the 7-carbon position of Sia was induced to migrate to the 9-carbon position by incubation of the purified Sia pool in 20 mM ammonium hydroxide at 37 °C for 30 min followed by rapid evaporation.
DMB derivatization and HPLC analysis of Sias
Sias were derivatized in 7 mM DMB (1,2-diamino-4,5-methylene dioxybenzene), 18 mM sodium hydrosulfite, 1.4 M acetic acid, and 0.7 M 2-mercaptoethanol for 2 h at 50 °C in the dark. Typically, fresh 2 × DMB reagent was added to an equal volume of released Sias or reaction mixture. Derivatized Sias were resolved on a reverse phase C18 Microsorb-MV column (Varian, 4.6-mm internal diameter, 25 cm, 5 μm) using a Rainin Dynamax SD-200 HPLC, and a 50-min isocratic elution in 8% acetonitrile and 7% methanol in water at a flow rate of 0.9 ml/min (45). C18 separation of Sias was followed by fluorescence detection using a Jasco FP-2020 Plus Intelligent Fluorescence Detector. Standard Sias prepared from bovine submaxillary mucin as previously described (46) were derivatized in parallel for peak assignment. Integration of HPLC peaks was performed using Rainin R software, allowing calculation of O-acetylation as a percent of total Sias.
NeuA esterase assays
Purified NeuA enzyme was incubated at a concentration of 1–4 μM with Neu5,9Ac2 or [9-O-acetyl-3H]Neu5,9Ac2 in 100 mM Tris-HCl, pH 7.5 in the presence or absence of 5 mM CTP and 20 mM MgCl2 at 37 °C. Control reactions in the absence of enzyme or in the presence of an equimolar concentration of BSA showed little background hydrolysis. When (nonradioactive) Neu5,9Ac2 was used, reactions were quenched at the designated time interval by addition of 2 × DMB derivatization reagent (see below) followed by incubation at 50 °C for 2 h, then HPLC separation, and quantitation of starting material (Neu5,9Ac2) and reaction product (Neu5Ac). When [9-O-acetyl-3H]Neu5,9Ac2 was used, reactions were performed with 7,000–12,000 cpm of substrate (3–4 μM final concentration) and were quenched with an equal volume of 125 mM chloroacetic acid dissolved in a 1:1 mixture of 1 M NaOH and 4 M NaCl. Reactions to assess linearity of hydrolysis with varying enzyme concentration were performed in separate tubes; however, a single large reaction was set up to measure hydrolysis over time, with removal of reaction aliquots at various time intervals. Quenched reactions were allowed to sit on ice for 15 min, followed by a 5 min high-speed centrifugation step to remove any precipitated protein. Typically, 80/100 μl of quenched reaction were added to 10 ml of scintillation cocktail consisting of 0.5% PPO (2,5-Diphenyloxazole) and 0.03% POPOP (both from USB, Cleveland OH) dissolved in toluene with subsequent addition of 20% isoamyl alcohol. Released (protonated) free acetate is taken into the organic phase scintillation cocktail, whereas the non-hydrolyzed starting material remains in the aqueous phase of the reaction mixture and is not counted. This is based on an assay originally reported for analysis of acetylcholinesterase activity (47).
Reduction of free Sias in reaction products with sodium borohydride (NaBH4)
CMP-Sias are cleaved under the acidic conditions of DMB derivatization. For this reason, the DMB-HPLC method alone cannot distinguish between free and CMP-activated Sias produced during the NeuA reaction. We modified a previously described method (48) to detect CMP-activated Sias. Products of the NeuA reaction (~400 pmol) were treated with 80 mM sodium borohydride at room temperature for 20 min, then quenched with the addition of 5 M acetic acid (1:7 by volume). The DMB reagent was added directly to NaBH4-treated or untreated samples. Parallel controls included commercially produced Neu5Ac (Pfanstiehl Laboratories, Waukegan, IL) and CMP-Neu5Ac (Sigma). In control reactions, Neu5Ac was destroyed by NaBH4 and CMP-Neu5Ac remained >90% intact. Untreated Neu5Ac was used as a standard for quantitation of CMP activation and de-O-acetylation reactions.
1H NMR assays
Experiments were carried out in a total volume of 0.7 mL using 2.5 μM GBS NeuA and 2.5 mM substrate in the presence of cofactors (5 mM CTP, 20 mM MgCl2) in 20 mM deuterated phosphate buffer pH 7.5. Spectra were acquired every 2 minutes using 600 MHz 1H NMR. Peak assignments were made by comparison with the 1HNMR spectra of standards.
Strains and culture conditions
All GBS strains are isolates from human neonates with invasive infection. GBS were propagated on Todd-Hewitt agar plates (THA) or liquid broth (THB) at 37 °C without shaking; with 5–10 μg/ml erythromycin (Erm) or 2 μg/ml chloramphenicol (Cm) selection when indicated. Allelic exchange mutagenesis of neuA was performed in WT serotype III strains COH1 and D136, and serotype Ia strain A909. In addition, NeuA over-expression was performed in serotype Ib (H36B), V (NCTC), and Ia (KL90) strains.
Release of GBS Sias
Total bacterial Sias were released using 2 M acetic acid for 3 h at 80 °C (45). Alternatively, surface Sias were released using AUS (EY Labs) in 50 mM sodium acetate for 3 h at 37 °C.
Deletion, complementation, and overexpression of NeuA in GBS
The GBS neuA knockout vector (pNeuA-KO) was constructed as described previously (17) and used for precise, in frame replacement of GBS neuA for a chloramphenicol (Cm) acetyltransferase gene (cat), which confers resistance to Cm. Briefly, wild-type (WT) GBS strains were transformed by electroporation with pNeuA-KO with selection on Erm at 30 °C. Chromosomal integration events were identified by a shift to the non-permissive temperature (37 °C) under Erm selection, followed by relaxation in the absence of antibiotics to identify double crossover mutants with Cm resistance but Erm sensitivity. Precise in-frame allelic replacement of neuA by cat in the GBS chromosome was confirmed by PCR. A plasmid bearing GBS NeuA under a constitutive promoter (pGBS-NeuA) was generated as described previously (17) and introduced by electroporation to transform WT or NeuA mutant strains.
Site-directed mutagenesis of neuA
Site-directed mutagenesis of NeuA asparagine 301 to alanine in pGBS-NeuA was accomplished with PAGE-purified primer 5′-ctataggggtagctgatctcattacagg-3′ and its reverse complement in the QuikChange reaction (Stratagene) followed by DpnI digestion to remove residual template. DNA isolated by ethanol precipitation was used to transform electrocompetent E. coli MC1061, and plasmid pGBS-NeuA-N301A was confirmed by sequencing to harbor only the single residue change in the neuA coding region.
RESULTS
GBS NeuA de-O-acetylates free Neu5,9Ac2 under conditions that maintain the NeuA CMP-Sia synthetase activity
The GBS O-acetyltransferase, NeuD, acts at the level of free intracellular Sia (17, 18). We hypothesized that the NeuA C-terminal esterase domain could act on free intracellular O-acetylated Sias to regulate capsular O-acetylation on the bacterial surface. Assays were designed to test this hypothesis in vitro, using purified recombinant GBS NeuA and synthetic 9-O-acetylated N-acetylneuraminic acid (Neu5,9Ac2). Reaction products were monitored by reverse phase HPLC after fluorescent derivatization of Sias using DMB.
Initial experiments used reaction conditions identical to those previously published for the (presumably non-native) substrates pNP-acetate and PAF (37). However, little difference was observed between HPLC profiles of the Neu5,9Ac2 substrate in the presence or absence of purified NeuA enzyme (Fig. 1A). Given the putative bi-functional nature of this enzyme, cofactors of the CMP-Sia synthetase domain (CTP and Mg2+) were added in an attempt to boost the proposed secondary activity. The new conditions did in fact, stimulate de-O-acetylation of Neu5,9Ac2 as evidenced by a NeuA-dependent increase in the Neu5Ac reaction product by DMB-HPLC (Fig. 1A). Upon removal of either CTP or MgCl2 from the reaction, esterase activity returned to near base-line levels (Fig. 1B).
Spontaneous migration and hydrolysis of Sia-O-acetyl esters has been reported in several contexts, particularly under basic conditions (17, 41). The current experiments were carried out at pH 7.5 with little background hydrolysis. De-O-acetylation did not occur in the presence of an equivalent concentration of a control protein, bovine serum albumin (data not shown). Additionally, hydrolysis of [9-O-acetyl-3H]-Neu5,9Ac2 was monitored over time and with different NeuA concentrations for further verification that the observed reaction had typical enzymatic properties, with product formation being approximately linear over time and with added enzyme (data not shown).
Taken together, these data suggested that the CMP-synthetase activity of NeuA might be required for in vitro esterase-mediated de-O-acetylation of free Neu5,9Ac2. To explore the reaction mechanism further, we monitored CMP-activation and de-O-acetylation reactions using a modified DMB-derivatization procedure, as well as by 600 MHz 1H NMR.
CMP-activation of Sia is not a prerequisite of the Sia-O-acetyl esterase reaction
Conditions of DMB derivatization result in the combined analysis of both free and CMP-activated Sias (since the CMP-Sia bond is hydrolyzed during derivatization). CMP-Sias can be distinguished from free Sias in a mixture, by selectively reducing the anomeric C-2 position of the free molecules using sodium borohydride (NaBH4) (48). Borohydride reduction of free Sias renders them invisible in the DMB derivatization, as this reaction requires the intact α-keto (C-1/C-2) moiety of Sias and related sugars. Unfortunately, sodium borohydride can also cleave O-acetyl groups on Sia. Therefore, NeuA reactions were analyzed by DMB-HPLC with or without prior borohydride treatment to compare overall levels of O-acetyl hydrolysis and CMP-activation during in vitro NeuA assays (Fig 2).
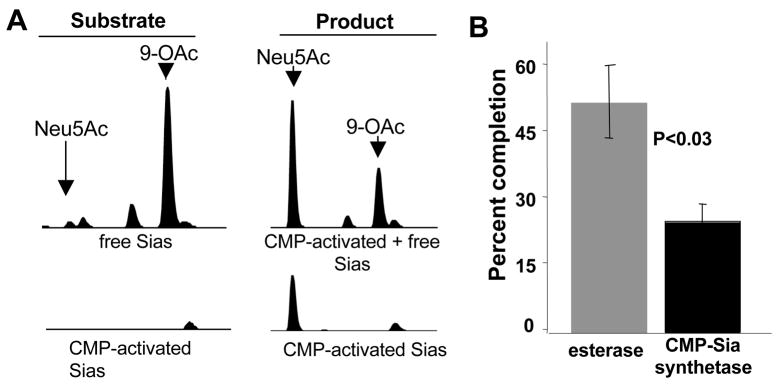
Figure 2. GBS NeuA can directly de-O-acetylate Neu5,9Ac2.
NeuA esterase and CMP-Sia synthetase activities were monitored to test the hypothesis that CTP/Mg2+ dependence is due to a processive mechanism. A. NeuA enzyme assays were performed in 100 mM Tris pH 7.5 with (right) or without (left) 2 μM enzyme in the presence of 8 μM substrate and allowed to proceed for 30 min. The CMP-Sia bond is labile under the acidic conditions of DMB derivatization, thereby resulting in combined detection of free and CMP-bound Sias (top). Half of each reaction was treated with 80 mM freshly prepared NaBH4 to destroy free Sias and detect only CMP-activated Sias (bottom). See Figure 1 legend for complete description of peaks. B. Percent completion of CMP-synthetase and esterase reactions in A. Sia-O-acetyl hydrolysis was measured as a percentage of total Sia substrate in the reaction, using integration of Neu5Ac peaks in samples +/− enzyme without NaBH4 treatment. CMP-Sia activation was likewise measured by integration of peaks in the NaBH4-treated samples. While 52% of the Neu5,9Ac2 substrate was de-O-acetylated, only 25% of the free Sias were CMP-activated. These data are representative of 2 independent experiments.
Reaction products were incubated with or without NaBH4 to reveal the degree of enzymatic de-O-acetylation (−NaBH4) and CMP-activation (+NaBH4) (Fig 2A). Quantitation of each reaction showed that when Neu5,9Ac2 is used as substrate, de-O-acetylation occurs more readily than CMP-activation (Fig. 2B). If CMP-Neu5,9Ac2 were the only substrate of the esterase, the extent of CMP-Sia formation should be greater than or equal to the extent of de-O-acetylation. The dominance of de-O-acetylation over CMP-activation suggests that NeuA-mediated hydrolysis of Neu5,9Ac2 does not require CMP-activation, and that free Neu5,9Ac2 can be a direct substrate of the esterase reaction.
GBS NeuA can activate Neu5,9Ac2, followed by de-O-acetylation of CMP-Neu5,9Ac2
Next, 600 MHz proton NMR was used to monitor the progress of NeuA reactions over time. Assays employed purified GBS NeuA with cofactors in deuterated phosphate buffer. Spectra were acquired every 2 min using 600 MHz 1H NMR to monitor reaction progress. Assays using Neu5,9Ac2 as the substrate showed immediate formation of CMP-Neu5,9Ac2 (Fig. 3A). Although CMP-Neu5,9Ac2 was observed at early time points, levels of the activated, O-acetylated molecule remained low and disappeared by 205 min (Fig 3A). It was apparent that the level of substrate Neu5,9Ac2 decreased over time; however, NMR was unable to determine whether this happened directly or via a CMP-activated O-acetylated intermediate. As expected, the de-O-acetylation reaction was mirrored by corresponding increases in the level of free acetate. The formation of CMP-Neu5Ac was also observed over time by increasing signal associated with protons at the C-3 position (not shown). These results showed that NeuA can activate Neu5,9Ac2 to CMP-Neu5,9Ac2 prior to de-O-acetylation. To determine whether this pathway (i.e. activation then de-O-acetylation) is the predominant NeuA mechanism, 1H NMR-monitoring was also performed on reactions containing both Neu5,9Ac2 and CMP-Neu5,9Ac2.
CMP-Neu5,9Ac2 is de-O-acetylated rapidly by GBS NeuA
CMP-Neu5,9Ac2 was generated enzymatically from Neu5,9Ac2 using the N. meningitidis NeuA enzyme, which does not possess esterase activity (Fig. 4B). This product was then used as a tool to study the GBS NeuA esterase reaction. Protons of the 9-O-acetyl substituent of CMP-Neu5,9Ac2 clearly decreased over time, with about half of the CMP-Neu5,9Ac2 hydrolyzed by 10 min and the remaining substrate hydrolyzed by 99 min (Fig. 3B). Notably, some Neu5,9Ac2 was also present in the starting material, allowing internal comparison of the two potential pathways. The signal arising from the 9-O-acetyl group of Neu5,9Ac2 also decreased with time, yet much of this substrate remained at 99 min (Fig. 3B). Again, de-O-acetylation was accompanied by an increasing acetate signal. Importantly, CMP hydrolysis was not observed in this reaction, as evidenced by the equatorial hydrogen on C-3 that remained on both CMP-Neu5Ac and CMP-Neu5,9Ac2 (Fig. 3B). These data showed that CMP-Neu5,9Ac2 serves as a direct substrate of the NeuA O-acetyl esterase in vitro, a reaction that appears to occur more rapidly than de-O-acetylation of free Neu5,9Ac2. Taken together, these results suggest that while there are two possible pathways in vitro, the activated molecule may be the preferred substrate in vivo.
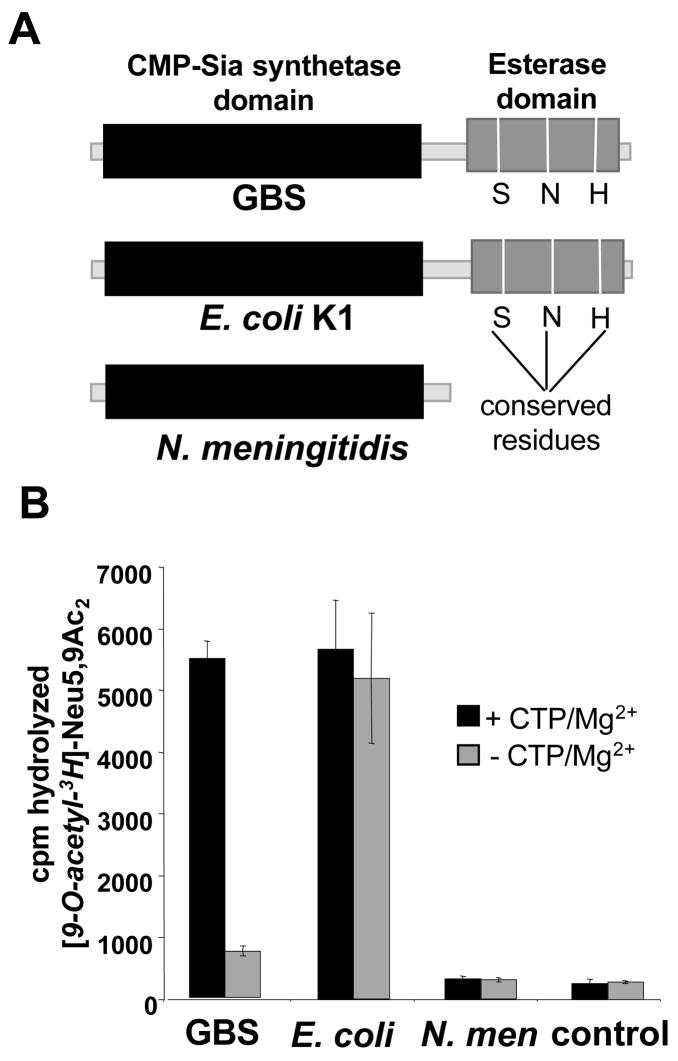
Figure 4. Divergence of co-factor dependence between the homologous bi-functional NeuA enzymes of GBS and E. coli.
A. Schematic representation of NeuA homologs in GBS, E. coli K1 and N. meningitidis. B. Radioligand hydrolysis assay comparing requirements of NeuA esterase activity between the three homologs. Reactions were performed as described in Figure 2 with (or without) 4 μM enzyme in the presence or absence of 5 mM CTP and 20 mM MgCl2. Bars represent standard deviation of 2 experiments performed on separate days.
Structure-activity comparison of NeuA homologs
Some sialylated bacterial pathogens like E. coli K1 have a NeuA enzyme composed of two domains homologous to those of GBS. Others, like serogroup B N. meningitidis, maintain only the CMP-Sia synthetase domain and are thus not expected to possess Sia-O-acetylesterase activity (Fig. 4A). Despite their overall structural similarity, we noted a striking difference between the activities of the GBS and E. coli Sia O-acetylesterases. Whereas the GBS enzyme strongly favored conditions that maintain the CMP-Neu5Ac synthetase function, the E. coli enzyme hydrolyzed [9-O-acetyl-3H]Neu5,9Ac2 equally well in the presence or absence of CTP and Mg2+. This observation suggests that the bi-functional nature of NeuA may have diverged mechanistically along the two bacterial lineages.
Analysis of GBS Sias from mutant bacteria that over-express or lack NeuA
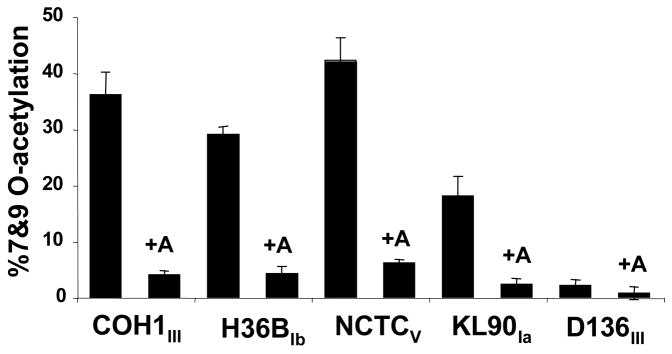
To determine whether GBS NeuA esterase activity is important for O-acetyl modification in vivo, the focus of our studies turned toward characterization of NeuA in intact bacteria. First, a plasmid construct was used to deliver and over-express GBS NeuA in five different WT GBS strains. Consistent with the observed in vitro esterase activity, fluorescent derivatization and HPLC analysis of acid-released Sias shows that overexpression of GBS NeuA resulted in decreased Sia O-acetylation in each of the GBS strains (Fig. 5).
Figure 5. Over-expression of GBS NeuA decreases Sia-O-acetylation on GBS strains of multiple serotypes.
WT GBS strains were transformed with an expression construct encoding the full-length NeuA enzyme “+A” and O-acetylation was compared to the parent strain. Total cellular Sias were isolated by mild acid hydrolysis and fluorescently derivatized with DMB as described in Experimental Procedures. Derivatized Sias were resolved by reverse phase HPLC and percent O-acetylation determined by software-assisted integration of HPLC peaks corresponding to O-acetylated and non-O-acetylated Neu5Ac peaks. HPLC profiles of the COH1 strain and corresponding NeuA-overexpressing strain were published previously (17).
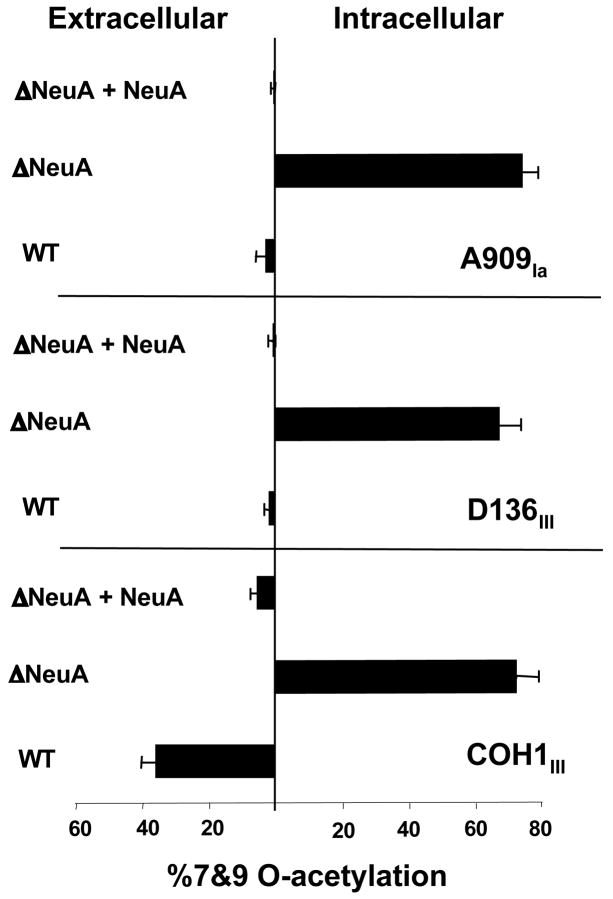
In addition to NeuA over-expression, chromosomal deletion of neuA was accomplished in three different WT GBS strains, through precise, in-frame allelic replacement of neuA with an antibiotic resistance cassette. Following DMB-HPLC analysis of total bacterial Sias, we found that deletion of neuA resulted in a significant increase in overall levels of Sia O-acetylation in all 3 strains. Consistent with the concomitant inability of GBS to activate Sias, (i.e. loss of CMP-Sia synthetase activity), all Sias in the ΔNeuA strains were found in the intracellular compartment (Fig. 6), as we have previously described (17). Complementation of the mutant strains with plasmid-expressed NeuA reversed the intracellular hyper-O-acetylated phenotype into a capsular hypo-O-acetylated phenotype (Fig. 6).
Figure 6. Deletion of GBS NeuA increases intracellular Sia-O-acetylation in both “high-OAc” and “low-OAc” strains.
Elimination of NeuA (both CMP-Sia synthestase and esterase domains) in different GBS strains was accomplished by precise allelic replacement of the neuA gene with chloramphenicol acetyltransferase (cat) to produce the ΔNeuA or “ΔA” strains as described in Experimental Procedures. Mutant strains were complemented by plasmid-based expression of NeuA (“+A”) as in Figure 4. Intracellular and extracellular Sias were separated as previously described (17) and percent O-acetylation was determined by DMB-HPLC analysis. HPLC profiles of the wild-type COH1 and corresponding ΔNeuA strain were published previously (17). Bars represent standard deviation of 2 or more independent experiments.
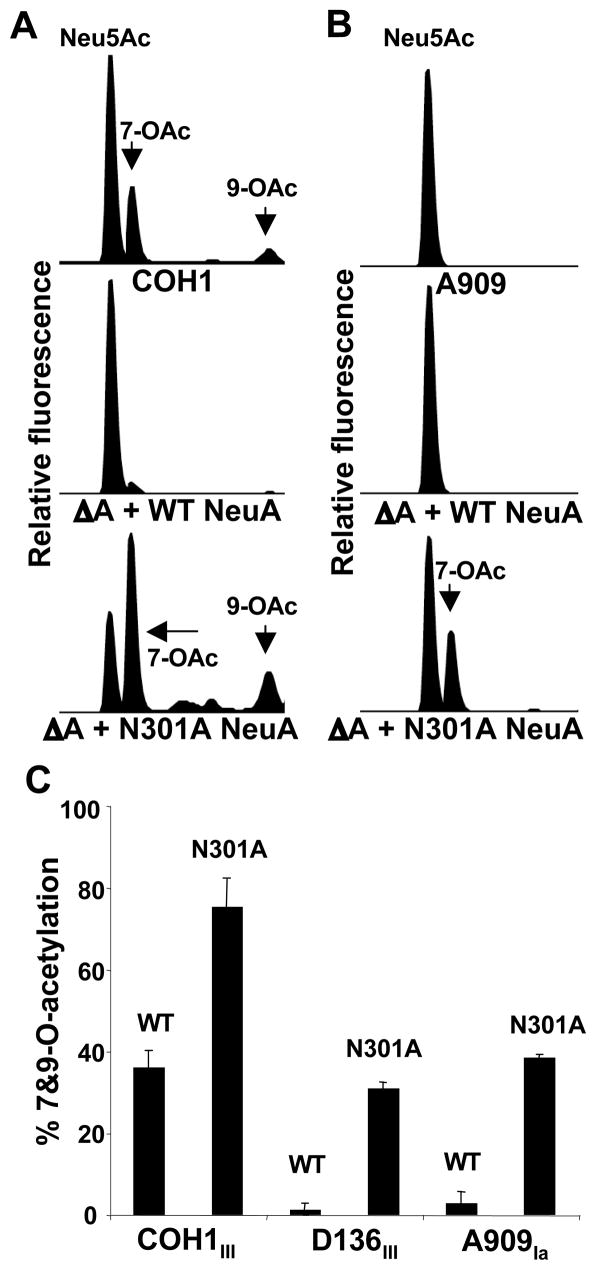
Asn301 is important for NeuA esterase activity, which limits capsular O–acetylation in intact GBS
Because of the dual domains and activities of NeuA, the above experiments cannot definitively exclude the possibility that effects of (full-length) NeuA deletion and over-expression represent a pathway artifact introduced by alteration of the CMP-Neu5Ac synthetase activity. Whereas complete neuA deletion resulted in intracellular hyper-O-acetylation with no capsular Sia, we predicted that a specific disruption of NeuA esterase functionality, while preserving CMP-Neu5Ac synthetase activity, would result in a hyper-O-acetylated surface capsule.
Initial attempts to truncate the NeuA C–terminal esterase domain also affected CMP-Sia synthetase activity, as evidenced by the lack of capsular Sia upon introduction of the truncated NeuA enzymes into the GBS neuA mutant strains (data not shown). Thus, site-directed mutagenesis was employed to target NeuA esterase activity while avoiding (or minimizing) effects on the N-terminal CMP-Sia synthetase. Others have identified conserved residues within the GDSL family of esterases that are required for esterase/lipase activity (49, 50). In order to verify an essential role for one of these residues in GBS NeuA esterase activity, the asparagine at amino acid position 301 was mutated to alanine using site-directed mutagenesis. Following direct sequence confirmation of the mutated expression vector, NeuA N301A was introduced by transformation into the GBS NeuA mutant strains. Capsular Sias were released using AUS for analysis by DMB-HPLC.
As predicted, capsular O-acetylation was significantly increased in the N301A-complemented strains (Fig. 7). These data clearly demonstrate an important role for conserved residue Asn301 in NeuA esterase activity, consistent with structural observations of E. coli thioesterase I, where the analogous residue participates in stabilization of the oxyanion generated during nucleophilic attack of the substrate (50). These data also confirm the essential role of NeuA esterase activity in limiting GBS capsular Sia-O-acetylation. Separation of intracellular (free) from extracellular (capsular) Sias as previously described (17), revealed no evidence of intracellular Sia accumulation, confirming preservation of CMP-Neu5Ac synthetase activity in the N301A-complemented strains (data not shown).
Figure 7. Active site mutagenesis of the NeuA esterase increases capsular O-acetylation.
Site-directed mutagenesis of putative esterase active site residue Asn301 was performed using the WT NeuA expression plasmid as template. “WT NeuA” and mutated “N301A NeuA” constructs were then used to complement all three GBS NeuA-deficient “ΔA” strains, followed by DMB-HPLC analysis of sialidase-released (capsular) Sias. Raw HPLC data for “WT”, “ΔA + WT NeuA”, and “ΔA + N301A NeuA” strains in the A. COH1 (high-OAc) and B. A909 (low-OAc) GBS backgrounds. C. Percent O-acetylation of N301A-complemented NeuA-deficient “ΔA + N301A NeuA” strains is substantially higher than the respective isogenic WT NeuA-complemented strains. Further experiments employing separation of intracellular and extracellular Sias from each strain validated the lack of intracellular Sia accumulation and capsular hyper-O-acetylation of the “ΔA + N301A NeuA” strains (data not shown). Bars represent standard deviation of 2 or more independent experiments.
DISCUSSION
These studies define a role for NeuA Sia O-acetylesterase activity in controlling the level of capsular Sia O-acetylation in GBS. In vitro, purified NeuA can act through two distinct pathways: de-O-acetylation of free Neu5,9Ac2, followed by activation of Neu5Ac; or, activation of free Neu5,9Ac2, then de-O-acetylation of CMP-Neu5,9Ac2. Taken together, our data suggest that CMP-Neu5,9Ac2 is the preferred substrate for the acetylesterase activity of GBS NeuA in vivo. Over-expression and deletion of neuA in GBS have the effect of decreasing and increasing total Sia-O-acetylation respectively, findings consistent with those observed in vitro esterase activity. Furthermore, site-directed mutagenesis of a conserved active-site residue within the NeuA esterase domain (N301A) abolishes esterase activity while retaining CMP-Sia synthetase activity in vivo, resulting in GBS that expresses a hyper-O-acetylated CPS. Taken together, these data conclusively demonstrate that O-acetylated Sias are a native substrate of the NeuA O-acetylesterase, which acts prior to CPS assembly, thereby modulating the degree of GBS CPS Sia-O-acetylation.
Two distinct strategies of Sia-O-acetyl regulation in GBS
WT GBS strains characterized to date modify between 5 and 55% of total Sias by O-acetylation (17). A polymorphism within the GBS O-acetyltransferase encoded by neuD is at least partially responsible for controlling the degree of O-acetylation in GBS, where 88C corresponds to the “low-O-Ac” phenotype (< 5% O-Ac) and 88F corresponds to the “high-O-Ac” phenotype (>30% Sia-O-Ac) (18).(18). Here we show that in high-O-Ac GBS strains, NeuA prevents even greater levels O-acetylation (> 70%) as observed in the NeuA esterase-inactive mutant (Fig. 5). In the low-O-Ac strains, NeuA also prevents further O-acetylation, maintaining capsular Sia-O-Ac at a low level. These data indicate that the two phenotypes of capsular O-acetylation in GBS are governed by the combined action of two molecular strategies: 1) the genetic polymorphism in neuD and 2) NeuA esterase activity. It remains a possibility that polymorphisms within the coding sequence of neuA also contribute to natural differences in O-acetylation between GBS strains. The two apparently distinct phenotypes of CPS O-acetyl modification suggest that GBS strains may have differentially optimized this biochemical parameter based on varied (and perhaps opposing) selective pressures exerted within the animal host.
New understanding enriches model of GBS Sia O-acetylation
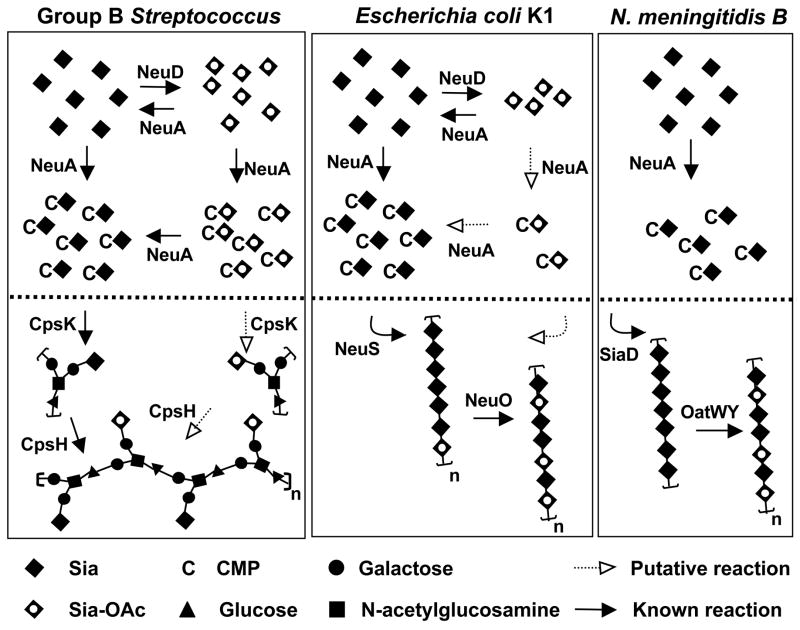
In a previous publication, we suggested that NeuA over-expression may decrease Sia O-acetylation by altering pathway flux away from the O-acetyltransferase (NeuD), which competes for the same intracellular substrate, Neu5Ac (17). Taken together, our current analyses of the NeuA esterase reveal that the effects of neuA deletion and over-expression are unlikely to result simply from the architecture of the Sia biosynthetic pathway, but instead may largely result from the active removal of some Sia-O-acetyl groups prior to capsular assembly. Our current studies now allow us to further enrich the model (Fig. 8) by introduction of a new reaction (de-O-acetylation), which may occur at the level of free or CMP-activated intracellular Sias.
Figure 8. NeuA esterase activity in the context of capsule biosynthetic evolution in three prominent Sia-decorated pathogens GBS, E. coli K1, and serogroup B N. meningitidis.
Bacterial Sia biosynthesis can be divided into three main parts: 1) Sia biosynthesis and degradation, 2) intracellular Sia O-acetylation and de-O-acetylation, and 3) polysaccharide biosynthesis, polymerization, and O-acetylation. For simplicity, only 2) and 3) are shown (above and below the dotted line respectively). With respect to 1), these Sia-expressing pathogens use a homologous 3-enzyme pathway to generate N-acetylmannosamine (ManNAc) by epimerization of UDP-N-acetylglucosamine, condense phosphoenolpyruvate with ManNAc to generate N-acetylneuraminic acid (Neu5Ac), then activate Neu5Ac by transfer of the anomeric oxygen to the α-phosphate of CTP. Only E. coli possesses Sia degradation machinery. In GBS, cpsK and cpsH encode the sialyltransferase and repeating unit polymerase respectively. Intracellular Sia O-acetylation and de-O-acetylation is limited to GBS and E. coli, both of which possess the NeuD Sia O-acetyltransferase and NeuA Sia-O-acetyl esterase. The N. meningitidis pathway is more stream-lined in appearance due to the lack of an endogenous O-acetylation/de-O-acetylation cycle. Despite the pronounced difference between N. meningitidis (serogroup B) and E. coli K1 in intracellular O-acetylation cycling, these pathogens share an identical poly-Sia antigen made up of α2–8-linked Neu5Ac residues (polymerized by SiaD and NeuS respectively) that can be O-acetylated after capsule polymerization (by OatWY or NeuO respectively). See text for further discussion. Model is based on references (17, 18, 38, 51–61).
Interestingly, when Sias are released from the GBS surface using a sialidase at physiological pH, O-acetylation is found to occur predominantly at the 7-C position (Fig. 7). 7-O-acetyl groups are prone to migration along the exocyclic side chain of sialic acid (C-7 to C-9) over time under physiological conditions (41). This behavior has also been noted for Sias released from the GBS surface by mild acid hydrolysis (17, 18). While spontaneous migration of the O-acetyl position makes the hypothesis difficult to test, we speculate that de-O-acetylation of 9-O-acetylated free and CMP-Sias intracellularly may allow the dominant expression of 7-O-acetylated capsular polysaccharide.
Evolution of Sia-O-acetyl regulation: Comparison of 3 sialylated pathogens
From an evolutionary perspective, the NeuA de-O-acetylation reaction provides an interesting vantage point for observing biochemical pathway optimization among different bacterial species. GBS, E. coli K1, and serogroup B N. meningitidis are all known to carry the O-acetyl modification on their sialylated capsular polysaccharides (17, 19, 21). However, these bacteria employ different combinations of O-acetyltransferase- and O-acetylesterase-encoding genes (18, 38), which may themselves produce enzymes with varied mechanisms and/or activity levels (Fig. 4) (51). We can compare and contrast the known parameters of O-acetyl regulation in these three pathogens, with reference to a simplified model of capsule biosynthesis (Fig. 8).
GBS, E. coli, and N. meningitidis generate the O-acetyl modification by different mechanisms involving O-acetyltransferases that act at the level of free intracellular Sia and/or the fully assembled CPS (17, 51). Whereas GBS uses only an intracellular O-acetylation mechanism and N. meningitidis likely uses only a capsular O-acetylation mechanism, E. coli can employ both intracellular and capsular O-acetylation mechanisms to arrive at the final level of surface modification (38, 51). All three bacteria can modulate O-acetylation levels by varying the amino acid sequence of their O-acetyltransferases. However, whereas E. coli K1 and N. meningitidis accomplish this by slipped-strand mis-pairing of homopolymeric DNA tracts within their poly-Sia O-acetyltransferase genes (17, 51, 52), GBS strains appear to encode a single specific residue polymorphism in the free-Sia O-acetyltransferase gene that results in differential O-acetylation (18).
Despite the similarity of intracellular Sia anabolic pathways in GBS and E. coli, these pathogens appear to differentially regulate the O-acetylation/de-O-acetylation cycle catalyzed by NeuD and NeuA respectively. This differential regulation is evidenced by the wide divergence of capsular O-acetylation specifically attributable to the intracellular process in GBS and E. coli. In GBS, NeuD-dependent O-acetylation typically results in >30% capsular Sia O-acetylation (18), indicating that the NeuA esterase does not hydrolyze O-acetylated Sias to completion in vivo. In E. coli, the intracellular O-acetylation mechanism results in only 2–4% O-acetylation of capsular Sias (38). As reviewed by Steenbergen et al., intracellular O-acetylation in E. coli may be further regulated by several E. coli-specific parameters (38). Overall differences in intracellular Sia-O-acetylation between GBS and E. coli may also be related to our observation that unlike GBS NeuA, E. coli NeuA operates independently of the co-factors necessary to maintain CMP-Sia synthetase activity (Fig 4B).
In conclusion, our studies show that GBS NeuA displays native Sia-de-O-acetylation activity and demonstrate for the first time that O-acetyl removal prior to capsular assembly is a mechanism controlling the extent of capsular O-acetylation in living bacteria. Identification and site-directed mutation of the Sia-O-acetyl transferase (NeuD K123A) (18) and Sia O-acetylesterase (NeuA N301A) have yielded inactive enzymes that produce a full-range of capsular Sia O-acetylation when introduced into GBS mutants where the corresponding WT genes have been deleted. This panel of isogenic GBS strains represent a key step toward understanding the role(s) of Sia O-acetylation and the mechanisms of Sia-dependent virulence at the host-pathogen interface.
Acknowledgments
This work was supported by NIH grants P01-HL57345 and GM32373 (AV), R01-HD051796 (VN), R01-GM076360 (XC), a Gianinni Family Foundation Postdoctoral Fellowship Award (AL), and an American Heart Association Established Investigator Award (VN).
Abbreviations used
- GBS
Group B Streptococcus
- Sia
sialic acid
- Sia-OAc
O-acetylated Sia
- Neu5Ac
N-acetylneuraminic acid (Sia)
- Neu5
9Ac2, 9-O-acetylated N-acetylneuraminic acid
- DMB
1,2-Diamino-4,5-methylene Dioxybenzene
- CMP
cytidine monophosphate
- CPS
capsular polysaccharide
- AUS
Arthrobacter urafaciens sialidase
- PAF
platelet activating factor
- WT
wild-type
References
- 1.Angata T, Varki A. Chem Rev. 2002;102:439–469. doi: 10.1021/cr000407m. [DOI] [PubMed] [Google Scholar]
- 2.Gagneux P, Varki A. Glycobiology. 1999;9:747–755. doi: 10.1093/glycob/9.8.747. [DOI] [PubMed] [Google Scholar]
- 3.Vimr E, Lichtensteiger C. Trends Microbiol. 2002;10:254–257. doi: 10.1016/s0966-842x(02)02361-2. [DOI] [PubMed] [Google Scholar]
- 4.Edwards MS, Kasper DL, Jennings HJ, Baker CJ, Nicholson W. J Immunol. 1982;128:1278–1283. [PubMed] [Google Scholar]
- 5.Marques MB, Kasper DL, Pangburn MK, Wessels MR. Infect Immun. 1992;60:3986–3993. doi: 10.1128/iai.60.10.3986-3993.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogel U, Weinberger A, Frank R, Muller A, Kohl J, Atkinson JP, Frosch M. Infect Immun. 1997;65:4022–4029. doi: 10.1128/iai.65.10.4022-4029.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ram S, Sharma AK, Simpson SD, Gulati S, McQuillen DP, Pangburn MK, Rice PA. J Exp Med. 1998;187:743–752. doi: 10.1084/jem.187.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charland N, Kobisch M, Martineau-Doizé B, Jacques M, Gottschalk M. FEMS Immunol Med Microbiol. 1996;14:195–203. doi: 10.1111/j.1574-695X.1996.tb00287.x. [DOI] [PubMed] [Google Scholar]
- 9.Jurcisek J, Greiner L, Watanabe H, Zaleski A, Apicella MA, Bakaletz LO. Infect Immun. 2005;73:3210–3218. doi: 10.1128/IAI.73.6.3210-3218.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards MS, Nizet V, Baker CJ. In: Infectious Diseases of the Fetus and Newborn Infant. Remington J, Klein J, Baker CJ, Wilson CB, editors. Elselvier Saunders; Philadelphia: 2006. pp. 404–464. [Google Scholar]
- 11.Paoletti LC, Kasper DL. Expert Opin Biol Ther. 2003;3:975–984. doi: 10.1517/14712598.3.6.975. [DOI] [PubMed] [Google Scholar]
- 12.Wessels MR, Rubens CE, Benedí VJ, Kasper DL. Proc Natl Acad Sci USA. 1989;86:8983–8987. doi: 10.1073/pnas.86.22.8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aoyagi Y, Adderson EE, Min JG, Matsushita M, Fujita T, Takahashi S, Okuwaki Y, Bohnsack JF. J Immunol. 2005;174:418–425. doi: 10.4049/jimmunol.174.1.418. [DOI] [PubMed] [Google Scholar]
- 14.Baker CJ, Kasper DL. N Engl J Med. 1976;294:753–756. doi: 10.1056/NEJM197604012941404. [DOI] [PubMed] [Google Scholar]
- 15.Jones C, Virji M, Crocker PR. Mol Microbiol. 2003;49:1213–1225. doi: 10.1046/j.1365-2958.2003.03634.x. [DOI] [PubMed] [Google Scholar]
- 16.Carlin AF, Lewis AL, Varki A, Nizet V. J Bacteriol. 2007;89:1231–1237. doi: 10.1128/JB.01155-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis AL, Nizet V, Varki A. Proc Natl Acad Sci U S A. 2004;101:11123–11128. doi: 10.1073/pnas.0403010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis AL, Hensler ME, Varki A, Nizet V. J Biol Chem. 2006;281:11186–11192. doi: 10.1074/jbc.M513772200. [DOI] [PubMed] [Google Scholar]
- 19.Bhattacharjee AK, Jennings HJ, Kenny CP, Martin A, Smith IC. Can J Biochem. 1976;54:1–8. doi: 10.1139/o76-001. [DOI] [PubMed] [Google Scholar]
- 20.Knirel YA, Vinogradov EV, Shashkov AS, Dmitriev BA, Kochetkov NK, Stanislavsky ES, Mashilova GM. Eur J Biochem. 1986;157:129–138. doi: 10.1111/j.1432-1033.1986.tb09648.x. [DOI] [PubMed] [Google Scholar]
- 21.Orskov F, Orskov I, Sutton A, Schneerson R, Lin W, Egan W, Hoff GE, Robbins JB. J Exp Med. 1979;149:669–685. doi: 10.1084/jem.149.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thibault P, Logan SM, Kelly JF, Brisson JR, Ewing CP, Trust TJ, Guerry P. J Biol Chem. 2001;276:30674–30666. doi: 10.1074/jbc.M104529200. [DOI] [PubMed] [Google Scholar]
- 23.Knirel YA, Kocharova NA, Shashkov AS, Dmitriev BA, Kochetkov NK, Stanislavsky ES, Mashilova GM. Eur J Biochem. 1987;163:639–652. doi: 10.1111/j.1432-1033.1987.tb10913.x. [DOI] [PubMed] [Google Scholar]
- 24.Knirel YA, Rietschel ET, Marre R, Zahringer U. Eur J Biochem. 1994;221:239–245. doi: 10.1111/j.1432-1033.1994.tb18734.x. [DOI] [PubMed] [Google Scholar]
- 25.Gamian A, Romanowska E, Ulrich J, Defaye J. Carbohydr Res. 1992;236:195–208. doi: 10.1016/0008-6215(92)85016-s. [DOI] [PubMed] [Google Scholar]
- 26.Feng L, Senchenkova SN, Tao J, Shashkov AS, Liu B, Shevelev SD, Reeves PR, Xu J, Knirel YA, Wang L. J Bacteriol. 2005;187:758–764. doi: 10.1128/JB.187.2.758-764.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butor C, Diaz S, Varki A. J Biol Chem. 1993;268:10197–10206. [PubMed] [Google Scholar]
- 28.Fahr C, Schauer R. J Invest Dermatol. 2001;116:254–260. doi: 10.1046/j.1523-1747.2001.01237.x. [DOI] [PubMed] [Google Scholar]
- 29.Hueso P, Cabezas JA, Reglero A. Ital J Biochem. 1988;37:302–309. [PubMed] [Google Scholar]
- 30.Klein A, Krishna M, Varki NM, Varki A. Proc Natl Acad Sci USA. 1994;91:7782–7786. doi: 10.1073/pnas.91.16.7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krishna M, Varki A. J Exp Med. 1997;185:1997–2013. doi: 10.1084/jem.185.11.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen Y, Tiralongo J, Kohla G, Schauer R. Biol Chem. 2004;385:145–152. doi: 10.1515/BC.2004.033. [DOI] [PubMed] [Google Scholar]
- 33.Malisan F, Franchi L, Tomassini B, Ventura N, Condo I, Rippo MR, Rufini A, Liberati L, Nachtigall C, Kniep B, Testi R. J Exp Med. 2002;196:1535–1541. doi: 10.1084/jem.20020960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi WX, Chammas R, Varki NM, Powell L, Varki A. J Biol Chem. 1996;271:31526–31532. doi: 10.1074/jbc.271.49.31526. [DOI] [PubMed] [Google Scholar]
- 35.Ambrose MG, Freese SJ, Reinhold MS, Warner TG, Vann WF. Biochemistry. 1992;31:775–780. doi: 10.1021/bi00118a019. [DOI] [PubMed] [Google Scholar]
- 36.Liu G, Jin C, Jin C. J Biol Chem. 2004;279:17738–17749. doi: 10.1074/jbc.M400143200. [DOI] [PubMed] [Google Scholar]
- 37.Yu H, Ryan W, Yu H, Chen X. Biotechnol Lett. 2006;28:107–113. doi: 10.1007/s10529-005-4955-z. [DOI] [PubMed] [Google Scholar]
- 38.Steenbergen SM, Lee YC, Vann WF, Vionnet J, Wright LF, Vimr ER. J Bacteriol. 2006;188:6195–6206. doi: 10.1128/JB.00466-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu H, Yu H, Karpel R, Chen X. Bioorg Med Chem. 2004;12:6427–6435. doi: 10.1016/j.bmc.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 40.Yu H, Huang S, Chokhawala H, Sun M, Zheng H, Chen X. Angew Chem Int Ed Engl. 2006;45:3938–3944. doi: 10.1002/anie.200600572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamerling JP, Schauer R, Shukla AK, Stoll S, van HH, Vliegenthart JFG. Eur J Biochem. 1987;162:601–607. doi: 10.1111/j.1432-1033.1987.tb10681.x. [DOI] [PubMed] [Google Scholar]
- 42.Higa HH, Paulson JC. J Biol Chem. 1985;260:8838–8849. [PubMed] [Google Scholar]
- 43.Varki A, Diaz S. J Biol Chem. 1985;260:6600–6608. [PubMed] [Google Scholar]
- 44.Leelavathi DE, Estes LW, Feingold DS, Lombardi B. Biochim Biophys Acta. 1970;211:124–138. [Google Scholar]
- 45.Varki A, Diaz S. Anal Biochem. 1984;137:236–247. doi: 10.1016/0003-2697(84)90377-4. [DOI] [PubMed] [Google Scholar]
- 46.Manzi AE, Diaz S, Varki A. Anal Biochem. 1990;188:20–32. doi: 10.1016/0003-2697(90)90523-c. [DOI] [PubMed] [Google Scholar]
- 47.Johnson CD, Russell RL. Anal Biochem. 1975;64:229–238. doi: 10.1016/0003-2697(75)90423-6. [DOI] [PubMed] [Google Scholar]
- 48.Kean EL, Roseman S. J Biol Chem. 1966;241:5643–5650. [PubMed] [Google Scholar]
- 49.Akoh CC, Lee GC, Liaw YC, Huang TH, Shaw JF. Prog Lipid Res. 2004;43:534–552. doi: 10.1016/j.plipres.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 50.Lo YC, Lin SC, Shaw JF, Liaw YC. J Mol Biol. 2003;330:539–551. doi: 10.1016/s0022-2836(03)00637-5. [DOI] [PubMed] [Google Scholar]
- 51.Deszo EL, Steenbergen SM, Freedberg DI, Vimr ER. Proc Natl Acad Sci U S A. 2005;102:5564–5569. doi: 10.1073/pnas.0407428102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Claus H, Borrow R, Achtman M, Morelli G, Kantelberg C, Longworth E, Frosch M, Vogel U. Mol Microbiol. 2004;51:227–239. doi: 10.1046/j.1365-2958.2003.03819.x. [DOI] [PubMed] [Google Scholar]
- 53.Haft RF, Wessels MR, Mebane MF, Conaty N, Rubens CE. Mol Microbiol. 1996;19:555–563. doi: 10.1046/j.1365-2958.1996.395931.x. [DOI] [PubMed] [Google Scholar]
- 54.Chaffin DO, McKinnon K, Rubens CE. Mol Microbiol. 2002;45:109–122. doi: 10.1046/j.1365-2958.2002.02988.x. [DOI] [PubMed] [Google Scholar]
- 55.Daines DA, Wright LF, Chaffin DO, Rubens CE, Silver RP. FEMS Microbiol Lett. 2000;189:281–284. doi: 10.1111/j.1574-6968.2000.tb09244.x. [DOI] [PubMed] [Google Scholar]
- 56.Chaffin DO, Beres SB, Yim HH, Rubens CE. J Bacteriol. 2000;182:4466–4477. doi: 10.1128/jb.182.16.4466-4477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vann WF, Liu TY, Robbins JB. J Bacteriol. 1978;133:1300–1306. doi: 10.1128/jb.133.3.1300-1306.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steenbergen SM, Vimr ER. Mol Microbiol. 1990;4:603–611. doi: 10.1111/j.1365-2958.1990.tb00629.x. [DOI] [PubMed] [Google Scholar]
- 59.Edwards U, Frosch M. FEMS Microbiol Lett. 1992;96:161–166. doi: 10.1016/0378-1097(92)90397-7. [DOI] [PubMed] [Google Scholar]
- 60.Ganguli S, Zapata G, Wallis T, Reid C, Boulnois G, Vann WF, Roberts IS. J Bacteriol. 1994;176:4583–4589. doi: 10.1128/jb.176.15.4583-4589.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hammerschmidt S, Müller A, Sillmann H, Mühlenhoff M, Borrow R, Fox A, Van PJ, Zollinger WD, Gerardy-Schahn R, Frosch M. Mol Microbiol. 1996;20:1211–1220. doi: 10.1111/j.1365-2958.1996.tb02641.x. [DOI] [PubMed] [Google Scholar]