Abstract
Background
Diuretics are recommended as initial treatment for hypertension. Several studies have suggested suboptimal persistence and adherence to thiazide diuretic monotherapy; this study compared patient persistence and adherence with hydrochlorothiazide (HCTZ) monotherapy to fixed-dose combinations containing HCTZ.
Methods
Patients with at least one prescription claim during 2001 to 2003 for either HCTZ or one of the following fixed-dose combinations: angiotensin-receptor blockers/HCTZ (ARB/HCTZ), angiotensin-converting enzyme inhibitor/HCTZ (ACEI/HCTZ), or beta blockers/HCTZ (BB/HCTZ) were identified. Patients were required to be continuously benefit-eligible six months pre- and one year post-index date, and to have no prescription claims for any antihypertensive therapy six months prior to the index date. Patients were followed for one year to assess persistence, medication possession ratio (MPR), adherence (MPR >80%), and proportion of days covered (PDC) with initial antihypertensive therapy. Logistic regression was used to calculate adjusted odds ratios for persistence, adherence and PDC, adjusted for age, gender, business segment, RxRisk disease categories, average co-pay and concurrent cardiovascular-related medication utilization.
Results
The study cohort consisted of 48,212 patients; 72.5% used HCTZ, 13.2% ACEI/HCTZ, 9.3% ARB/HCTZ, and 5.0% BB/HCTZ. Mean age was 53.7 years and 66.5% were female. A significantly lower proportion of patients using HCTZ (29.9%) remained persistent with therapy at 12 months compared with ARB/HCTZ (52.6%; OR = 0.37, CI = 0.36, 0.38), ACEI/HCTZ (51.4%; OR = 0.38, CI = 0.37, 0.39), and BB/HCTZ (51.9%; OR = 0.38, 0.37, 0.40). Similarly, PDC was lower for HCTZ patients (32.5%) as compared to ARB/HCTZ (53.7%; OR = 0.39, CI = 0.37, 0.40), ACEI/HCTZ (50.9%; OR = 0.42, CI = 0.40, 0.43), and BB/HCTZ (51.3%; OR = 0.44, CI 0.42, 0.45). MPR was also significantly lower for HCTZ patients as compared to those using fixed-dose combination therapies.
Conclusion
Initiating HCTZ fixed-dose combination therapy with an ACEI, ARB, or BB was associated with greater persistence and adherence as compared to HCTZ monotherapy. Further research is needed to determine the relationship between improved persistence and adherence with blood pressure control.
Background
Hypertension affects almost one in three American adults; age-adjusted prevalence in 2005 was estimated at 33.6% [1,2]. Only 37% of patients with hypertension, and only slightly more than half (57%) of those receiving antihypertensive treatment currently have their blood pressure (BP) controlled [1]. The Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure published its seventh report (JNC 7) in 2003 [3]. JNC 7 included the recommendation of thiazide diuretics, either alone or in combination with drugs from other classes, as initial therapy treatment of most patients with hypertension.
Patient persistence and adherence to prescribed antihypertensive therapy is a key component of hypertension management. Greater persistence with antihypertensive therapy has been associated with lower rates of long-term hypertension sequelae [4], as well lower health care resource use [5-8] and hospitalization rates [6]. In usual-practice settings, less than optimal persistence with antihypertensive monotherapy regimens has been well documented [9-13]. Several studies have indicated that initial monotherapy treatment with diuretics is associated with poorer patient persistence, compared to angiotensin-converting enzyme inhibitors (ACEI), angiotensin-receptor blockers (ARB), and beta blockers (BB). [9,11,13]. Tolerability and perceived side effects associated with antihypertensive medications may play an important role in patient motivation, and thus affect medication persistence [14]. Patients may complain of frequent urination upon initiation of diuretic therapy, and diuretics have been associated with side effects such as weakness, fatigue, palpitations, and electrolyte disturbances. Patients are often prescribed a lower dose of diuretic when used in combination therapy with an agent from another antihypertensive class, and combination low-dose therapy has been shown to increase BP-lowering efficacy and reduce adverse side effects associated with higher-dose monotherapy regimens [15]. Thus, the addition of a medication from another antihypertensive class to a diuretic may attenuate the side effects often seen with diuretics when used as monotherapy [15-17].
The purpose of this study was to compare patient persistence and adherence to hydrochlorothiazide (HCTZ) monotherapy versus fixed-dose combinations containing HCTZ and an ACEI, ARB, or BB in a natural (non-clinical trial) setting. This retrospective, longitudinal cohort study employed administrative pharmacy claims data to examine drug utilization in patients previously naïve to antihypertensive therapy who initiated therapy with HCTZ monotherapy, or fixed-dose ACEI/HCTZ, ARB/HCTZ, or BB/HCTZ.
Methods
This was a retrospective, population-based study which employed a pharmacy claims database from MedImpact, a large US pharmacy benefits manager (PBM) which administers prescription benefits for about 27 million persons across the US. Adult participants (≥ 18 years) were eligible for study inclusion if they received ≥ 1 prescription for HCTZ or fixed-dose combination ACEI/HCTZ, ARB/HCTZ, or BB/HCTZ during the study identification period of January 1, 2001 through December 31, 2003. "Index drug" was defined as the first prescription therapy filled within the identification period, and the "index date" was defined as the date of the first index drug fill. Participants were required to be continuously benefit-eligible for at least 6 months preceding and 12 months subsequent to the index date. Patients were required to have no claims for any antihypertensive therapy during the 6 months prior to their index date.
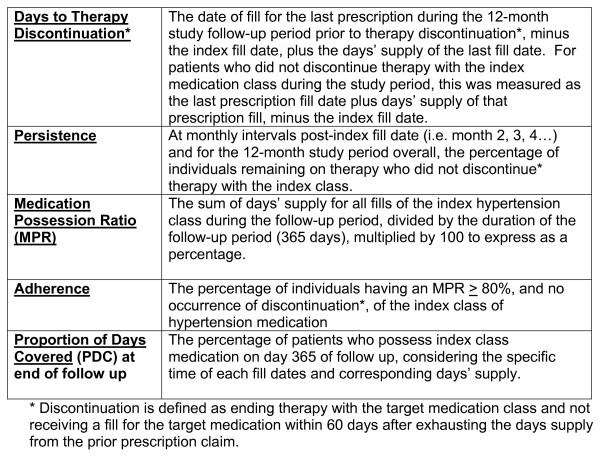
Drug utilization was followed for 1 year subsequent to the index date, and analyses were performed relative to the specific index drug classes. Figure 1 includes definitions of utilization metrics, including persistence, medication possession ratio (MPR), adherence, proportion of days covered (PDC) and time to therapy discontinuation. For index drug classification purposes, patients who received prescription fills for antihypertensive medications in addition to the study index medication on their study index date were excluded.
Figure 1.
Study utilization metrics and definitions.
RxRisk [18] methodology was used to identify the presence of other diseases. RxRisk identifies claims during the 6 months prior to the index date for medications used to treat specific conditions to identify co-morbidities (e.g. a pharmacy claim for an oral antidiabetic agent is used to identify the presence of diabetes). RxRisk disease categories were used (e.g. diabetes yes/no) to describe patient clinical characteristics, and for adjustment of statistical comparisons for the presence of co-morbid conditions.
To control for potential physician's treatment selection bias, a propensity score adjustment was performed. A multinomial regression model was constructed to estimate the probability of the observed treatment choice based on patient characteristics, including patient age, gender, RxRisk disease categories, and type of benefit coverage (i.e., Commercial, Medicaid, Medicare, and self-insured). The inverse of this probability, or propensity score weight, was then used in study multivariable logistic and linear regressions. For MPR, multiple variable linear regression techniques were used in pairwise comparisons of means adjusted for independent variables, which included propensity weight, patient age, gender, RxRisk disease categories, type of benefit coverage, average co-pay, and concurrent cardiovascular-related medications utilized (i.e., other antihypertensive drugs not equal to the initial therapy subsequent to the index pharmacy claim date; and digitalis, nitrates, antiplatelet agents and antihyperlipidemics). Pairwise multiple logistic regression was used to calculate odds ratios to compare persistence, adherence, and proportion of days covered (PDC), adjusted for the same independent variables, using HCTZ monotherapy as the reference group. Although these statistical methods were used to control for important variables, crude statistics are included in the text and the tables for descriptive purposes, since statistical adjustment did not appreciably alter any values.
Persistence was evaluated using multivariable survival analysis techniques. Cox proportional hazards regression was employed to calculate hazard ratios (HR) to compare discontinuation for antihypertensive medications studied, adjusted for covariates. Discontinuation of therapy with the index medication was modeled using HCTZ as the referent. All analyses for this study were performed using SAS version 9.1.3 (SAS Institute, Inc, Cary, NC).
Results
The final study population was comprised of 48,212 patients newly initiated on antihypertensive therapy. Cohort descriptive characteristics are summarized in Table 1. Overall, 67% were females and the mean age was 53.7 years. HCTZ users comprised 72.5% of the study cohort, while 13.2% used ACEI/HCTZ, 9.3% ARB/HCTZ, and 5.0% BB/HCTZ. HCTZ and BB/HCTZ patients were generally younger than ACEI/HCTZ or ARB/HCTZ patients. Patients receiving HCTZ were more likely to be female than those utilizing fixed-dose combination therapy. The proportion of cohort patients with cardiovascular-related conditions or diabetes was higher for ACEI/HCTZ and ARB/HCTZ than for HCTZ or BB/HCTZ.
Table 1.
Study cohort descriptive characteristics (N = 48,212)
| Initial anti-hypertensive therapy | ||||
| Characteristics | HCTZ (N = 34,934, 72.5%) | ARB/HCTZ (N = 4,469, 9.3%) | ACEI/HCTZ (N = 6,388, 13.2%) | BB/HCTZ (N = 2,421, 5.0%) |
| Mean age in years ± SD | 53.6 ± 15.3 | 54.6 ± 13.8 | 54.3 ± 13.9 | 52.2 ± 13.8 |
| Age in categories | ||||
| 18 – 44 (%) | 10,296 (29.5) | 1,006 (22.5) | 1,486 (23.3) | 712 (29.4) |
| 45 – 54 (%) | 9,769 (28.0) | 1,457 (32.6) | 2,021 (31.6) | 780 (32.2) |
| 55 – 64 (%) | 6,423 (18.4) | 1,006 (22.5) | 1,469 (23.0) | 505 (20.9) |
| 65 – 74 (%) | 4,500 (12.9) | 570 (12.8) | 824 (12.9) | 256 (10.6) |
| 75 – 84 (%) | 2,925 (8.4) | 327 (7.3) | 443 (6.9) | 115 (4.8) |
| 85 and above (%) | 1,021 (2.9) | 103 (2.3) | 145 (2.3) | 53 (2.2) |
| Frequency female (%) | 24,495 (70.1) | 2,573 (57.6) | 3,629 (56.8) | 1,356 (56.0) |
| RxRiska, b | ||||
| Behavioral Health | 6,413 (18.4) | 653 (14.6) | 810 (12.7) | 332 (13.7) |
| Cardiovascular Conditions | 2,012 (5.8) | 435 (9.7) | 874 (13.7) | 128 (5.3) |
| Gastric acid disorder, IBS | 2,768 (7.9) | 250 (5.6) | 351 (5.5) | 147 (6.1) |
| Asthma, Allergic Rhinitis | 2,590 (7.4) | 196 (4.4) | 301 (4.7) | 79 (3.3) |
| Diabetes | 843 (2.4) | 147 (3.3) | 281 (4.4) | 23 (1.0) |
| Thyroid Disease | 2,193 (6.3) | 230 (5.1) | 321 (5.0) | 108 (4.5) |
| Rheumatoid Arthritis, Gout | 1,273 (3.6) | 120 (2.7) | 171 (2.7) | 50 (2.1) |
| Primary market segment | ||||
| HMO | 24,562 (70.3) | 2,644 (59.2) | 4,656 (72.9) | 1,631 (67.4) |
| Medicaid | 4,103 (11.8) | 125 (2.8) | 300 (4.7) | 112 (4.6) |
| Medicare | 2,285 (6.5) | 227 (5.1) | 355 (5.6) | 122 (5.0) |
| Self-insured | 3,984 (11.4) | 1,473 (33.0) | 1,077 (16.9) | 556 (23.0) |
| Average copay ($)c | 5.2 ± 4.2 | 22.8 ± 19.0 | 14.0 ± 13.0 | 9.2 ± 8.7 |
a The 49 RxRisk categories were consolidated into 21 categories of which the top 7 most prevalent groups (3% threshold) are presented here. Behavioral health = anxiety and tension, bipolar, depression, psychotic illness, attention deficit disorder; Cardiovascular conditions: Cardiac disease, coronary & peripheral vascular disease, heart disease, hypertension (does not include the target medications, digitalis, nitrates, anti-platelet agents or anti-hyperlipemic agents); Gastrointestinal (GI) disorders = Gastric acid disorder, irritable bowel syndrome
b Limited to antihypertensive medications of the same class as the index drug
Unadjusted results for antihypertensive medication utilization metrics are displayed in Table 2. Patients receiving HCTZ had the lowest persistence (29.9%), compared to 52.6% for ARB/HCTZ, 51.9% for BB/HCTZ, and 51.4% for ACEI/HCTZ. The MPR was highest for BB/HCTZ (62.1%), followed by ARB/HCTZ (60.5%), ACEI/HCTZ (58.3%), and HCTZ (44.5%). Adherence was lowest for HCTZ patients (24.2%) and highest for BB/HCTZ (43.9%). HCTZ patients had the lowest proportion of days covered (32.5%), and time to therapy discontinuation was longest for ARB/HCTZ patients (240.1 ± 140.3 days).
Table 2.
Unadjusted antihypertensive persistence and adherence
| Outcome measures | HCTZ | ARB/HCTZ | ACEI/HCTZ | BB/HCTZ |
| Persistence (%) | 29.9 | 52.6 | 51.4 | 51.9 |
| Adherence (%) | 24.2 | 39.2 | 38.8 | 43.9 |
| PDCa at end of follow-up (%) | 32.5 | 53.7 | 50.9 | 51.3 |
| MPRb ± SD | 44.5 ± 34.5 | 60.5 ± 32.7 | 58.3 ± 34.2 | 62.1 ± 34.1 |
| Days to therapy discontinuation ± SD | 164.5 ± 141.8 | 240.1 ± 140.3 | 235.9 ± 140.8 | 238.2 ± 140.9 |
aPDC denotes proportion of days covered at the end of the follow-up period. The percentage of patients who possess index class medication on day 365 of follow-up period.
bMPR denotes medication possession ratio.
Adjusted odds ratios for persistence, adherence, and days covered are found in Table 3. Compared to all fixed-dose combination therapy users, HCTZ patients were significantly (p < 0.01, all comparisons) less likely to be persistent, adherent, and to have medication "on hand" at the end of the study period. Patients receiving HCTZ were approximately 63% less likely to be persistent compared to ARB/HCTZ patients, 62% less likely to be persistent than ACEI/HCTZ patients, and 61% less likely to be persistent than BB/HCTZ patients. Similarly, HCTZ patients were 60%, 54%, and 50% less likely to be adherent as compared to BB/HCTZ, ARB/HCTZ, and ACEI/HCTZ patients, respectively. Odds ratios for days' covered indicated that HCTZ users were 61%, 59%, and 56% less likely to have medication on hand at the end of the study as compared to ARB/HCTZ, ACEI/HCTZ, and BB/HCTZ patients, respectively. Comparisons for MPR (Table 3) also revealed that HCTZ patients had lower adjusted mean MPR as compared to BB/HCTZ (difference = -17.0, p < 0.0001), ARB/HCTZ (difference = -16.6, p < 0.0001), and ACEI/HCTZ (difference = -12.9, p < 0.0001).
Table 3.
Outcome measures – adjusted pairwise comparison of HCTZ monotherapy versus each fixed-dose combination therapy
| HCTZ fixed-dose combination therapy | |||
| Outcome measures | ARB/HCTZ | ACEI/HCTZ | BB/HCTZ |
| Persistence (Odds Ratio, 95% CI) | 0.369a (0.356, 0.383) |
0.380a (0.368, 0.393) |
0.382a (0.370, 0.395) |
| Adherence (Odds Ratio, 95% CI) | 0.457a (0.440, 0.475) |
0.495a (0.478, 0.513) |
0.398a (0.385, 0.412) |
| PDC at end of follow-up period (Odds Ratio, 95% CI) | 0.388a (0.374, 0.403) |
0.415a (0.402, 0.429) |
0.435a (0.421, 0.449) |
| MPR (HCTZ vs. fixed-dose) | 44.4 vs 61.0 Δ = -16.6b |
44.6 vs 57.5 Δ = -12.9b |
44.5 vs 61.5 Δ = -17.0b |
a Significant difference in outcome measures between pairwise comparison with HCTZ users at p < 0.05.
b p < 0.0001
Cox proportional hazards model results are included in Table 4. Patients receiving HCTZ were more likely to discontinue the index medication class as compared to users of ACEI/HCTZ, ARB/HCTZ, and BB/HCTZ, adjusted for demographic and clinical covariates. Cardiovascular-related conditions and patient copay were not significant predictors of therapy discontinuation in the proportional hazards model. An increased hazard of therapy discontinuation was observed for Medicaid and Medicare patients as compared to managed care patients, and for patients with diabetes.
Table 4.
Results of Cox proportional hazards model for therapy discontinuation
| Study population (N = 48,212) | ||
| Variable | Hazards ratio | p-value |
| Age | 0.989 | <.0001* |
| Female | 1.048 | 0.0002* |
| RxRisk categoriesa | ||
| Anxiety and Tension, Bipolar Disorder, Depression, Psychotic Illness, ADD | 1.079 | <.0001* |
| Asthma, Allergic Rhinitis | 1.031 | 0.1891 |
| Cardiac Disease, Coronary & Peripheral Vascular Disease, Heart Disease, Hypertensionb | 0.973 | 0.2432 |
| Gastric acid disorder, IBS | 1.004 | 0.8477 |
| Rheumatoid Arthritis, Gout | 1.147 | <.0001* |
| Thyroid Disease | 0.951 | 0.0426* |
| Diabetes | 1.158 | <.0001* |
| Business Type | ||
| HMO | N/A | N/A |
| Medicaid | 1.339 | <.0001* |
| Medicare | 1.451 | <.0001* |
| Self | 1.056 | 0.0013* |
| Concomitant Other CVD-related Medications Used | ||
| Digitalis | 1.138 | 0.0086* |
| Nitrates | 1.224 | <.0001* |
| Antiplatelet Medications | 1.097 | 0.0502 |
| Antihyperlipidemic Medications | 0.797 | <.0001* |
| Average copayc | 1.001 | 0.3831 |
| Target medication classesd | ||
| ARB/HCTZ | 0.529 | <.0001* |
| ACEI/HCTZ | 0.536 | <.0001* |
| BB/HCTZ | 0.532 | <.0001* |
* Significant p-value
a RxRisk categories shown in this table include the 7 most frequent disease states in the study cohort. All RxRisk disease categories were included in the model.
b This RxRisk category does not include the target medications, digitalis, nitrates, antiplatelet medications or antihyperlipidemics.
c Limited to HTN medications of the same class as the index drug.
d Reference Medication Class = HCTZ
Discussion
Our retrospective study found that the combination of another antihypertensive medication with HCTZ via fixed-dose combination therapy was associated with better patient persistence and adherence as compared to HCTZ monotherapy. HCTZ patients were 61–63% less likely to be persistent, and 50–60% less likely to be adherent, than patients who initiated antihypertensive therapy with fixed-dose ACEI/HCTZ, ARB/HCTZ, or BB/HCTZ therapy. Patients were more likely to stay on fixed-dose combinations longer than monotherapy, as mean time to therapy discontinuation was, on average, two and a half months longer for fixed-dose combinations than for monotherapy. To our knowledge, the current study is the first to compare persistence with HCTZ monotherapy to fixed-dose HCTZ combination therapy in a "usual care" setting in the US.
A few other studies have used retrospective methods to evaluate combination therapy in "usual-care" settings outside of the US. Van Wijk et al, in a recent community-based retrospective study using pharmacy dispensing records in the Netherlands, studied 2325 previously naïve patients who newly initiated antihypertensive therapy with a ACEI, BB, calcium channel blocker (CCB), or diuretic [19]. Only 39% of patients used antihypertensive therapy consistently during 10 years of follow-up. More patients who initiated therapy with diuretics and BB discontinued compared to those who started with a CCB or ACEI. In this study, comparing patients who initiated with diuretics, those who started with fixed-dose combination therapy were 70% more likely to be persistent with therapy (OR 0.29; 95% CI 0.14–0.54).
Another recent study comparing utilization for antihypertensive monotherapy versus fixed-dose combination therapy used a Canadian database, and found that during the first 6 months after treatment was initiated, persistence declined to 75%; at the end of 3 years, only 55% were persistent [20]. During the first year of follow-up, compared to diuretic monotherapy, patients prescribed other antihypertensive classes or fixed-dose combination therapy (HR 0.71; 95% CI 0.67 to 0.75) were found to have higher persistence [20]. Other studies comparing monotherapy regimens conducted in naturalistic settings have consistently found poorer compliance and/or persistence for diuretics as compared to other antihypertensive therapeutic classes [11-13,21,22]. Conlin and colleagues [9] followed patients from a large pharmacy benefits manager (PBM) and demonstrated that at 4 years post-therapy initiation, only 16% of diuretic patients were persistent, compared to 61% of ARB, 47% of ACEI, 41% of CCB, and 35% of BB patients.
Improving patient adherence and persistence with antihypertensive therapy may have a beneficial effect on blood pressure control. One recent retrospective study of 840 patients using antihypertensive monotherapy assessed the relationship between medication compliance and blood pressure control (<140/90 mmHg or < 130/85 mmHg for diabetic hypertensive patients) [23]. Patients received monotherapy with an ACEI (27%), or CCB (22%), BB (20%), or diuretic (11%) and were classified as having high (80–100%), medium (50–79%), or low (<50%) medication compliance. High-compliance patients were 45% more likely to achieve blood pressure control than those with medium or low compliance after controlling for age, gender, and comorbidities.
Differences between antihypertensive drug regimens for patient medication adherence and persistence may have cost implications. Increased healthcare expenditures for nonadherent patients with hypertension in usual-care settings have been well documented [5-8]. In one study, Medicaid patients with an interruption of antihypertensive therapy consumed extra healthcare costs of $873/patient during the first year [5]. Another study of both MCO and traditional fee-for-service patients from a large PBM found that better compliance with antihypertensive therapy was associated with a decreased risk of hospitalization and, thus, lower medical costs [6]. Addressing patient noncompliance with antihypertensive medication can thus play an important role in managing the costs of patient care for MCOs and other healthcare providers.
Retrospective analyses of administrative databases can provide information about patient behavior in a naturalistic setting that is difficult to assess in clinical trials, but it is important to note some study limitations. Since definitions of adherence and persistence may be expected to differ somewhat between studies, comparisons of medication utilization results across studies should be interpreted cautiously. As medical claims were unavailable to confirm hypertension diagnosis, some of the patients included may not have had a diagnosis of hypertension, and may have been prescribed a study medication for an unrelated diagnosis. This may have particularly influenced patient selection for BB/HCTZ users, as this combination has multiple indications and may be used more frequently in patients with stable angina or acute coronary syndromes. Drug utilization metrics were measured by prescription refill patterns and not actual drug taken by the patient; however, other studies have supported the evaluation of pharmacy claims data for such purposes [24,25]. Propensity score weighting was used in statistical analyses to control for selection bias, but it is possible that factors that were not available for analysis may have played a role in physician treatment selection. Since patient BP was not available for analysis, patients prescribed HCTZ in combination with a drug from another antihypertensive class may have had a higher baseline BP than those prescribed HCTZ alone; this information was unavailable for inclusion in the propensity score adjustment and may have influenced our study's results. It could be hypothesized that patients prescribed HCTZ alone may be "healthier" patients than those prescribed HCTZ in combination. While analysis of the prevalence of comorbid conditions between treatment groups suggests that this may be somewhat true for HCTZ monotherapy patients as compared to ARB/HCTZ and ACEI/HCTZ patients, frequency of cardiovascular diagnoses and diabetes were similar for HCTZ and BB/HCTZ patients, yet persistence and adherence for BB/HCTZ patients was much greater than for HCTZ patients. Statistical analyses were employed to control for such differences in patient co-morbidity and demographic characteristics, and significant differences in therapy discontinuation rates persisted. Since we identified patients using a 6-month prior eligibility criteria, it is possible that some patients may have discontinued previous AHY therapy prior to the 6 months or received another AHY therapy while a member of another health plan. It is possible that a patient who discontinued a study medication did so based on physician instructions, and this would have been incorrectly classified as non-persistence and may have influenced study results. Finally, our study included only fixed-dose combinations with HCTZ and excluded free combination regimens. Other studies have demonstrated improved persistence with fixed-dose antihypertensive combination regimens as compared to free combinations [26]; therefore, some of the persistence advantages found in our study of fixed-dose combinations may not be evident when using free combination regimens.
This study sought to evaluate patient utilization of HCTZ monotherapy as compared to HCTZ used in combination in patients new to antihypertensive therapy. Our findings are not meant to imply that all patients prescribed HCTZ as monotherapy are candidates for initial combination antihypertensive therapy; important prescriber and patient clinical factors play a role in the choice of initial antihypertensive therapy. However, our study provides additional information regarding the use of these regimens in a non-clinical trial setting, and when interpreted in the context of other literature suggesting poor patient adherence and persistence with HCTZ monotherapy versus monotherapy with other antihypertensive classes, provides important information to prescribers of antihypertensive medication.
Conclusion
In a naturalistic, non-clinical trial setting, our study suggested that patients who initiated new antihypertensive therapy with HCTZ in a fixed-dose combination with an ACEI, ARB, or BB may be more persistent than patients who initiated antihypertensive therapy with HCTZ alone. HCTZ patients were also less likely to be adherent as compared to fixed-dose combination patients, and to stay on therapy an average of 2.5 months less during the first year than patients using fixed-dose combinations. While additional research is needed to evaluate the impact of improved persistence and adherence with blood pressure control across practice settings, improving patient adherence and persistence with antihypertensive medication is an important approach to improving blood pressure control in the US.
Competing interests
Dr. Plauschinat is employed by Novartis Pharmaceuticals Corporation, the manufacturer of several medications indicated for the treatment of hypertension, and also owns stock in Novartis. At the time of the study, Dr. Preblick was employed by Novartis Pharmaceuticals Corp., and Dr. Preblick owns Novartis stock. Dr. Patel, Ms. Remigio-Baker, and Dr. Thiebaud have no competing interests to disclose.
Authors' contributions
BP and RP participated in conception and design of the study. BP, CP, RP, and RRB participated in writing and editing portions of the manuscript. RRB and PT participated in statistical analysis. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
The authors would like to acknowledge Jenifer Wogen, MedMentis Consulting, LLC, for medical writing assistance in preparing this manuscript and R. Scott Leslie, MedImpact, for his assistance with the data analysis and statistical support. Ms. Wogen received financial compensation from Novartis for her medical writing assistance. Mr. Leslie is an employee of MedImpact.
This study was funded by Novartis Pharmaceuticals Corporation. Dr. Plauschinat is employed by Novartis Pharmaceuticals Corporation, the manufacturer of several medications indicated for the treatment of hypertension, and also owns stock in Novartis. Dr. Preblick is currently employed by Bayer Pharmaceuticals; at the time of the study, he was employed by Novartis Pharmaceuticals. Dr. Thiebaud and Ms. Remigio-Baker were employees during the time of data analysis and manuscript preparation, but are no longer employed with MedImpact. Ms. Remigio-Baker is currently employed by University of California San Diego. Dr. Thiebaud is currently employed by Acumen, LLC.
Dr. Preblick and Dr. Plauschinat, as employees of the funding body, Novartis Pharmaceuticals Corporation, were involved in the aspects of the study listed above under 'Authors' contributions'.
Portions of this study were presented as a poster at the American Society for Hypertension's 21st Annual Scientific Meeting and Exposition, New York, NY, May 2006.
Contributor Information
Bimal V Patel, Email: bimal.patel@medimpact.com.
Rosemay A Remigio-Baker, Email: remigio_baker@hotmail.com.
Patrick Thiebaud, Email: ptrck_thbd@yahoo.com.
Ronald Preblick, Email: ronald.preblick@bayer.com.
Craig Plauschinat, Email: craig.plauschinat@novartis.com.
References
- Ong KL, Cheung BMY, Man YB, Lau CP, Lam KSL. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension. 2007;49:69–75. doi: 10.1161/01.HYP.0000252676.46043.18. [DOI] [PubMed] [Google Scholar]
- Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y, American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart Disease and Stroke Statistics 2008 Update. A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ, National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- Cramer JA. Consequences of intermittent treatment for hypertension: the case for medication compliance and persistence. Am J Managed Care. 1998;4:1563–1568. [PubMed] [Google Scholar]
- McCombs JS, Nichol MB, Newman CM, Sclar DA. The costs of interrupting antihypertensive drug therapy in a Medicaid population. Med Care. 1994;32:214–226. doi: 10.1097/00005650-199403000-00003. [DOI] [PubMed] [Google Scholar]
- Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43:521–530. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- Rizzo JA, Simons WR. Variations in compliance among hypertensive patients by drug class: implications for health care costs. Clin Ther. 1997;19:1446–1457. doi: 10.1016/S0149-2918(97)80018-5. [DOI] [PubMed] [Google Scholar]
- Caro JJ, Speckman JL. Existing treatment strategies: does noncompliance make a difference? J Hypertens-Supplement. 1998;12:533–537. [PubMed] [Google Scholar]
- Conlin PR, Gerth WC, Fox J, Roehm JB, Boccuzzi SJ. Four-year persistence patterns among patients initiating therapy with the angiotensin II receptor antagonist losartan versus other antihypertensive drug classes. Clin Ther. 2001;23:1999–2010. doi: 10.1016/S0149-2918(01)80152-1. [DOI] [PubMed] [Google Scholar]
- Wogen J, Kreilick CA, Livornese RC, Yokoyama K, Frech F. Patient adherence with amlodipine, lisinopril, or valsartan therapy in a usual-care setting. J Managed Care Pharm. 2003;9:424–429. doi: 10.18553/jmcp.2003.9.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marentette MA, Gerth WC, Billings DK, Zarnke KB. Antihypertensive persistence and drug class. Can J Cardiol. 2002;18:649–656. [PubMed] [Google Scholar]
- Caro JJ, Speckman JL, Salas M, Raggio G, Jackson JD. Effect of initial drug choice on persistence with antihypertensive therapy: the importance of actual practice data. Can Med Assoc J. 1999;160:41–46. [PMC free article] [PubMed] [Google Scholar]
- Elliott WJ, Plauschinat CA, Skrepnek GH, Gause D. Persistence, adherence, and risk of discontinuation associated with commonly prescribed antihypertensive drug monotherapies. J Am Board Fam Med. 2007;20:72–80. doi: 10.3122/jabfm.2007.01.060094. [DOI] [PubMed] [Google Scholar]
- Gregoire JP, Moisan J, Guibert R, Ciampi A, Milot A, Gaudet M, Côté I. Determinants of discontinuation of new courses of antihypertensive medications. J Clin Epidemiol. 2002;55:727–734. doi: 10.1016/S0895-4356(02)00400-6. [DOI] [PubMed] [Google Scholar]
- Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326:1427–34. doi: 10.1136/bmj.326.7404.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash DT. Rationale for combination therapy in hypertension management: focus on angiotensin receptor blockers and thiazide diuretics. South Med. 2007;100:386–392. doi: 10.1097/SMJ.0b013e318038174e. [DOI] [PubMed] [Google Scholar]
- Neutel JM, Black HR, Weber MA. Combination therapy with diuretics: an evolution of understanding. Am J Med. 1996;101:61S–70S. doi: 10.1016/S0002-9343(96)00269-0. [DOI] [PubMed] [Google Scholar]
- Fishman PA, Goodman MJ, Hornbrook MC, Meenan RT, Bachman DJ, O-Keeffe Rosetti MC. Risk adjustment using automated ambulatory pharmacy data: the RxRisk model. Med Care. 2003;41:84–99. doi: 10.1097/00005650-200301000-00011. [DOI] [PubMed] [Google Scholar]
- Van Wijk BL, Klungel OH, Heerdink ER, de Boer A. Rate and determinants of 10-year persistence with antihypertensive drugs. J Hypertens. 2005;23:2101–7. doi: 10.1097/01.hjh.0000187261.40190.2e. [DOI] [PubMed] [Google Scholar]
- Perreault S, Lamarre D, Blais L, Dragomir A, Berbiche D, Lalonde L, Laurier C, St-Maurice F, Collin J. Persistence with treatment in newly treated middle-aged patients with essential hypertension. Ann Pharmacother. 2005;39:1401–8. doi: 10.1345/aph.1E548. [DOI] [PubMed] [Google Scholar]
- Thaker DJ, Frech F, Gause D, Zhang W. Patient adherence and persistence with antihypertensive agents: a comparison of agents in different therapeutic classes. Am J Hypertens. 2005;18:222A. [Google Scholar]
- Patel BV, Remigio-Baker RA, Mehta D, Thiebaud P, Frech-Tamas F, Preblick R. Effects of initial antihypertensive drug class on patient persistence and compliance in a usual-care setting in the United States. J Clin Hypertens. 2007;9:692–700. doi: 10.1111/j.1524-6175.2007.07194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramley TJ, Gerbino PR, Nightengale BS, Frech-Tamas F. Relationship of blood pressure control to adherence with antihypertensive monotherapy in 13 managed care organizations. J Manag Care Pharm. 2006;12:239–45. doi: 10.18553/jmcp.2006.12.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen DB, Williams B, Goldberg HI, Martin DP, Engelberg R, LoGerfo JP. Comparison of prescription and medical records in reflecting patient antihypertensive drug therapy. Ann Pharmacother. 1994;28:99–104. doi: 10.1177/106002809402800119. [DOI] [PubMed] [Google Scholar]
- Choo PW, Rand CS, Inui TS, Lee ML, Cain E, Cordeiro-Breault M, Canning C, Platt R. Validation of patient reports, automated pharmacy records, and pill counts with electronic monitoring of adherence to antihypertensive therapy. Med Care. 1999;37:846–857. doi: 10.1097/00005650-199909000-00002. [DOI] [PubMed] [Google Scholar]
- Dezii CM. A retrospective study of persistence with single-pill combination therapy vs. concurrent two-pill therapy in patients with hypertension. Manag Care. 2000;9:2–6. [PubMed] [Google Scholar]