Abstract
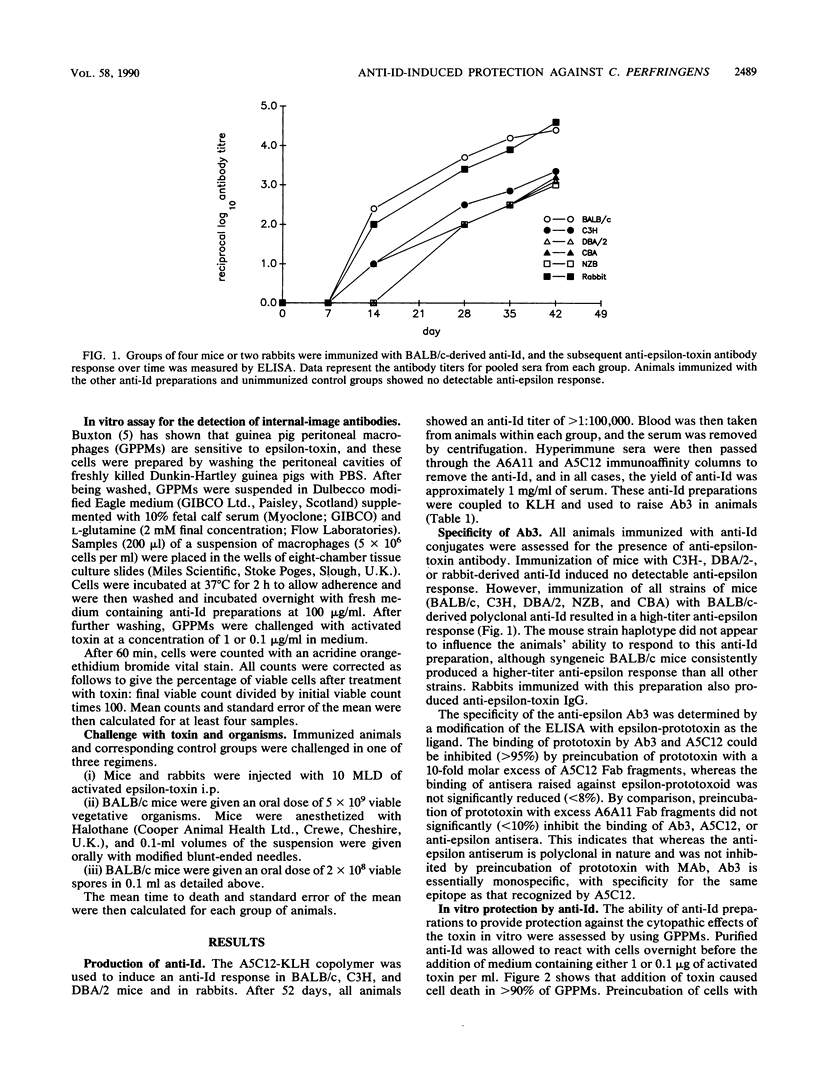
A monoclonal antibody (BALB/c mouse) with specificity for a neutralizing epitope on the epsilon-toxin produced by Clostridium perfringens type D was used to raise anti-idiotypic antibodies (anti-Id) in different strains of mice and rabbits. These were purified and used in cross-immunization studies to induce anti-(anti-idiotype). All strains of mice and rabbits immunized with BALB/c-derived anti-Id showed a high-titer antibody response directed towards the active site of the toxin. This protected the animals against toxin challenge and against an oral dose of the vegetative organisms. Animals immunized with other anti-Id preparations showed no specific antibody response and were not protected. Guinea pig peritoneal macrophages have a cell surface receptor for the toxin, and incubation of these cells with BALB/c anti-Id allowed them to survive toxin challenge, indicating that occupation of the receptors by the anti-Id prevented binding by the toxin. In conclusion, we have shown that an internal-image anti-Id preparation will induce protective immunity in syngeneic and xenogeneic animals and furthermore that immunity to a single epitope on the exotoxin is sufficient to protect against the toxin and clinical sequelae evoked by the disease-causing organism itself.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BATTY I., BULLEN J. J. The effect of Clostridium welchii type D culture filtrates on the permeability of the mouse intestine. J Pathol Bacteriol. 1956 Apr;71(2):311–323. doi: 10.1002/path.1700710206. [DOI] [PubMed] [Google Scholar]
- Buxton D. In-vitro effects of Clostridium welchii type-D epsilon toxin on guinea-pig, mouse, rabbit and sheep cells. J Med Microbiol. 1978 Aug;11(3):299–302. doi: 10.1099/00222615-11-3-299. [DOI] [PubMed] [Google Scholar]
- Buxton D. The use of an immunoperoxidase technique to investigate by light and electron microscopy the sites of binding of Clostridium welchii type-D epsilon toxin in mice. J Med Microbiol. 1978 Aug;11(3):289–292. doi: 10.1099/00222615-11-3-289. [DOI] [PubMed] [Google Scholar]
- Darakhshan H., Lauerman L. H. Some properties of beta toxin produced by Clostridium haemolyticum strain IRP-135. Comp Immunol Microbiol Infect Dis. 1981;4(3-4):307–316. doi: 10.1016/0147-9571(81)90017-5. [DOI] [PubMed] [Google Scholar]
- Gefter M. L., Margulies D. H., Scharff M. D. A simple method for polyethylene glycol-promoted hybridization of mouse myeloma cells. Somatic Cell Genet. 1977 Mar;3(2):231–236. doi: 10.1007/BF01551818. [DOI] [PubMed] [Google Scholar]
- Habeeb A. F. Studies on epsilon-prototoxin of Clostridium perfringens type D. I. Purification methods: evidence for multiple forms of epsilon-prototoxin. Arch Biochem Biophys. 1969 Mar;130(1):430–440. doi: 10.1016/0003-9861(69)90055-1. [DOI] [PubMed] [Google Scholar]
- Jerne N. K., Roland J., Cazenave P. A. Recurrent idiotopes and internal images. EMBO J. 1982;1(2):243–247. doi: 10.1002/j.1460-2075.1982.tb01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerne N. K. Towards a network theory of the immune system. Ann Immunol (Paris) 1974 Jan;125C(1-2):373–389. [PubMed] [Google Scholar]
- Kaufmann S. H., Eichmann K., Müller I., Wrazel L. J. Vaccination against the intracellular bacterium Listeria monocytogenes with a clonotypic antiserum. J Immunol. 1985 Jun;134(6):4123–4127. [PubMed] [Google Scholar]
- Kennedy R. C., Melnick J. L., Dreesman G. R. Antibody to hepatitis B virus induced by injecting antibodies to the idiotype. Science. 1984 Mar 2;223(4639):930–931. doi: 10.1126/science.6198721. [DOI] [PubMed] [Google Scholar]
- McNamara M. K., Ward R. E., Kohler H. Monoclonal idiotope vaccine against Streptococcus pneumoniae infection. Science. 1984 Dec 14;226(4680):1325–1326. doi: 10.1126/science.6505692. [DOI] [PubMed] [Google Scholar]
- NIILO L. BOVINE "ENTEROTOXEMIA". 3. FACTORS AFFECTING THE STABILITY OF THE TOXINS OF CLOSTRIDIUM PERFRINGENS TYPES A, C AND D. Can Vet J. 1965 Feb;6(2):38–42. [PMC free article] [PubMed] [Google Scholar]
- Nisonoff A., Lamoyi E. Implications of the presence of an internal image of the antigen in anti-idiotypic antibodies: possible application to vaccine production. Clin Immunol Immunopathol. 1981 Dec;21(3):397–406. doi: 10.1016/0090-1229(81)90228-2. [DOI] [PubMed] [Google Scholar]
- Noseworthy J. H., Fields B. N., Dichter M. A., Sobotka C., Pizer E., Perry L. L., Nepom J. T., Greene M. I. Cell receptors for the mammalian reovirus. I. Syngeneic monoclonal anti-idiotypic antibody identifies a cell surface receptor for reovirus. J Immunol. 1983 Nov;131(5):2533–2538. [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck B., Scheuber P. H., Londong W., Sailer-Kramer B., Bartsch K., Hammer D. K. Protection against the staphylococcal enterotoxin-induced intestinal disorder in the monkey by anti-idiotypic antibodies. Proc Natl Acad Sci U S A. 1988 May;85(9):3170–3174. doi: 10.1073/pnas.85.9.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo C., Callegaro L., Lanza E., Ferrone S. Re.: Purification of IgG monoclonal antibody by caprylic acid precipitation. J Immunol Methods. 1983 Dec 16;65(1-2):269–271. doi: 10.1016/0022-1759(83)90324-1. [DOI] [PubMed] [Google Scholar]
- Sege K., Peterson P. A. Use of anti-idiotypic antibodies as cell-surface receptor probes. Proc Natl Acad Sci U S A. 1978 May;75(5):2443–2447. doi: 10.1073/pnas.75.5.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein K. E., Söderström T. Neonatal administration of idiotype or antiidiotype primes for protection against Escherichia coli K13 infection in mice. J Exp Med. 1984 Oct 1;160(4):1001–1011. doi: 10.1084/jem.160.4.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington R. W., Mülders M. S., Van Rensburg J. J. Enzymatic activation of Clostridium perfringens epsilon prototoxin and some biological properties of activated toxin. Onderstepoort J Vet Res. 1973 Dec;40(4):151–154. [PubMed] [Google Scholar]


