Abstract
Although gait disturbance is frequently documented among patients with traumatic brain injury (TBI), gait data from animal models of TBI are lacking. To determine the effect of TBI on gait function in adult mice, we assessed gait changes following unilateral controlled cortical impact (CCI) using a computer-assisted automated gait analysis system. Three days after CCI, intensity, area or width of paw contact were significantly decreased in forepaw(s) while the relative paw placement between the fore and hindpaws altered, suggesting that TBI affected sensorimotor status and reduced inter-limb coordination. Similar to TBI patients, CCI decreased gait velocity and stride length, and prolonged stance and swing phase in mice. Following CCI, step pattern was also changed with increasing use in the ipsilateral-diagonal limb sequence. Our results indicate that gait analysis provides great insight into both spatial and temporal aspects of limb function changes during overground locomotion in quadruped species with head injury that are valuable for the purpose of treatment and rehabilitation. Our study also provides additional functional validation for the established mouse CCI model that is relevant to human head injury.
Keywords: controlled cortical impact, catwalk, motor function, corticospinal tract, and neuroplasticity
Introduction
Apart from cognitive impairment, motor dysfunction is a common sequela in patients with moderate to severe traumatic brain injury (TBI) (Basford et al. 2003; van Loo et al. 2004; Wiese et al. 2004). A recent longitudinal study suggests that more than one-third of TBI patients still exhibit neuromotor impairment two years after injury with persistent gait abnormality (Walker and Pickett 2007). Patients with head trauma produce a gait pattern characteristics of temporal asymmetry, increased double-limb support time, reduced stride length and decreased walking speed (Johnk et al. 1999; Ochi et al. 1999). Dual-task walking test reveals an intriguing interplay between gait and executive functions (Vallee et al. 2006; Yogev-Seligmann et al. 2008), both are frequently affected by TBI. Consistent with this notion, a recent report indicated that TBI patients had more difficulty in maintaining dynamic stability during gait when performing more challenging walking task (Niechwiej-Szwedo et al. 2007).
Currently there are a number of systems available for measuring gait and posture changes in humans including GAITRite, the three-dimensional gait analysis, force-plate, and plantar pressure distribution system (Chen et al. 2005; Ng and Hui-Chan 2005). However, the technology in measuring gait and ambulation in laboratory rodents is still limited. Although rotor rod, gridwalk or the cylinder tests have been successfully used to detect motor deficits after experimental TBI (Baskin et al. 2003; Hamm et al. 1994), each of these manual tests only assesses a narrow spectrum of gait characteristics. Recently, the catwalk imaging method, similar to the concept of GAITRite, was adapted to provide an automated means to assess gait function with the benefit of measuring a number of locomotor-related parameters simultaneously. This method can not only detect the dynamic as well as static aspects of gait as the human gait analysis systems, but also the spatial and temporal aspects of interlimb coordination that are particularly valuable for quadrupeds. Although the catwalk method has been used in a variety of rodent studies for assessing impaired gait function following spinal cord injury (Hamers et al. 2006), pyramidotomy (Starkey et al. 2005), Parkinson’s disease (Vlamings et al. 2007), neurotmesis (Deumens et al. 2007), and neuropathy (Gabriel et al. 2007), it has not yet been explored in animal models of TBI. Assessing gait changes in quadrupeds following experimental TBI provides complementary information regarding motor function status that is directly relevant to clinical studies.
Using acute functional assessment as a reliable predictor for vocational outcome has been reported in a series of follow-up studies involving TBI patients (Cifu et al. 1997; Keyser-Marcus et al. 2002), reiterating the importance in developing sensitive and accurate methods in evaluating functional deficits at early stages after head trauma. The current study focuses on the quantification and analysis of gait function in mice with acute TBI by a newly developed gait imaging and analysis system for rodents. This computer-assisted catwalk method allows a comprehensive characterization of both spatial and temporal gait parameters that are related to sensorimotor status and interlimb coordination. Our data suggest that the automated catwalk system might be the instrument of choice to detect a wide range of motor function deficits in mice with head injury, thus become an invaluable tool to study the effect of therapeutic intervention on post trauma motor function recovery.
Materials and methods
Animals and housing
This study was conducted in accordance with the animal care guidelines issued by the National Institutes of Health and by the San Francisco Veterans Affairs Medical Center Animal Care and Use Committee. Adult male C57BL/6 mice 2.5 months of age, weighing 24 to 30 g, purchased from Charles River Laboratories, Inc. (Wilmington, MA, USA) were housed in institutional standard cages (4 mice per cage) on a 12-hr light/12-hr dark cycle with ad libitum access to water and food before and during experimental procedures. The animals were randomly assigned into two procedure groups for receiving traumatic brain injury (n = 15) or sham surgery (n = 12).
Surgical Procedure
Animals were anesthetized with isoflurane/O2/N2O (1.5/30/68.5%) during surgery and their core temperature was maintained within 37± 0.5°C with a heating blanket and rectal thermistor servo-loop during both the surgical and the postoperative recovery period.
Secured in the stereotaxic frame (Kopf instrument, Tujunga, CA, USA) followed by a midline skin incision, a 3 mm diameter circular craniotomy was performed with a dental drill, lateral (right side) to the mid-sagittal suture centering at [AP: -2.0 mm; ML: 2.0 mm] relative to Bregma. Through this opening, the operated animal was subjected to a controlled cortical impact using a generic CNS injury device which was employed previously for inducing TBI in mouse (Bilgen 2005). The impactor, operated by a linear motor and microprocessor controller (Linmot, Zurich, Switzerland), was equipped with a polished stainless steel tip of 2.0 mm in diameter. The impactor tip was first centered over the craniotomy and was slowly lowered till the tip just contacted the dura (confirmed by a operating microscope). The impact injury was generated using the following parameters: 1.5 m/sec strike velocity, 1.5 mm depth of penetration, and a 155 msec contact time. Pilot experiments suggest that the combination of these parameters produces a moderate to severe level of injury in mice that is likely to induce significant motor asymmetry without mortality. The scalp was then closed with sutures and each animal was given 1.0 ml of isotonic saline subcutaneously after the operation to prevent dehydration. Sham animals received craniotomy but no impact from the CCI device.
Computer-assisted method for gait analysis
Mice were subjected to gait assessment at 3 days after CCI using the CatWalk automated gait analysis system (Noldus Information Technology, Wageningen, The Netherlands). The apparatus is made of a 1.3-meter-long glass plate with dim fluorescent light beamed into the glass from the side. In a darkened environment (below 1 LUX of illumination), the light is reflected downward and the images of the footprints recorded by the camera under the walkway when the animal’s paws come in contact with the glass surface. Mice were subjected to 3 consecutive runs of gait assessment at 3 days after CCI or sham surgery. The identity of each animal with respect to treatment was concealed to experimenters who conducted the trials and analysis. The images from each trial were converted into digital signals and processed with a threshold set at 30 arbitrary units (a. u., ranging from 0 to 225, meaning all pixels brighter than 30 a.u. were used). The imaging setting used for this study defines 1 pixel to be 0.085 mm. Following the identification and labeling of each footprint, a wide range of gait data was generated including the 1) spatial parameters related to individual paws (intensity, maximum area, print area, print width and print length); 2) relative spatial relationship between different paws (base of support, relative paw placement, and stride length); 3) interlimb coordination (step pattern and regularity index); and 4) temporal parameters (swing, stance, cadence, and walk speed), summarized as the following:
Intensity: This parameter describes the mean pressure exerted by one individual paw during the floor contact when crossing the walkway. Data analysis was performed with a threshold value of 30 (ranging from 0 to 225) and expressed in arbitrary unit (a.u.).
Maximum area: This parameter describes the total floor area contacted by the paw during the stance phase and is expressed in square pixel.
Print area: Unlike the maximum area, the print area represents the complete print including all frames that makes up a stance.
Print width: It is a measurement of the width of the print area and is expressed in pixel.
Print length: It is a measurement of the length of the print area and is expressed in pixel.
Relative paw placement: It is defined as the position of the hind paw relative to the position of the fore paw in the previous step cycle.
Stance: It describes the time duration during which the paw is in contact with the glass plate. It is expressed in second.
Swing: It describes the time interval between two consecutive paw placements of the same paw in which the paw is not in contact with the glass plate. It is expressed in second.
Stride Length: This parameter describes the distance between two consecutive paw placements of the same paw and is expressed in pixel.
Cadence: It reflects the frequency of steps during a trial. It is calculated as the no. of steps divided by (Initial Contactlast step-Initial Contactfirst step).
Walk speed: It represents the Distance that the animals walk per second; It is calculated as the sum of stride length divided by (Max Contactlast step-Max Contactfirst step).
Swing Speed: This parameter is computed from stride length and swing duration and is expressed in pixel/second.
% Support time: This parameter displays the relative duration of contact with the glass plate of all combinations of number of paws. The combinations range from no paw contact (zero), using a single paw, diagonal two paws (RF & LH or LF & RH), lateral two paws (RF & RH or LF & LH), girdle two paws (RF & LF or RH & LH), three paws or four paws.
Base of support (BOS): This parameter describes the distance between the mass-midpoints of two fore or hind prints at max contact during each step cycle. The results from each step cycle are averaged and expressed in mm in each trial.
Step pattern: There are a total of six normal step sequence patterns observed in rodents (Ca: RF-LF-RH-LH; Cb: LF-RF-LH-RH; Aa: RF-RH-LF-LH; Ab: LF-RH-RF-LH; Ra: RF-LF-LH-RH; Rb: LF-RF-RH-LH) that fall into three categories (cruciate, alternate and rotary) or three types of limb sequences (girdle-diagonal, ipsilateral-diagonal and ipsilateral-girdle).
Regularity Index (RI) (%): An % index for the degree of interlimb coordination during gait, as measured by the number of normal step sequence patterns (NSSP), multiplied by the number of paws and divided by the number of paw placements; RI = 100% × (NSSP × 4) / no. of paw placements.
Tissue preparation
Animals were anesthetized with ketamine (80 mg/kg; Parke-Davis, Morris Plains, NJ, USA) and xylazine (20 mg/kg; Butler, Columbus, OH, USA) and perfused transcardially with 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB), pH 7.4. The brains were removed, fixed overnight in 4% PFA-PB and placed in 20% sucrose for 48 hrs. Coronal sections were cut at 40 μm on a microtome and collected serially.
Immunohistochemistry staining and reconstruction of impact area
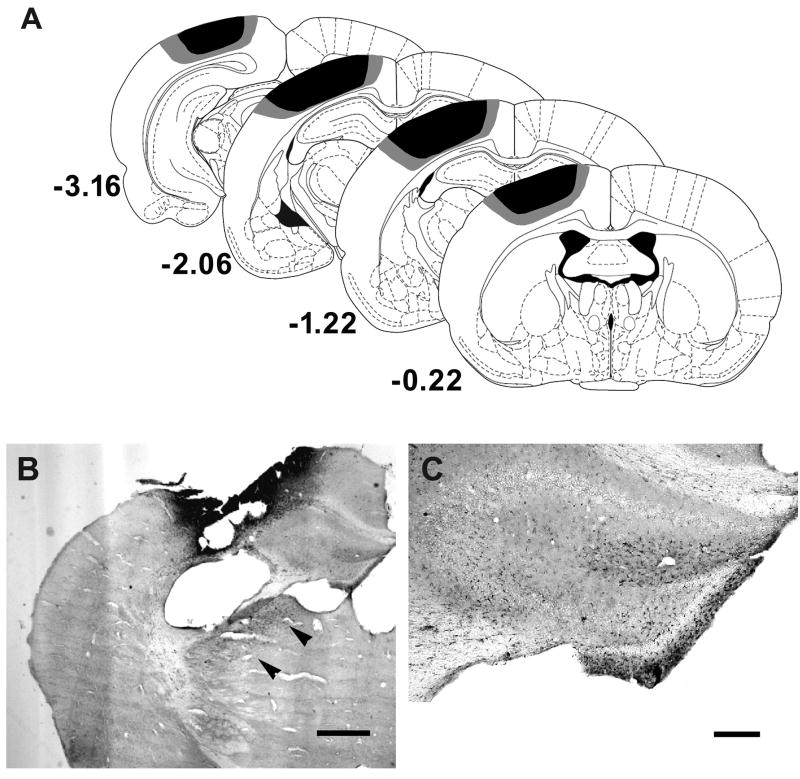
Serial coronal sections (480 μm apart) were immunostained with anti-NeuN and anti-ED1 antibodies as described previously (Liu et al. 2007; Matsumori et al. 2006) for detection of impact area and inflammation. The extent of injury in mice treated with CCI was illustrated on coronal atlas plates produced by Paxinos and Watson. Smallest and largest damaged areas were highlighted in black and gray, respectively.
Statistical analyses
Data were expressed as mean ± standard error of mean (SEM). All statistical tests were carried out with Statview 5.0.1 software (SAS Institute Inc., Cary, NC, USA). Statistical significance was evaluated using one-way and two-way analyses of variance (ANOVAs) followed by post hoc paired comparisons using the Fisher’s PLSD or Student-Newman-Keuls tests when appropriate. Linear correlations between weight, walk speed, cadence, and the other measures of gait were evaluated by determination of the Spearman’s correlation coefficient with Bonferroni’s correction using the STATA 10.0 software (StataCorp, TX, USA). Values of P < 0.05 were considered as significant.
Results
We evaluated the extent of gait impairment at the acute stage following the impact using an established rodent model that produces consistent and reliable injury. Consistent with previous findings, CCI produced a direct impact in the parasagittal cortex (Fox et al. 1998; Hannay et al. 1999) including forelimb, hindlimb areas and a portion of the parietal 1 area (Figure 1A). In addition to the contusion seen in the sensorimotor cortex, focal areas of inflammation were observed in the hippocampus and thalamus (Figure 1B&C) at 3 days following CCI, suggesting the presence of diffused secondary injury. There was no significant difference in body weight between the sham (22.7 ± 1.4 g) and the TBI mice (22.2 ± 1.3 g) at this time. The majority of the gait parameters described in this study were expressed for each paw, namely the left fore (LF), left hind (LH), right fore (RF), and right hind (RH) paw.
Figure 1.
The extent of injury by controlled cortical impact at the time of gait assessment. A, Reconstructions of coronal sections (adapted from Paxinos and Watson) showing the extent of injury in mice with CCI. Smallest and largest damaged areas appear in black and gray, respectively. Numbers indicate the section distance in millimeters from Bregma. Representative photomicrographs of brain sections show the expression of ED-1 inflammatory marker in the thalamus (B, arrowheads) and hippocampus (C). Scale bar: 0.5 mm (B) and 250 μm (C).
TBI led to a reduction in paw pressure and area of paw contact
Intensity
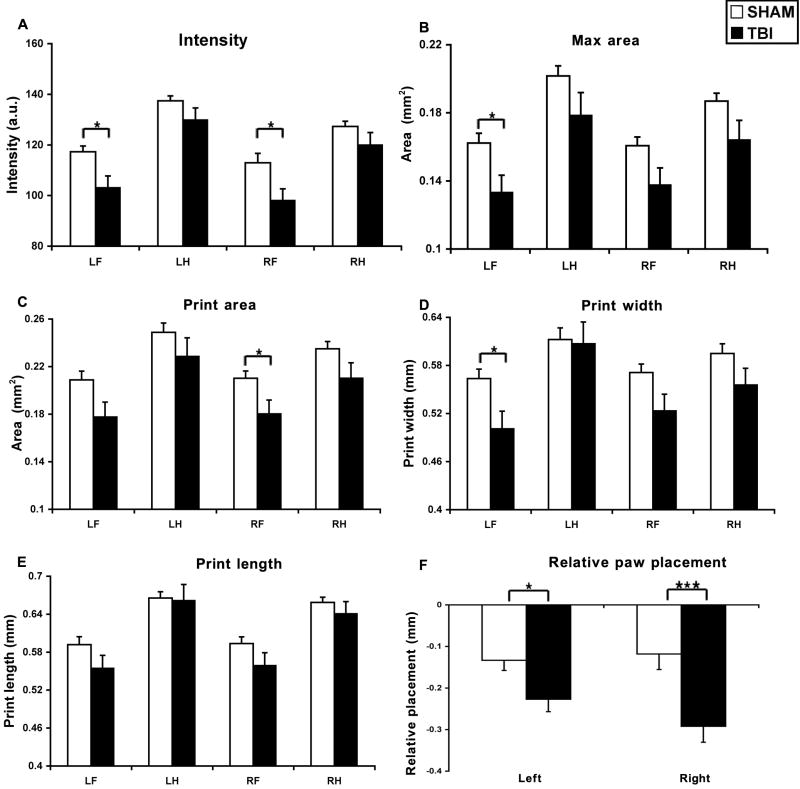
Three days after CCI, the paw pressure during floor contact, as measured by the intensity of the footprint, was significantly affected (TBI effect: F1, 100 = 14.92, P < 0.0002; paw effect: F3, 100 = 20.99, P < 0.0001). The intensity of all four paws in TBI mice was generally lower than that of sham mice (interaction TBI × paw, F3, 100 = 0.54, P > 0.65, NS) with a statistically significant decrease detected in both the LF (affected) paw (P < 0.01) and RF paw (P < 0.05) (Figure 2A).
Figure 2.
Effects of TBI on gait spatial parameters including paw pressure, area of paw contacts, and relative paw placement. The max areas (B) were reduced at all paws, and the intensity (A) and print area (C) of fore paws was also decreased acutely after TBI. TBI reduced the width (D) but not the length (E) of the paw print. Following TBI, the distance between fore and hindpaw placement (F) increased, suggesting that the hindpaws of TBI mice were lagging behind compared to sham mice. *: P < 0.05; ***: P < 0.005. Abbreviations: TBI, traumatic brain injury; RF, right fore; RH, right hind; LF, left fore; LH, left hind; a.u., arbitrary units.
Max area
The size of the paw print area during maximal floor contact in TBI mice was smaller than that of sham mice at 3 days after CCI (TBI effect: F1, 100 = 13.2, P < 0.0005; paw effect: F3, 100 = 9.62, P < 0.0001). The max areas of all 4 paws were by and large reduced by CCI (interaction TBI × paw, F3, 100 = 0.05, P > 0.98, NS) with a significant reduction in the affected forepaw (LF) (P < 0.05) (Figure 2B).
Print area and dimensions
The results of print area were generally similar to that of max area at 3 days after CCI. Print areas of all four paws in CCI mice were in general smaller than those of sham mice (TBI effect: F1, 100 = 10.8, P < 0.005; paw effect: F3, 100 = 7.4, P < 0.0005), especially in the forepaws (Figure 2C). Detailed analysis further revealed that the reduction in print area seen after CCI likely resulted from a decrease in the width (called box width) (TBI effect: F1, 100 = 7.7, P < 0.01; paw effect: F3, 100 = 5.9, P < 0.001). The box width of all four paws in TBI mice was generally smaller than that of sham mice (interaction TBI × paw, F3, 100 = 0.76, P > 0.52, NS) with a statistically significant decrease detected in the affected LF paw (P < 0.05) (Figure 2D). CCI did not significantly affect the length (box length) of the paw print (TBI effect: F1, 100 = 3.3, P > 0.07; paw effect: F3, 100 = 5.9, P < 0.0001) (Figure 2E).
TBI altered the relative positions between ipsilateral paws
Relative paw placement
In both the sham and TBI mice, the hindpaws were always placed behind the forepaws of during the same cycle, producing a negative value when subtracting the x-coordinates of the mass midpoints of ipsilateral hindpaws from forepaws at maximal contact. Three days following CCI, the distance between ipsilateral paws of the adjacent cycles were increased (Right paw: F1, 25 = 3.2, P < 0.005; Left paw: F1, 25 = 2.2, P < 0.05), suggesting that the hindpaws of the TBI mice advanced in less distance from cycle to cycle compared to sham mice (Figure 2F).
TBI increased stance and swing duration, and decreased stride length and gait velocity
Temporal parameters
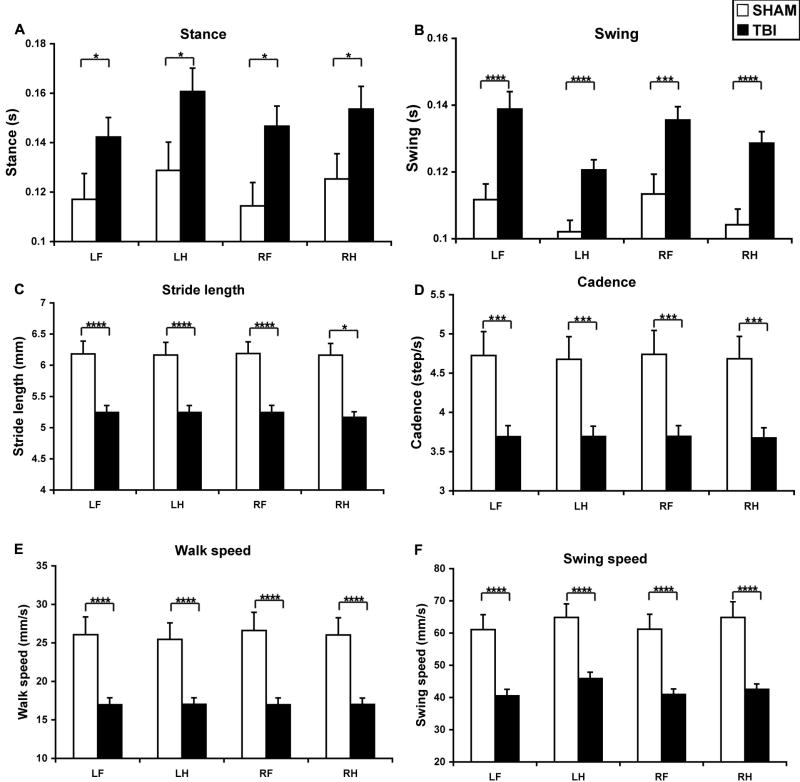
The duration of the stance increased significantly after CCI (TBI effect: F1, 100 = 19.1, P < 0.0001; paw effect: F3, 100 = 1.1, P > 0.32). The stance of all four paws in TBI mice was generally longer than that of sham mice (interaction TBI × paw, F3, 100 = 0.06, P > 0.97, NS) (Figure 3A). The swing phase was increased as well after TBI (TBI effect: F1, 100 = 55.4, P < 0.0001; paw effect: F3, 100 = 4.7, P < 0.005) (Figure 3B). The swing duration of all four paws in TBI mice was significantly longer than that of sham mice (interaction TBI × paw, F3, 100 = 0.34, P > 0.79) (LF: P < 0.0005; LH: P < 0.0005; RF: P < 0.005; RH: P < 0.0005).
Figure 3.
TBI affected temporal parameters and stride length. Following CCI, the swing duration (B) was significantly increased in all 4 paws and the stance (A) increased in the LH and RF. The stride length was significantly reduced in all 4 paws. All velocity related parameters including cadence (D), walk speed (E) and swing speed (F) were also reduced after TBI. Abbreviations: TBI, traumatic brain injury; RF, right fore; RH, right hind; LF, left fore; LH, left hind. *: P < 0.05; ***: P < 0.005; ****: P < 0.001.
Stride length
TBI reduced the distance between successive placements of the same paw during maximal contact, known as stride length (TBI effect: F1, 100 = 74.3, P < 0.0001; paw effect: F3, 100 = 0.003, P > 0.99) (Figure 3C). The stride length of all four paws in TBI mice was significantly shorter than that of sham mice (interaction TBI × paw, F3, 100 = 0.004, P > 0.99) (LF: P < 0.0005; LH: P < 0.0005; RF: P < 0.0005; RH: P < 0.0005).
Velocity
Since many gait parameters are influenced by the speed of walking, we determined the effect of CCI on cadence (steps/sec) (Figure 3D), walk velocity (distance moved/sec) (Figure 3E) and swing speed (Figure 3F). TBI significantly reduced cadence (TBI effect: F1, 100 = 45.1, P < 0.0001; paw effect: F3, 100 = 0.015, P > 0.99) in all 4 paws (P < 0.005 for all), walk speed (TBI effect: F1, 100 = 65.5, P < 0.0001; paw effect: F3, 100 = 0.04, P > 0.98) in all 4 paws (P < 0.005 for all) and swing speed (TBI effect: F1, 100 = 79.9, P < 0.0001; paw effect: F3, 100 = 0.9, P > 0.44) in all 4 paws (LF: P < 0.0005; LH: P < 0.0005; RF: P < 0.0005; RH: P < 0.0001).
TBI changed step pattern but did not affect the overall support time or step width
Step pattern
There are 6 step-sequence patterns described in rodents, as indicated in Figure 4A. The most frequently step pattern observed in mice was the alternate b pattern (Ab), which accounted for 48.8% of all step patterns in the sham mice and 41.1% in the TBI mice at 3 days after impact (P > 0.27, NS). However, there was a nearly two-fold increase in the cruciate b pattern (Cb) after CCI (P < 0.05) (Figure 4A). There were no rotary patterns (Ra or Rb) observed in our mice subjects. There was no significant difference in the regularity index (RI), the degree to which the animals use normal step sequence patterns, between the sham (98.8 ± 1.1) and TBI (99.4 ± 0.9) mice (P > 0.15).
Figure 4.
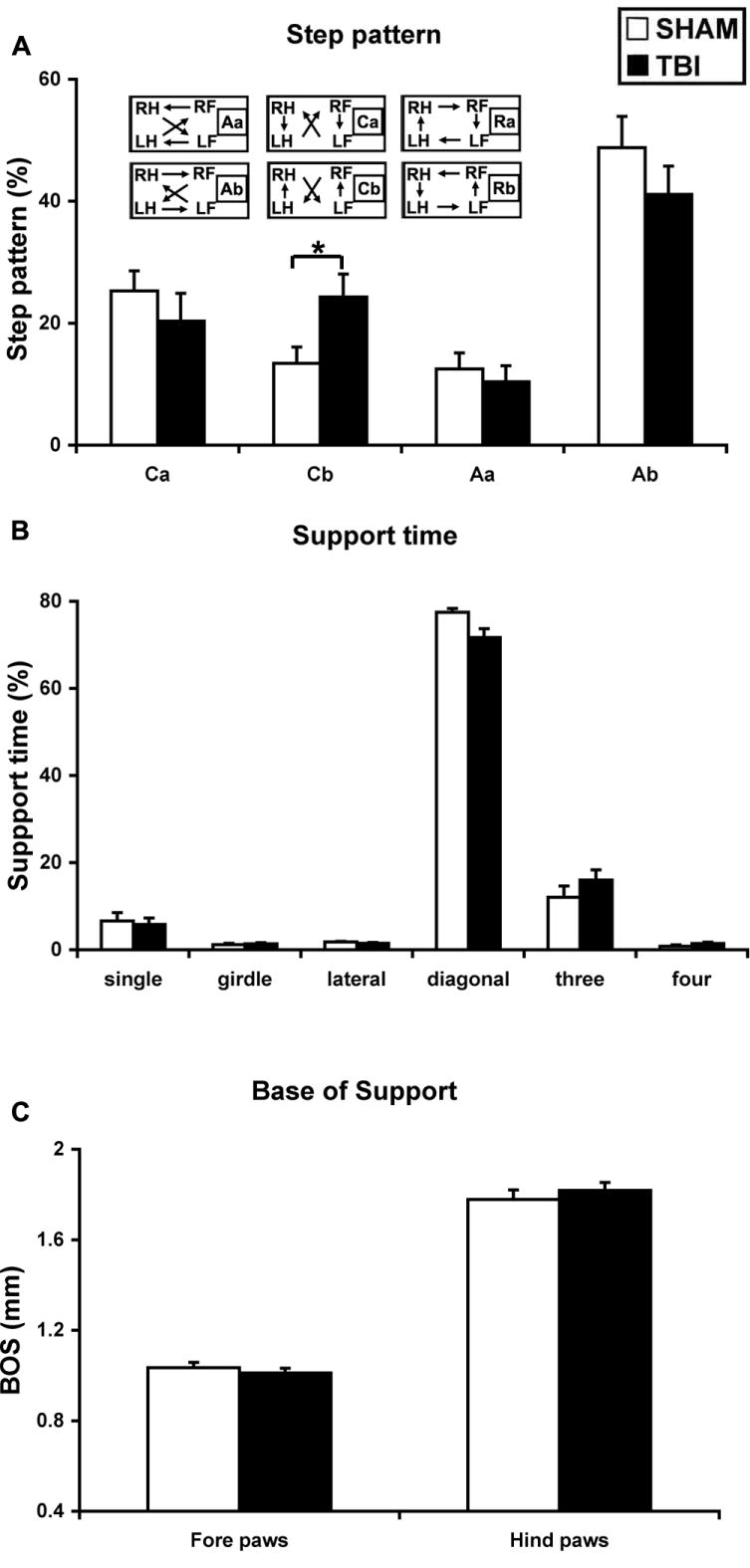
TBI altered step patterns without affecting simultaneous usage of paws during landing. There are a total of 6 step patterns observed in rodents (A-inset), classified under 3 categories as the alternate (Aa and Ab), cruciate (Ca and Cb) or the rotary (Ra and Rb) patterns which are absent in our mice subjects. CCI significantly increased the percentages in the cruciate b pattern (Cb) by almost two folds (A). *: P < 0.05. TBI had a borderline effect in increasing the % double support time using the diagonal paws (B). TBI did not affect simultaneous usage of 3 or 4 paws, nor did it affect single support time. C, TBI did not affect the base of support (BOS) either in the fore or hindlimbs.
Support time
The most frequent simultaneous usage of paws during floor contact was with 2 paws (diagonal, lateral or girdle pairs), followed by 3, 1, then 4 paws, in both sham and TBI mice (Figure 4B). There was a borderline significant decrease in the double support time using the diagonal pair of paws (P = 0.052) among the TBI mice compared to sham mice (Figure 4B). There was also a trend towards an increase in triple-limb support time in the TBI mice, but the difference was not statistically significant (P = 0.19). There was no difference in support time between groups with 1 or 4 paws (P > 0.75 or P > 0.14, respectively).
Base of support
There was no significant difference in the distance between the bilateral paws, known as base of support (BOS) or step width, between the sham and TBI mice (forepaw: P > 0.46; hindpaw: P > 0.46) (Figure 4C).
The correlation among the parameters
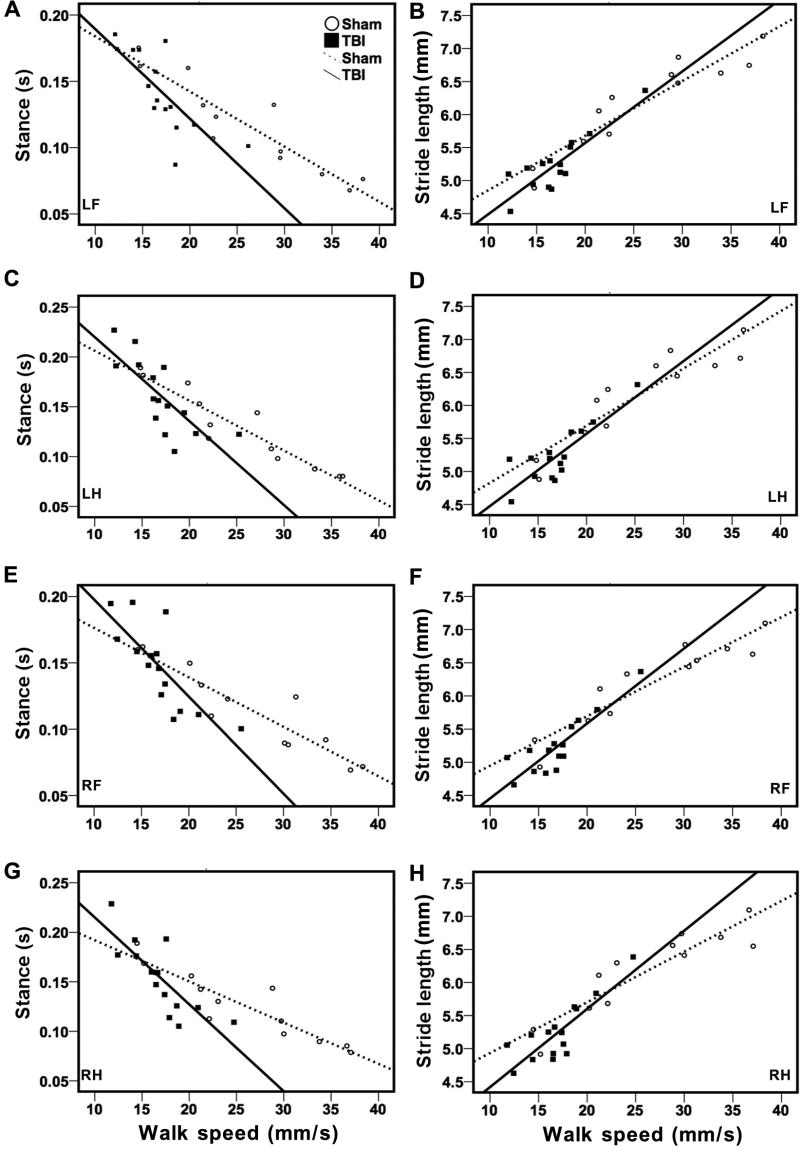
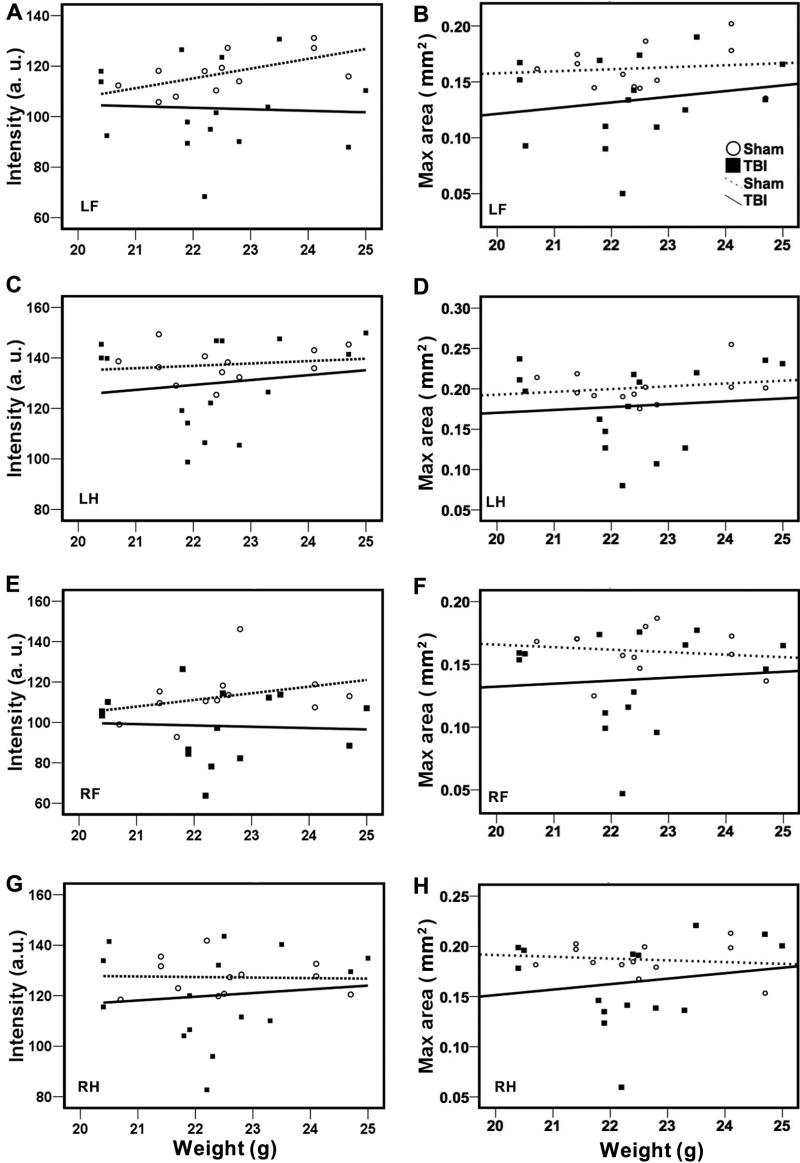
Because walking velocity is a very important determinant that can potentially influence several parameters of gait function (Gorska et al. 1998), we determined the relationship between walk speed and the other gait parameters by Spearman’s correlation analysis (Table 1). Our results showed that walk speed, highly correlated with cadence, were interdependent with stride length and temporal parameters such as stance, and swing. In general, stance and swing duration showed a negative correlation but stride length displayed a positive correlation with walk speed (Figure 5). Walk speed was also positively correlated with single support and negatively correlated with triple-limb support time in the sham animals. However, walk speed did not affect step pattern (Table 1), paw intensity, area of paw contact, and other spatial parameters such as relative paw placement or BOS (data not shown). Besides, there was no correlation between walk speed and body weight (not shown). Body weight also did not influence paw intensity or area of paw contact in either sham or TBI animals (Figure 6).
Table 1.
Relationship between walk speed and stance, stride length, swing, cadence, support time and step pattern.
| Walk speed | ||||||
|---|---|---|---|---|---|---|
| Sham
|
TBI
|
|||||
| Spearman cca | P value | Spearman cca | P value | |||
| Stance | LF | -0.9371 | p < 0.001**** | 0.80 | p < 0.001**** | |
| LH | -0.9650 | p < 0.001**** | -0.8464 | p < 0.001**** | ||
| RF | -0.8741 | p < 0.001**** | -0.8286 | p < 0.001**** | ||
| RH | -0.9510 | p < 0.001**** | -0.8321 | p < 0.001**** | ||
| Stride length | LF | 0.9580 | p < 0.001**** | 0.71 | p < 0.001**** | |
| LH | 0.9301 | p < 0.001**** | 0.6429 | p < 0.01** | ||
| RF | 0.9091 | p < 0.001**** | 0.7821 | p < 0.001**** | ||
| RH | 0.8671 | p < 0.001**** | 0.7107 | p < 0.005*** | ||
| Swing | LF | -0.9650 | p < 0.001**** | -0.4964 | 0.0598 | |
| LH | -0.8322 | p < 0.001**** | -0.3714 | 0.1728 | ||
| RF | -0.8811 | p < 0.001**** | -0.5857 | p < 0.05* | ||
| RH | -0.9560 | p < 0.001**** | -0.3214 | 0.2427 | ||
| Cadence | LF | 0.9650 | p < 0.001**** | 0.8464 | p < 0.001**** | |
| LH | 0.9790 | p < 0.001**** | 0.9036 | p < 0.001**** | ||
| RF | 0.9930 | p < 0.001**** | 0.8036 | p < 0.001**** | ||
| RH | 0.9790 | p < 0.001**** | 0.8929 | p < 0.001**** | ||
| Support time | Zero | 0.3817 | 1.0000 | 0.2458 | 1.0000 | |
| Single | 0.8392 | p < 0.05* | 0.5214 | 1.0000 | ||
| Double | ||||||
| Diagonal | 0.4895 | 1.0000 | 0.4214 | 1.0000 | ||
| Lateral | -0.4266 | 1.0000 | -0.0786 | 1.0000 | ||
| Girdle | 0.0946 | 1.0000 | -0.1071 | 1.0000 | ||
| Three | -0.9231 | p < 0.001**** | -0.7179 | 0.0723 | ||
| Four | -0.6737 | 0.4563 | -0.5893 | 0.5822 | ||
| Step pattern | Aa | 0.5734 | 0.5127 | 0.3009 | 1.0000 | |
| Ab | -0.6025 | 0.3816 | -0.3393 | 1.0000 | ||
| Ca | 0.6923 | 0.1259 | 0.5786 | 0.2385 | ||
| Cb | 0.1436 | 1.0000 | -0.5357 | 0.3957 | ||
| Ra | NAb | NA | NA | NA | ||
| Rb | NA | NA | NA | NA | ||
Spearman correlation coefficient;
no data available for correlation analysis.
p < 0.05;
p < 0.01;
p < 0.005;
p < 0.001.
Figure 5.
Significant correlations between velocity and stance (A, C, E, &G) or stride length (B, D, F, &H) in both sham and TBI mice at 3 days after CCI. Variation in stance or stride length with walk speed in sham (open circles) and TBI mice (filled squares) is shown in the plot. Linear regression lines were plotted in each treatment group (dotted lines for sham and solid lines for TBI). Stance duration showed a negative correlation with walk speed, while stride length showed a positive correlation with walk speed in each paw.
Figure 6.
Lack of correlation between body weight and intensity (A, C, E, &G) or max area (B, D, F, &H) in either sham or TBI mice at 3 days after CCI. Variation in intensity or max contact area with body weight in sham (open circles) and TBI mice (filled squares) is shown in the plot. Linear regression lines were plotted in each treatment group (dotted lines for sham and solid lines for TBI). There was no relationship between body weight and intensity or max area.
Discussion
The present study provides a thorough analysis of gait function in mice after TBI using the computer-assisted automated gait analysis method. Most of the spatial parameters related to individual paw placements and interlimb coordination were altered in mice following unilateral CCI. Similar to human TBI patients, stride length and the temporal parameters such as stance, swing or gait velocity were also significantly affected in mice after CCI. In addition, changes in the sensitivity of plantar surface of the paw were observed in TBI mice, possibly due to an impairment of the sensorimotor function. To our knowledge, this is the first study to characterize the gait impairment in laboratory quadrupeds with traumatic brain injury, representing an important validation for the detection of motor deficits in this model.
Gait impairment is not only associated with neurodegenerative diseases such as Alzheimer’s disease (AD) and Parkinson’s disease (PD), but also commonly observed in victims of central or peripheral nervous system injury. Ischemic stroke, unilateral spinal cord injury or pyramidotomy all share a compromised corticospinal tract (CST) with various degrees of hemiparalysis and distinct gait features. Due to the location of impact, mice also exhibited temporal hemiparalysis following unilateral CCI (unpublished observation), suggesting the involvement of CST. In rats with pyramidotomy, the stride length of all four paws is typically reduced as compared to sham controls, possibly because of a general decrease in walk speed (Starkey et al. 2005). Following unilateral spinal cord injury, the maximal contact area of the affected forepaws is often reduced most likely because of reduced forelimb weight bearing (Gensel et al. 2006). Stride length has also been reported to decrease in the unaffected, but not the affected, forepaw, pointing to compensatory phenomena in the affected limbs. Following experimental stroke using the distal middle cerebral artery occlusion model, paw intensity and contact area was decreased and double support time increased, while stride length and walk speed unaffected (unpublished observation). While frequently examined in TBI patients, it is surprising that detailed gait changes in quadrupeds commonly used in animal models of TBI are lacking. Using the catwalk automated gait analysis method, we found that CCI led to a reduction in paw pressure and area of paw contact. These gait impairments are likely due to an altered use of the plantar surface of the paw, a pattern often observed in other models of central nervous system injury including sciatic nerve resection, spinal cord injury, Parkinson’s disease, pain and experimental stroke (Gabriel et al. 2007; Gensel et al. 2006; Vlamings et al. 2007; Vogelaar et al. 2004). The impairment in intensity and contact area was only observed in the forepaws rather than hindpaws of TBI mice, possibly because the forepaws play a more important role in supporting the body weight during walking. However, there was no direct correlation between body weight and any of these parameters.
Step pattern can reflect interlimb coordination. Among the six step patterns identified in rodents, the ‘Ab’ alternate pattern is the most common walking pattern, constituting 80-95% of the total step cycles in intact rats (Cheng et al. 1997). The Ab pattern remains predominant even in animals with nerve injury (Hamers et al. 2006) and ischemic stroke (unpublished observation). However, unlike the patterns observed in rats, only 40-50% of step sequences of our mice were of Ab pattern. Following CCI, cruciate b pattern, significantly increased. The change in step pattern might be related to alteration in the sequence of limb placement due to motor asymmetry. For example, unilateral brain injury following CCI has significantly increased the preference in using ipsilateral-diagonal limb sequence (Cb), while it has a tendency to decrease the girdle-diagonal sequence (Ab). Regularity index (RI) is a common, but not a very sensitive parameter for assessing interlimb coordination. Theoretically, the value of RI is 100% in healthy (fully coordinated) animals, and the loss of interlimb coordination leads to a decrease in RI. Because only extra or loss of paw placement of one or multiple limbs are defined as ‘irregular’ steps (Vrinten and Hamers 2003), resulting in almost certainly normal RI in most cases as long as all the four paws were used in one single step cycle no matter what the step sequence was or how the paws were placed. This likely explains why abnormal RI has rarely been detected, except in some cases of spinal cord injury models in which RI was temporarily decreased during the early phase after surgery, followed by a full recovery at a later time point (Hamers et al. 2006). The RIs of both groups in the current study were more than 98.5%, making it unlikely that we would detect differences between groups.
The distance between fore and hind paws between adjacent step cycles (called relative paw placement) can influence walking velocity. Following CCI, relative paw placement decreased on both sides, likely led to a reduced walking speed and cadence observed in the TBI mice. Brain injury such as ischemic stroke or TBI often increases the double support time in humans, due to increased gait asymmetry and instability. Although there was a trend in increased triple-limb support time in our CCI mice, the result did not reach a statistical significance. Velocity by itself is a major determinant that can potentially influence several parameters of gait function (Gorska et al. 1998). Consistent with a previous finding (Gillis and Biewener 2001), our results show that both walk speed and cadence directly affected stride length and temporal parameters including stance, and swing time. Walk speed is in inversely correlated with triple support time in the sham but not in TBI mice. TBI independently affected all parameters related to velocity. A number of previous reports suggest that body weight might affect the value of intensity, contact area, and print length. Koopmans et al. compared the differences of gait parameters among different stains of rats of the same age (Koopmans et al. 2007). They found differences in stride length, BOS, stance duration, and even step cycle due to differences in body weight among strains. Dellon and colleagues reported a significant increase in paw print length, toe spread, and intermediate spread with increasing body weight, but only when the body weight exceeded 300 to 350 grams (Dellon and Dellon 1991). In our study, we failed to find significant correlations between body weight and any gait parameters, including intensity and contact area, possibly due to a small range of difference in body weight among our cohort. Thus, the reduction of paw intensity and maximal contact area observed in TBI mice was unrelated to body weight.
Although the kinetic mechanisms in gait and body weight support in quadrupeds might differ from humans, the types of gait impairment between mice and human after TBI are strikingly similar. The main reason accounts for this similarity is probably due to injury to the CST in both human TBI patients and the CCI mice in the current study. Nevertheless, it suggests that the catwalk method used in the current study to assess gait function for laboratory animals in the setting of head trauma validates previous observations in humans. However, difference in head injury-induced gait change between human TBI patients and our CCI mice was also observed. Unlike the increase in the BOS commonly observed in human patients, there was no apparent difference in the BOS in either fore or hind paws detected between mice with CCI and sham surgery, although we can not predict if change in BOS only becomes apparent in animals with much more severe head injury. The variability in stride length or stride time has been reported in human TBI patients (Katz-Leurer et al. 2008; Zverev et al. 2002), particularly when the complexity of the gait task increased (Niechwiej-Szwedo et al. 2007), suggesting that the ability to maintain a relationship between the center of mass and BOS was compromised after head injury. However, gait variability in mice with CCI injury did not differ from that in sham mice in our current study (data not shown), possibly due to the limited number of steps allowed in the current catwalk setup. In order to obtain valuable dynamic gait data in laboratory animals, a longer walkway without optical distortion is desirable. The option in comparing gait data between overground locomotion and motorized treadmill walking with speed control is also a plus. Lastly, because TBI also produces cognitive impairment, future studies investigating how cognitive function affects the recovery of gait function are warranted.
Acknowledgments
The authors are grateful for the technical assistance and overall expertise on experimental TBI model provided by Drs. Linda J. Noble-Haeusslein and Kyoko Tsuru. This work was supported by a VA Merit award (JL), Department of Defense (JL) and infrastructural fund by Northern California Institute for Research and Education and the Department of Defense collaborative in Neuroscience Center of Excellence.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Basford JR, Chou LS, Kaufman KR, Brey RH, Walker A, Malec JF, Moessner AM, Brown AW. An assessment of gait and balance deficits after traumatic brain injury. Arch Phys Med Rehabil. 2003;84(3):343–349. doi: 10.1053/apmr.2003.50034. [DOI] [PubMed] [Google Scholar]
- Baskin YK, Dietrich WD, Green EJ. Two effective behavioral tasks for evaluating sensorimotor dysfunction following traumatic brain injury in mice. J Neurosci Methods. 2003;129(1):87–93. doi: 10.1016/s0165-0270(03)00212-7. [DOI] [PubMed] [Google Scholar]
- Bilgen M. A new device for experimental modeling of central nervous system injuries. Neurorehabil Neural Repair. 2005;19(3):219–226. doi: 10.1177/1545968305278635. [DOI] [PubMed] [Google Scholar]
- Chen G, Patten C, Kothari DH, Zajac FE. Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds. Gait Posture. 2005;22(1):51–56. doi: 10.1016/j.gaitpost.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Cheng H, Almstrom S, Gimenez-Llort L, Chang R, Ove Ogren S, Hoffer B, Olson L. Gait analysis of adult paraplegic rats after spinal cord repair. Exp Neurol. 1997;148(2):544–557. doi: 10.1006/exnr.1997.6708. [DOI] [PubMed] [Google Scholar]
- Cifu DX, Keyser-Marcus L, Lopez E, Wehman P, Kreutzer JS, Englander J, High W. Acute predictors of successful return to work 1 year after traumatic brain injury: a multicenter analysis. Arch Phys Med Rehabil. 1997;78(2):125–131. doi: 10.1016/s0003-9993(97)90252-5. [DOI] [PubMed] [Google Scholar]
- Dellon ES, Dellon AL. Functional assessment of neurologic impairment: track analysis in diabetic and compression neuropathies. Plast Reconstr Surg. 1991;88(4):686–694. doi: 10.1097/00006534-199110000-00020. [DOI] [PubMed] [Google Scholar]
- Deumens R, Jaken RJ, Marcus MA, Joosten EA. The CatWalk gait analysis in assessment of both dynamic and static gait changes after adult rat sciatic nerve resection. J Neurosci Methods. 2007;164(1):120–130. doi: 10.1016/j.jneumeth.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Fox GB, Fan L, Levasseur RA, Faden AI. Sustained sensory/motor and cognitive deficits with neuronal apoptosis following controlled cortical impact brain injury in the mouse. J Neurotrauma. 1998;15(8):599–614. doi: 10.1089/neu.1998.15.599. [DOI] [PubMed] [Google Scholar]
- Gabriel AF, Marcus MA, Honig WM, Walenkamp GH, Joosten EA. The CatWalk method: a detailed analysis of behavioral changes after acute inflammatory pain in the rat. J Neurosci Methods. 2007;163(1):9–16. doi: 10.1016/j.jneumeth.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Gensel JC, Tovar CA, Hamers FP, Deibert RJ, Beattie MS, Bresnahan JC. Behavioral and histological characterization of unilateral cervical spinal cord contusion injury in rats. J Neurotrauma. 2006;23(1):36–54. doi: 10.1089/neu.2006.23.36. [DOI] [PubMed] [Google Scholar]
- Gillis GB, Biewener AA. Hindlimb muscle function in relation to speed and gait: in vivo patterns of strain and activation in a hip and knee extensor of the rat (Rattus norvegicus) J Exp Biol. 2001;204(Pt 15):2717–2731. doi: 10.1242/jeb.204.15.2717. [DOI] [PubMed] [Google Scholar]
- Gorska T, Majczynski H, Zmyslowski W. Overground locomotion in intact rats: contact electrode recording. Acta Neurobiol Exp (Wars) 1998;58(3):227–237. doi: 10.55782/ane-1998-1277. [DOI] [PubMed] [Google Scholar]
- Hamers FP, Koopmans GC, Joosten EA. CatWalk-assisted gait analysis in the assessment of spinal cord injury. J Neurotrauma. 2006;23(34):537–548. doi: 10.1089/neu.2006.23.537. [DOI] [PubMed] [Google Scholar]
- Hamm RJ, Pike BR, O’Dell DM, Lyeth BG, Jenkins LW. The rotarod test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J Neurotrauma. 1994;11(2):187–196. doi: 10.1089/neu.1994.11.187. [DOI] [PubMed] [Google Scholar]
- Hannay HJ, Feldman Z, Phan P, Keyani A, Panwar N, Goodman JC, Robertson CS. Validation of a controlled cortical impact model of head injury in mice. J Neurotrauma. 1999;16(11):1103–1114. doi: 10.1089/neu.1999.16.1103. [DOI] [PubMed] [Google Scholar]
- Johnk K, Kuhtz-Buschbeck JP, Stolze H, Serocki G, Kalwa S, Ritz A, Benz B, Illert M. Assessment of sensorimotor functions after traumatic brain injury (TBI) in childhood - Methodological aspects. Restor Neurol Neurosci. 1999;14(23):143–152. [PubMed] [Google Scholar]
- Katz-Leurer M, Rotem H, Lewitus H, Keren O, Meyer S. Relationship between balance abilities and gait characteristics in children with post-traumatic brain injury. Brain Inj. 2008;22(2):153–159. doi: 10.1080/02699050801895399. [DOI] [PubMed] [Google Scholar]
- Keyser-Marcus LA, Bricout JC, Wehman P, Campbell LR, Cifu DX, Englander J, High W, Zafonte RD. Acute predictors of return to employment after traumatic brain injury: a longitudinal follow-up. Arch Phys Med Rehabil. 2002;83(5):635–641. doi: 10.1053/apmr.2002.31605. [DOI] [PubMed] [Google Scholar]
- Koopmans GC, Deumens R, Brook G, Gerver J, Honig WM, Hamers FP, Joosten EA. Strain and locomotor speed affect over-ground locomotion in intact rats. Physiol Behav. 2007;92(5):993–1001. doi: 10.1016/j.physbeh.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Liu Z, Fan Y, Won SJ, Neumann M, Hu D, Zhou L, Weinstein PR, Liu J. Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke. 2007;38(1):146–152. doi: 10.1161/01.STR.0000251791.64910.cd. [DOI] [PubMed] [Google Scholar]
- Matsumori Y, Hong SM, Fan Y, Kayama T, Hsu CY, Weinstein PR, Liu J. Enriched environment and spatial learning enhance hippocampal neurogenesis and salvages ischemic penumbra after focal cerebral ischemia. Neurobiol Dis. 2006;22(1):187–198. doi: 10.1016/j.nbd.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Ng SS, Hui-Chan CW. The timed up & go test: its reliability and association with lower-limb impairments and locomotor capacities in people with chronic stroke. Arch Phys Med Rehabil. 2005;86(8):1641–1647. doi: 10.1016/j.apmr.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Niechwiej-Szwedo E, Inness EL, Howe JA, Jaglal S, McIlroy WE, Verrier MC. Changes in gait variability during different challenges to mobility in patients with traumatic brain injury. Gait Posture. 2007;25(1):70–77. doi: 10.1016/j.gaitpost.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Ochi F, Esquenazi A, Hirai B, Talaty M. Temporal-spatial feature of gait after traumatic brain injury. J Head Trauma Rehabil. 1999;14(2):105–115. doi: 10.1097/00001199-199904000-00002. [DOI] [PubMed] [Google Scholar]
- Starkey ML, Barritt AW, Yip PK, Davies M, Hamers FP, McMahon SB, Bradbury EJ. Assessing behavioural function following a pyramidotomy lesion of the corticospinal tract in adult mice. Exp Neurol. 2005;195(2):524–539. doi: 10.1016/j.expneurol.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Vallee M, McFadyen BJ, Swaine B, Doyon J, Cantin JF, Dumas D. Effects of environmental demands on locomotion after traumatic brain injury. Arch Phys Med Rehabil. 2006;87(6):806–813. doi: 10.1016/j.apmr.2006.02.031. [DOI] [PubMed] [Google Scholar]
- van Loo MA, Moseley AM, Bosman JM, de Bie RA, Hassett L. Test-re-test reliability of walking speed, step length and step width measurement after traumatic brain injury: a pilot study. Brain Inj. 2004;18(10):1041–1048. doi: 10.1080/02699050410001672314. [DOI] [PubMed] [Google Scholar]
- Vlamings R, Visser-Vandewalle V, Koopmans G, Joosten EA, Kozan R, Kaplan S, Steinbusch HW, Temel Y. High frequency stimulation of the subthalamic nucleus improves speed of locomotion but impairs forelimb movement in Parkinsonian rats. Neuroscience. 2007;148(3):815–823. doi: 10.1016/j.neuroscience.2007.06.043. [DOI] [PubMed] [Google Scholar]
- Vogelaar CF, Vrinten DH, Hoekman MF, Brakkee JH, Burbach JP, Hamers FP. Sciatic nerve regeneration in mice and rats: recovery of sensory innervation is followed by a slowly retreating neuropathic pain-like syndrome. Brain Res. 2004;1027(12):67–72. doi: 10.1016/j.brainres.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Vrinten DH, Hamers FF. ‘CatWalk’ automated quantitative gait analysis as a novel method to assess mechanical allodynia in the rat; a comparison with von Frey testing. Pain. 2003;102(12):203–209. doi: 10.1016/s0304-3959(02)00382-2. [DOI] [PubMed] [Google Scholar]
- Walker WC, Pickett TC. Motor impairment after severe traumatic brain injury: A longitudinal multicenter study. J Rehabil Res Dev. 2007;44(7):975–982. doi: 10.1682/jrrd.2006.12.0158. [DOI] [PubMed] [Google Scholar]
- Wiese H, Stude P, Nebel K, Osenberg D, Volzke V, Ischebeck W, Stolke D, Diener HC, Keidel M. Impaired movement-related potentials in acute frontal traumatic brain injury. Clin Neurophysiol. 2004;115(2):289–298. doi: 10.1016/s1388-2457(03)00348-1. [DOI] [PubMed] [Google Scholar]
- Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord. 2008;23(3):329–342. doi: 10.1002/mds.21720. quiz 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zverev Y, Adeloye A, Chisi J. Quantitative analysis of gait pattern in hemiparetic patients. East Afr Med J. 2002;79(8):420–422. doi: 10.4314/eamj.v79i8.8828. [DOI] [PubMed] [Google Scholar]