Abstract
Background
Children with fetal alcohol spectrum disorders (FASD) display many problems ranging from deficits in intelligence to behavioral difficulties. Thus, many studies have aimed to better define the neuropsychological characteristics of children with FASD. The current article describes the neuropsychological characteristics of Italian children with severe diagnosis within FASD and compares them with controls. It was expected that intellectual functioning, language comprehension, academic skills, and inattention/hyperactivity would discriminate children with FASD from randomly-selected peers without FASD.
Methods
This paper presents data from a second cohort of children examined in 2005 as part of an in-school epidemiological study of FASD in Italy. Eighty children, 23 diagnosed with a FASD, and 57 randomly-selected control children from the same 1st grade classes, participated. After screening for FASD via growth and dysmorphology, the children were administered a test of general intelligence (WISC-R) as well as tests of nonverbal reasoning (Raven Colored Progressive Matrices), language comprehension (Rustioni), academic achievement (IPDA), and problem behavior (Disruptive Behavior Disorder Rating Scale).
Results
Children diagnosed with a FASD achieved lower scores than control children on Verbal, Performance, and Full Scale IQ. Profile analysis of the WISC-R indicates overall differences between the groups. However, some intact functioning within the FASD group was found, as the Similarities and Vocabulary subtests were similar to the controls. After an alpha adjustment to .004, the Block Design, Object Assembly, and Mazes subtests were significantly different from controls. On tests of nonverbal reasoning, language comprehension, and academic achievement, the children with a FASD scored significantly lower. Moreover, teachers rated children with a severe diagnosis within FASD as showing more inattentive symptoms than controls, while hyperactive/impulsive characteristics among children with a FASD were comparable with the control children. Significant correlations between head circumference, child dysmorphology, WISC-R, and Raven CPM scores are also reported.
Conclusions
This study indicates that a sample of Italian children with a FASD, when compared to control children, display poorer functioning on measures of general intelligence, nonverbal reasoning, academic achievement, and teacher-rated problem behaviors. The findings also contribute to the formulation of a neuropsychological profile of children diagnosed with a FASD.
Keywords: fetal alcohol syndrome, fetal alcohol spectrum disorders, neuropsychological characteristics, Italian children
Defining the neuropsychological characteristics of children with fetal alcohol syndrome (FAS) or another diagnosis within the fetal alcohol spectrum disorders (FASD) has been an important focus for researchers for decades. Not only do these children display deficits in intelligence, with average IQ scores in the borderline range (Mattson, Riley, Gramling, Delis, Jones, 1997; Streissguth, Barr, Sampson, 1990), they also have been shown to perform more poorly than controls on tasks that assess information processing (Jacobson, 1998), number processing (Kopera-Frye, Dehaene, Streissguth, 1996), visual-spatial reasoning (Carmichael Olson, Feldman, Streissguth, Sampson, Bookstein, 1998), visual memory (Uecker & Nadel, 1996), verbal learning and memory (Mattson, Riley, Delis, Stern, Jones, 1996), language (Abkarin, 1992), and motor function (Kalberg et al., 2006; Roebuck, Simmons, Richardson, Mattson, Riley, 1998). Children with FASD have also been shown to display difficulties with behavior and emotion (Bailey et al., 2004; Steinhausen & Spohr, 1998). In 1994, Streissguth and colleagues found that children affected by alcohol exposure scored lower on arithmetic tests than on other academic tests, and there is also evidence that the same children have impaired ability on tests that measure attention and executive functioning (Coles et al., 1997; Kodituwakku, Kalberg, May, 2001). Moreover, neurobehavioral studies of children with FAS from South Africa reveal that tests of fluid intelligence and verbal comprehension discriminated FASD children from controls (Adnams et al., 2001).
Researchers have consistently found intellectual deficits in children with fetal alcohol syndrome (FAS), the most severe form of FASD, reporting the average IQ scores in the mild retardation to borderline range (Mattson et al., 1997; Streissguth, Randels, & Smith, 1991). Given that subtest scores provide useful information pertinent to defining behavioral phenotypes, some researchers have conducted “profile analyses” of subtest scores from standardized intellectual batteries (Adnams et al., 2001; Mattson et al., 1997). Results from these studies reveal that children with FAS achieved lower scores than controls on all subtests. Having reviewed the literature on intellectual performance of alcohol-exposed children, Mattson and colleagues (1998) also concluded that there was no significant discrepancy between verbal and non-verbal abilities.
Although some progress has been made in the study of neuropsychological functioning of children who are exposed to alcohol prenatally, a complete and definite neuropsychological phenotype has not yet been defined. Having a set of intellectual and behavioral characteristics that are consistently associated with FASD would allow researchers to better discriminate between alcohol-affected children and controls.
The current study evaluates the neuropsychological functioning of children with severe FASD that was conducted in the context of a larger epidemiology project in Italy. This was the second time in two consecutive years, that neuropsychological testing was carried out with a well-diagnosed cohort of Italian children. The project was partially funded under the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), and has provided the researchers with an opportunity to explore the neuropsychological characteristics of children with a diagnosis within FASD in a Western European population. While some of the adverse effects of prenatal alcohol exposure in contemporary literature were first described by Lemoine and colleagues in 1968, drinking during pregnancy has not yet been recognized as a significant health risk factor in many countries of Western and Mediterranean Europe (Room, 2005). Thus far, a relatively limited number of studies on intellectual functioning of children with FAS or another type of FASD have been conducted in Western European Countries. For example, a large European study of 18-month-old children (N = 1240), found no relationship between maternal drinking and psychomotor abilities (EUROMAC, 1992). However, some other studies in France (Lemoine & Lemoine, 1992), Germany (Spohr, Willms, Steinhausen, 1993; Steinhausen, 1995; Steinhausen & Spohr, 1998), and Scandinavia and Finland (Aronson & Hagberg, 1998; Autti-Rämö, et al., 2006) have revealed a pattern of cognitive, intellectual, and physical deficiencies for children whose mothers drank moderate to heavy amounts of alcohol. One longitudinal study in Finland reported results that were somewhat variable in a cohort of alcohol-exposed children (Riley et al., 2003). While young children ages 5 and 9 years scored significantly lower on Verbal IQ than controls, the older children, 12-14 year olds, had significantly lower Performance IQ scores. Another study described various characteristics of six Italian subjects diagnosed with FAS (Roccella & Testa, 2003).
Thus far, only one Italian population-based study of intellectual functioning outcomes of prenatal alcohol exposure in children has been reported (Kodituwakku et al., 2006), where significant neurobehavioral differences in a cohort of Italian children were found. More specifically, the neurobehavioral characteristic most associated with children diagnosed with a FASD was inattention.
Problem behavior has been a significant area of interest with the FASD population (Bailey et al., 2004; Coles et al., 1997; Streissguth, Bookstein, Barr, Press, Sampson, 1998). The literature is consistent that children with FASD have a tendency to exhibit a cluster of problems that include: impulsivity, disorganization, short-term memory problems, and difficulty in understanding subtle social cues (Streissguth et al., 1998). Alcohol-exposed children also exhibit significantly more behavioral problems than their normally-developing peers (Kodituwakku et al., 2001; Streissguth et al., 1998). Other studies have reported that alcohol-exposed children are characterized by social deficits including difficulty in understanding the social consequences of behavior and inappropriate interactions (Kelly, Day, Streissguth, 2000). Oftentimes, this cluster of behavioral problems will overlap with poor executive functioning (Baddeley, Wilson, Kopelman, 2002; Wilson, Alderman, Burgess, Emslie, Evans, 1996).
Like children with attention-deficit/hyperactivity disorder (ADHD), inattentive type, who are often characterized as being forgetful, researchers have evidence that children with a FASD are slow learners and often make omission errors (Kodituwakku et al., 2001). A more recent study (Kodituwakku et al., 2006); found that teacher-rated symptoms of inattention, as opposed to hyperactivity, were more associated with FASD. In regard to academic performance, alcohol-affected children are deficient in academic skills in general, and particularly deficient in math (Streissguth et al., 1994).
The purpose of the current study was to compare children diagnosed with FASD with peers on a standardized measure of general intelligence, as well as on various neuropsychological and behavioral characteristics. It was hypothesized that intellectual functioning, language comprehension, academic skills, and inattention/hyperactivity would discriminate children with a FASD from their peers.
Materials and Methods
Sample
Eighty children, 23 diagnosed with a severe form of FASD and 57 control children (without any type of FASD), participated in this study. The children ranged in age from 6 to 7 years of age. The FASD group was composed of 11 males and 12 females with a mean age of 6.13 years. Of the 23 children diagnosed with a FASD, 19 were diagnosed with partial FAS (PFAS) (82.6%), while 4 (17.4%) were diagnosed with FAS (see Hoyme et al., 2005 for detailed and specific diagnostic criteria for FASD). One child in this 2005 study cohort was diagnosed with alcohol-related birth defects (ARBD) and was not included in the analysis for this study. In order to focus on the two most severe forms of FASD, we limited our affected sample to include only those children diagnosed either with PFAS or FAS. The not FASD control group was composed of 37 males and 20 females with a mean age of 6.15 years chosen randomly via a random number table from the same 1st grade cohort in the same schools.
These data came from active case ascertainment (May & Gossage, 2001) of children with explicit parental/guardian consent to participate. All children were from an in-school, first-grade study of the prevalence and characteristics of FASD in a public school district in the Lazio Region in central Italy. In 2004 and 2005, a bi-national team of Italian and U.S. researchers completed two waves of research on the prevalence of FASD among children in randomly-selected elementary schools in communities situated close to Rome in the Lazio region. This paper is comprised of data collected from wave II (2005) only. The school district is composed of 68 schools with first-grade classes across a district spanning 60 kilometers (km) which lies between 40 and 100 km from Rome. Twenty-five schools were selected using a random number table. Researchers obtained permission to conduct the research and contacted parents and guardians for consent via take-home notices. In this wave slightly less than half (433 of 902; 48%) participated in tier 1 screening. Most studies of FASD neurobehavioral characteristics do not use active case ascertainment, and less bias may be introduced in this type of study than in a clinic-based study using passive recruitment via referral. This is particularly true if no selection bias is introduced in the consent process. Similar methods have been used in South Africa (May et al., 2000; 2005; Viljoen, Croxford, Gossage, Kodituwakku, May, 2002) and in one previous study of a different cohort of children in Italy (Kodituwakku, et al., 2006; May et al., 2006). Also one in-school study in America using active case ascertainment was carried out in a county in Washington State (Clarren, Randels, Sanderson, Fineman, 2001), which used a passive consent procedure. Passive consent is no longer allowed in the United States, and is rarely allowed elsewhere.
Sampling and research procedures were approved by the Ethics Committee of the regional Italian Health Department and by The University of New Mexico Health Sciences Human Research Review Committee (HRRC), whose approval was contingent on the Italian approval.
Controls
Matched controls (for grade in school) for the children with a FASD were chosen from the same classes, in the same schools. Controls were randomly selected from those children for whom signed consent forms had been provided. All children received the same screening and were found not to meet criteria for any form of FASD. Controls were tested simultaneously with the index cases. Testers were affiliated with the University of Rome. Examiners were blinded as to the group membership of the children, and were not familiar with the particular communities or the individual children before the study. The psychological examiners were licensed Italian psychologists who were trained and experienced in the standardized administration of these psychological tests. The data collected are valid and reliable estimates of cognitive functioning of the Italian children examined. All students and their parents, either suspected subjects or controls, were contacted for testing, and the three-hour battery was administered in one session at the schools.
Data Collection
The data collection and diagnoses were made using three tiers of screening. First, the height, weight, and head circumference were measured for each child by local school physicians. If a child was at or below the 10th centile in height, weight, or head circumference on U.S. National Center for Health Statistics (NCHS) charts, then he/she was advanced to the second tier of the study. In this second tier, a dysmorphological examination was performed. Only the children who met the criteria for the diagnosis of any form of FASD, and the randomly-selected controls were advanced to the third tier of the study. In this third tier, psychological testing was carried out in the schools of each child by bachelors and master-level psychologists who were employed by the grant from the University of Rome. In addition, the mothers of the selected children completed the Parent/Teacher Disruptive Behavior Disorder Rating Scale (Pelham DBD Rating Scale; Pelham, Gnagy, Greenslade, Milich, 1992), and a maternal risk factor interview was administered to the entire sample. Teachers also completed the Parent/Teacher Disruptive Behavior Disorder Rating Scale (Pelham DBD Rating Scale; Pelham, Gnagy, Greenslade, Milich, 1992) and a standardized Italian questionnaire designed to identify difficulties in learning called the “Questionario Osservativo per L’Identificazione Precoce delle Difficoltà di Apprendimento” (IPDA; Terrini, Tretti, Corcella, Cornoldi, Tressoldi, 2002).
Under the diagnostic scheme used, a child meeting the specific criteria received a diagnosis within the FASD spectrum. These criteria represent specific operationalizations of the 1996 Institute of Medicine (IOM) criteria (Stratton, Howe, Battaglia, 1996), allowing their practical application in clinical settings (Hoyme et al., 2005). All growth centiles calculated were derived from US National Center for Health Statistics (NCHS) normative data. Under the revised IOM diagnostic guidelines, a child who has the following features/characteristics meets the criteria for the diagnosis of FAS: two or more of the specific facial anomalies of FAS (short palpebral fissures, thin vermilion border, and/or smooth philtrum), prenatal and/or postnatal growth retardation (≤ 10 centile), and small head circumference (≤ 10 centile) or other evidence of structural brain abnormalities with or without confirmation of maternal drinking. For these dysmorphology features a quantified checklist is used that provides a numerical value to each facial and anatomical feature required or common to the IOM criteria for an FASD diagnosis. For example, a head circumference ≤10th centile receives a score of 3 as does a palpebral fissure (eye opening) length ≤ 10th centile and a smooth philtrum, low weight (≤ 10th), midface hypoplasia, long philtrum, and other features receive a score of two. Other features receive a score of one. When the scores of each of these traits are added together, it provides a Total Dysmorphological Score as presented in Tables 1, 4, and 5 of this paper. The higher the score, the greater the dysmorphia. The entire revised IOM diagnostic system and the dysmorphology form utilized in this system is fully described elsewhere (Hoyme, et al., 2005) and its use illustrated in a number of studies in several populations (May, et al., 2007; 2006; 2005).
Table 1.
Maternal Background Information, Child Demographic and Dysmorphology, Means, Standard Deviations, and p values
| FASD | Control | p value | ||||
|---|---|---|---|---|---|---|
| Maternal demographics | ||||||
| Maternal age (y) on day of interview (SD) | 36.81 | (5.53) | 36.00 | (5.08) | .548a | |
| Maternal age (y) at birth of index child (SD) | 29.81 | (5.86) | 29.16 | (5.19) | .643a | |
| Maternal education attainment (%) | ||||||
| Elementary | 0 | 1.9 | .178b | |||
| Junior High | 52.4 | 26.4 | ||||
| Senior High | 23.8 | 50.9 | ||||
| Some College | 9.5 | 5.7 | ||||
| College Degree | 14.3 | 15.1 | ||||
| Mother’s monthly income (Euros), (%) | ||||||
| < 500 | 11.1 | 14.7 | .851b | |||
| 501 - 1,000 | 33.3 | 38.2 | ||||
| 1,001 - 1,500 | 44.4 | 29.4 | ||||
| 1,501 - 3,000 | 11.1 | 17.6 | ||||
| Child demographics and dysmorphology | ||||||
| Child gender | Males Females |
n = 11 n = 12 |
(47.8%) (52.2%) |
n = 37 n = 20 |
(64.9%) (35.1%) |
.157b |
| Child age (years) | 6.13 | (.34) | 6.15 | (.41) | .779a | |
| Child height (cm) | 118.13 | (4.71) | 121.71 | (5.20) | .005 a | |
| Child height (centile) | 42.82 | (29.56) | 64.40 | (27.11) | .002a | |
| Child weight (kg) | 21.21 | (3.09) | 24.35 | (3.67) | < .001a | |
| Child weight (centile) | 40.82 | (28.99) | 64.75 | (26.91) | < .001a | |
| Child head circumference (cm) | 49.90 | (1.62) | 52.01 | (1.50) | < .001a | |
| Child head circumference (centile) | 19.87 | (24.10) | 54.77 | (29.93) | < .001a | |
| Total child dysmorphology raw score c | 11.35 | (4.38) | 4.64 | (3.02) | < .001a | |
ANOVA
χ2test of data
Total child dysmorphology score is a composite raw score derived from a weighted scoring system with the most salient FAS diagnostic features (e.g., head circumference, palpebral fissure length and smoothness of philtrum) scored a three, followed by other features (e.g., weight, and specific facial features) scored with a two, and other physical features related to FASD scored with a one.
Table 4.
Correlations Between Dysmorphology Centiles, Total Dysmorphology Raw Score, WISC-R Summary Scores, and Raven CPM
| Height % | Weight % | Head Circumference % | Total Dysmorph Score | Full Scale IQ | VIQ | PIQ | CPM (%) | |
|---|---|---|---|---|---|---|---|---|
| Height % | - | .755** | .455** | -.427** | .200 | .241* | .137 | .090 |
| Weight % | - | - | .475** | -.482** | .276* | .299** | .221 | .112 |
| Head Circumference % | - | - | - | -.579** | .304** | .243* | .323** | .128 |
| Total Dysmorph Score | - | - | - | - | -.402** | -.333** | -.414** | -.182 |
| Full Scale IQ | - | - | - | - | - | .921** | .931** | .533** |
| VIQ | - | - | - | - | - | - | .723** | .431** |
| PIQ | - | - | - | - | - | - | - | .565** |
| CPM (%) | - | - | - | - | - | - | - | - |
Correlation is significant at the 0.01 level (2-tailed).
Correlation is significant at the 0.05 level (2-tailed).
Table 5.
Means, Standard Deviations, and ANOVAs on Dysmorphological, Neuropsychological and Behavioral Tests between Control Subjects with no Prenatal Alcohol Exposure Versus Control Subjects With Some Prenatal Exposure
| Controls with no prenatal exposure (n = 38) | Controls with prenatal exposure (n = 12) | F-test | p value | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Child height (centile) | 61.34 | 28.11 | 66.33 | 27.74 | F(1, 50) = 0.28 | .593 |
| Child weight (centile) | 63.84 | 28.20 | 62.08 | 27.50 | F(1, 50) = 0.03 | .851 |
| Child head circumference (centile) | 51.11 | 31.25 | 56.25 | 29.63 | F(1, 50) = 0.25 | .617 |
| Total child dysmorphology score | 4.97 | 3.10 | 5.08 | 2.64 | F(1, 50) = 0.01 | .913 |
| Verbal IQ | 99.62 | 18.08 | 103.25 | 17.83 | F(1, 49) = 0.36 | .548 |
| Performance IQ | 109.62 | 18.21 | 118.25 | 25.07 | F(1, 49) = 1.68 | .201 |
| Full scale IQ | 105.38 | 19.51 | 111.67 | 21.38 | F(1, 49) = 0.89 | .348 |
| Raven CPM | 21.24 | 4.59 | 20.58 | 2.84 | F(1, 50) = 0.21 | .645 |
| Raven CPM percentile score | 67.11 | 23.72 | 65.83 | 22.44 | F(1, 50) = 0.02 | .871 |
| Rustioni (total errors) | 6.66 | 2.67 | 5.33 | 1.96 | F(1, 50) = 2.50 | .120 |
| Rustioni qualitative | 4.13 | 1.94 | 5.08 | 1.88 | F(1, 50) = 2.21 | .143 |
| IPDA questionnaire | 20.17 | 6.23 | 24.27 | 5.13 | F(1, 45) = 3.87 | .055 |
| Teacher DBD rating of attention | .66 | 1.84 | .75 | 2.30 | F(1, 50) = 0.02 | .888 |
| Teacher DBD rating of hyperactivity/impulsivity | 1.10 | 2.59 | 1.25 | 2.45 | F(1, 50) = 0.02 | .865 |
| Parent DBD rating of attention | .60 | 1.68 | .25 | .62 | F(1, 50) = 0.50 | .481 |
| Parent DBD rating of hyperactivity/impulsivity | .89 | 2.02 | .41 | .79 | F(1, 50) = 0.63 | .431 |
For PFAS a child must have two or more typical facial features and one or more of the following characteristics: prenatal and/or postnatal growth retardation evidenced by height or weight ≤ 10 centile, evidence of abnormal brain growth or structure (e.g., microcephaly ≤ 10 centile), or evidence of characteristic behavioral or cognitive abnormalities, with or without evidence of maternal drinking during pregnancy. For a diagnosis of alcohol-related birth defects (ARBD), a child must have confirmed prenatal alcohol exposure, evidence characteristic pattern of minor facial anomalies, including two or more of the following: short palpebral fissures, thin vermilion, and/or smooth philtrum, as well as either major malformations or a pattern of minor malformations. For diagnosis of alcohol-related neurodevelopmental disorder (ARND), a child must have documented prenatal alcohol exposure, display neurological, or structural brain abnormalities (e.g., microcephaly), or manifest evidence of a characteristic complex pattern of behavioral or cognitive abnormalities inconsistent with developmental level not explained by genetic predisposition, family background, or environment alone (see Hoyme et al., 2005). Only children diagnosed with the two most severe manifestations of FASD: FAS and PFAS, were included in this study.
Instruments Used
Because of the challenge and importance of selecting a battery of tests that has the desired psychometric properties which can be used cross-culturally, several considerations were made when selecting the tests for the current study: (1) minimization of burden on test administrators and respondents; (2) sensitivity to the effects of prenatal alcohol exposure as demonstrated by previous research; and (3) administration of items of graded difficulty and high internal consistency in order to maximize the discrimination power of the test.
Because our research team completed a population-based epidemiology study the previous year (May et al., 2006; Kodituwakku, et al., 2006), we had the opportunity to revise, based on the previous results, our test battery for this second year study. In the first year of neuropsychological testing, among other measures, we included a fluid intelligence test (i.e., Raven Colored Progressive Matrices) and an Italian language measure (Rustioni). In order to increase the level of discrimination between groups and to better assess the full range of intellectual skills in the study cohort, we added the Wechsler Intelligence Scale for Children-Revised (WISC-R).
The psychological and developmental evaluations were completed using a battery of tests which included measures of intellectual functioning, perceptual nonverbal reasoning ability, language comprehension, academic achievement, and problem behavior. The following instruments were included: the Italian translation of the Wechsler Intelligence Scale for Children-Revised (WISC-R) (Rubini & Padovani, 1986); the Rustioni Test of Language Comprehension “Prove di Valutazione Della Comprensione Linguistica” (Rustioni, 1994); the Raven-Colored Progressive Matrices (CPM) (Raven, Court, Raven, 1947, 1985); a standardized Italian questionnaire identifying difficulties in learning: “Questionario Osservativo per L’Identificazione Precoce delle Difficoltà di Apprendimento” (IPDA) (Terrini et al., 2002); and the Parent/Teacher Disruptive Behavior Disorder (DBD) Rating Scale (Pelham et al., 1992).
Wechsler Intelligence Scale for Children-Revised (WISC-R; Rubini & Padovani, 1986)
The WISC-R utilizes twelve subscales (i.e., Information, Similarities, Arithmetic, Vocabulary, Comprehension, Memory, Picture Completion, Picture Arrangement, Block Design, Object Assembly, Coding, and Mazes) that test various aspects of intelligence. The subscales produce a Verbal, Performance, and a Full Scale IQ. The Verbal IQ measures verbal knowledge obtained informally and through formal education, while the performance IQ reflects the ability to interpret and organize nonverbal information within time constraints. The WISC-R is the only version of the Wechsler Intelligence Scale for Children that has been translated into Italian and that uses Italian-based norms (Termine, Balottin, Nicoli, Zopello, Lanzi, 2005). The Italian version of the WISC-R has demonstrated reliability equal to the English version (Grimaldi & Lisi, 1983).
Rustioni Test of Language Comprehension and IPDA Questionnaire
The Rustioni (1994) is an Italian-specific and Italian-normed test of linguistic understanding. It was modeled after the Test for the Reception of Grammar (TROG) (Bishop, 1989), is packaged in booklet form in a multiple-choice format, and is used to assess the understanding of grammatical contrasts in the Italian language. The child is shown a page with four picture choices and must select the picture that matches a spoken sentence; the test takes 10 to 20 minutes to administer. It has been standardized on over 2,622 children ages 3.6 to 8 years, and it assesses the comprehension level of the child by chronological age. Errors assessed by chronological age provide an estimate of the real age-graded comprehension of the child. In addition, the IPDA questionnaire (Terrini et al., 2002) was administered to measure a child’s current levels of academic achievement in the domains of language and math. The IPDA is also an Italian-normed test designed to identify difficulties in learning by measuring academic achievement.
Raven Colored Progressive Matrices (CPM)
The Raven CPM is a standardized test that assesses nonverbal reasoning ability; more specifically, reasoning in the visual modality and inductive reasoning (Alderton & Larson, 1990). The Raven CPM can take the place of a single test of intelligence when paired with a standardized test of language ability, and was designed for use with both young children and the elderly.
Parent/Teacher Disruptive Behavior Disorder Rating Scale (DBD)
The Parent/Teacher Disruptive Behavior Disorder Rating Scale (DBD) provides a measure of attention deficit hyperactivity disorder (ADHD), oppositional defiant disorder (ODD), and conduct disorder (CD) (Pelham et al., 1992). It was designed in the United State and translated into Italian. Only items assessing inattention and hyperactivity/impulsivity were used for this study.
Maternal Questionnaire
Mothers of the children with a severe FASD diagnosis and the control children became the subjects of a portion of the epidemiological study concerned with maternal risk. This maternal data set was utilized in case conference for the final diagnosis of each child (both cases and controls) for whom data are provided in this manuscript. The mothers of randomly selected control children who did not meet the diagnostic criteria for a FASD are believed to be representative of the average women in this region with regard to demographic variables, drinking patterns, nutrition, fertility and childbearing, and behavioral health issues.
The maternal drinking questions were asked in the context of a set of general health questions and immediately followed questions about daily diet and nutrition during the index pregnancy (May et al., 2005). Because of a general awareness in Italy that alcohol and pregnancy are not compatible and the highly sensitive nature of the questions, care was taken to ensure accuracy with the alcohol exposure information. The data were first collected, via time-line-follow-back methods (Sobell & Sobell, 1995; Sobell et al., 2001), on current drinking via a 7-day drinking log that began with the day before the interview and worked back in time for the previous week. The sequence of questions was designed to assist the interviewee in recall and to realistically calibrate their responses for accurate reporting (Graves & Kaskutas, 2002; Kaskutas & Graves, 2000, 2001). In some studies, retrospective reports of drinking levels during pregnancy have been reported to be higher and to be as accurate, or more accurate than the reporting of drinking during pregnancy (Alvik, Haldorsen, Groholt, Lindemann, 2006; Czarnecki, Russell, Cooper, Salter, 1990). However, Jacobson and colleagues (1991, 2002) also reported that prospective maternal reporting was likely to be more accurate in detecting behavioral effects than retrospective reports, and that retrospective reporting did not consistently detect neurobehavioral outcomes. Nonetheless, accurate maternal drinking reports are important in the determination of levels of drinking that are associated with deficits in psychological functioning (May et al., 2000; 2005; 2006; Viljoen et al., 2002). Every effort was made by the Italian interviewers, employees of the University of Rome, to elicit and record detailed and accurate alcohol consumption data as part of the overall epidemiology study.
Data Analysis
All data were entered, and statistical analyses performed using SPSS (Version 14.0; SPSS for Windows, SPSS Inc., Chicago, IL). The data were analyzed using Hotelling’s T2 via a multivariate analysis of variance (MANOVA), as there were only two groups (control versus FASD) as independent variables and there were several dependent variables within each domain (e.g., dysmorphology, intellectual, achievement, and behavior). The evaluations of assumptions of normality, linearity, homoscedasticity, and multicollinearity were all satisfactory. Further, Pearson product-moment correlation coefficients were performed on dysmorphology centiles, total dysmorphology raw score, as well as WISC-R summary scores, and the Raven CPM percentile score.
Results
A summary of both maternal and child demographic information, as well as child dysmorphology characteristics can be found in Table 1. The data indicate that the mothers of the children with a FASD and the mothers of controls were similar in age at interview, and at the age of the birth of the index child. Further, the two groups of mothers did not differ in education attainment or monthly income.
Child demographic and physical measurements, also found in Table 1, indicate that the two groups (FASD and control) were well matched to age and gender. As expected, the two groups were significantly different with respect to height, weight, and head circumference measurements and centiles, with significantly lower height, weight, and head circumference measures for the FASD group. Lastly, the FASD group had a significantly higher mean total dysmorphology raw score (11.35) than controls (4.65), indicating a greater number of FASD symptoms.
When comparing the two groups on the Verbal, Performance, and Full Scale mean IQ scores from the WISC-R (see Table 2.), the FASD group showed significantly lower scores on Verbal IQ (p = .015), Performance IQ (p < .001), and Full Scale IQ (p = .001). Additional post hoc within group Verbal IQ (mean = 90.91) and Performance IQ (mean = 93.70) comparisons revealed no differences for FASD group. However, significant differences between Verbal IQ and Performance IQ for controls were found, where control children had a significantly lower Verbal IQ (mean = 101.27) than Performance IQ (mean = 111.5) [t (55) = -5.64, p < .001].
Table 2.
Means, Standard Deviations, ANOVAs and effect sizes on WISC-R Summary Scores and Subscales
| FASD (n = 23) | Controls (n = 56) | F-test | p value | η2** | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| Verbal IQ | 90.91 | (14.93) | 101.27 | (17.55) | F(1, 79) = 6.15 | .015 | .074 |
| Performance IQ | 93.70 | (16.65) | 111.50 | (19.36) | F(1, 79) = 14.89 | < .001 | .162 |
| Full scale IQ | 91.26 | (15.39) | 107.25 | (19.38) | F(1, 79) = 12.40 | .001 | .139 |
| WISC-R Subscales | |||||||
| Information | 8.13 | (3.09) | 10.07 | (3.59) | F(1, 79) = 5.13 | .026 | .063 |
| Similarities | 10.65 | (3.32) | 11.30 | (3.20) | F(1, 79) = 0.66 | .419 | .008 |
| Arithmetic | 7.43 | (2.71) | 9.89 | (3.69) | F(1, 79) = 8.30 | .005 | .097 |
| Vocabulary | 8.43 | (3.23) | 9.91 | (3.65) | F(1, 79) = 2.84 | .096 | .036 |
| Comprehension | 8.56 | (1.75) | 9.77 | (2.50) | F(1, 79) = 4.39 | .039 | .054 |
| Memory | 8.13 | (3.42) | 10.23 | (3.10) | F(1, 79) = 7.03 | .010 | .084 |
| Picture Completion | 9.96 | (2.63) | 11.59 | (3.02) | F(1, 79) = 5.09 | .027 | .062 |
| Picture Arrangement | 9.48 | (4.73) | 12.13 | (3.62) | F(1, 79) = 7.23 | .009 | .086 |
| Block Design | 8.65 | (2.87) | 11.18 | (3.26) | F(1, 79) = 10.44 | .002* | .119 |
| Object Assembly | 7.61 | (3.35) | 10.80 | (3.24) | F(1, 79) = 15.50 | < .001* | .168 |
| Coding | 9.43 | (2.99) | 11.71 | (3.97) | F(1, 79) = 6.11 | .016 | .074 |
| Mazes | 9.78 | (4.03) | 12.66 | (3.58) | F(1, 79) = 9.76 | .003* | .113 |
p < .004 (for the WISC-R Subscales the adjusted Alpha = .004, 2-tailed test).
Partial Eta-squared (η2) .01 small; .06 medium; and .14 large effect size.
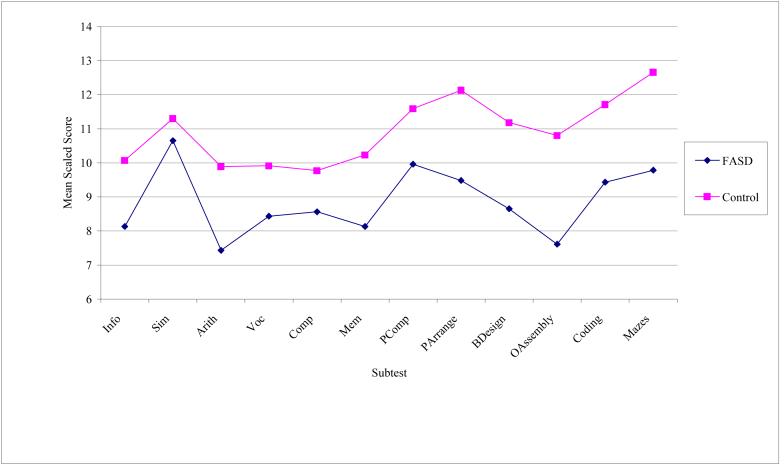
To determine whether our groups have different profiles on the WISC-R, a profile analysis was performed on the 12 subtests: information, similarities, arithmetic, vocabulary, comprehension, memory, picture completion, picture arrangement, block design, object assembly, coding, and mazes. Profile analysis is a special application of multivariate analysis of variance (MANOVA) where several dependent variables (e.g., WISC-R Subtests) are all measured on the same scale (Tabachnick & Fidell, 2007). The grouping variable was FASD and controls. The profiles, seen in Figure 1, deviated significantly from parallelism, F(1, 77) = 4.99, p = .028, partial η2 = .061. That is to say, the group profiles were not equivalent. For the levels test, significant differences were found among groups when scores were averaged over all subtests, F(1, 77) = 13.62, p < .001, partial η2 = .150. This suggests that the FASD group, on average, scored lower on the collected set of WISC-R subtest measures than the control group (e.g., the separation of the profiles was significant). In addition, when averaged over groups, subtests were found to deviate significantly from flatness, F(1, 77) = 14.74, p < .001, partial η2 = .161. This demonstrates that not all of the subtest scores elicited the same average response (e.g., the means of the subtest scores were not equal). Moreover, the partial Eta-squared (η2) effect sizes for these analyses ranged from medium (>.06) to large (>.14), emphasizing the magnitude of the findings.
Figure 1.
Profile of WISC-R subtest scores for FASD and control groups
To further evaluate deviation from parallelism, individual subtest mean scores between groups were then compared. Experimentwise α = .05 was achieved by setting α for each test at .004. As seen in Table 2, significant mean differences were evident on three of the twelve subscales of the WISC-R. The following WISC-R subtests proved to be significantly different between the affected children and the controls: Block Design (p = .002), Object Assembly (p < .001), and Mazes (p = .003). The subscales from the WISC-R where the groups performance was similar, were Similarities (p = .419), and Vocabulary (p = .096).
Differences between the FASD and control groups on a measure of nonverbal intellectual ability, language, learning, and teacher/parent-rated behavior were analyzed. A summary of mean ratings on the Raven CPM, Rustioni language comprehension, and behavioral symptoms are presented in Table 3. Examining the mean ratings between children with a FASD and controls reveals that the FASD group performed significantly poorer on almost all measures of nonverbal IQ, language comprehension and teacher-rated behavioral symptoms. The FASD group performed significantly lower than controls on the Raven CPM (a test of nonverbal abstract reasoning) (p = .007). This difference in performance is also illustrated by the significant difference in Raven CPM percentile scores of children with a FASD versus controls (p = .015). On the Rustioni test, children with FASD demonstrated poor performance (p = .028), with children with a FASD displaying a significantly lower mean score than controls. In contrast to the significant mean score differences between groups on the Rustioni qualitative measure, children with a FASD performed comparably to controls in the number of errors made (p = .072). When comparing the groups on a test of academic achievement (the IPDA questionnaire), the children with a FASD scored significantly lower than controls (p = .004). These analyses suggest that children diagnosed with a FASD demonstrate more difficulty on tests of inductive non-verbal reasoning, language comprehension, and academic achievement when compared to randomly-selected Italian control children.
Table 3.
Means, Standard Deviations, and ANOVAs on Neuropsychological and Behavioral Tests
| FASD (n = 23) | Controls (n = 57) | F-test | p value | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Raven CPM | 18.26 | (4.01) | 21.02 | (3.99) | F(1, 80) = 7.78 | .007 |
| Raven CPM percentile score | 53.04 | (26.48) | 67.37 | (21.98) | F(1, 80) = 6.17 | .015 |
| Rustioni (total errors) | 7.57 | (3.10) | 6.37 | (2.46) | F(1, 80) = 3.32 | .072 |
| Rustioni qualitative | 3.35 | (2.10) | 4.42 | (1.87) | F(1, 80) = 5.02 | .028 |
| IPDA questionnaire | 16.42 | (5.69) | 20.96 | (6.03) | F(1, 72) = 8.65 | .004 |
| Teacher DBD rating of attention | 2.17 | (3.02) | .60 | (1.83) | F(1, 80) = 8.17 | .005 |
| Teacher DBD rating of hyperactivity/impulsivity | 1.39 | (2.72) | 1.14 | (2.46) | F(1, 80) = 0.16 | .691 |
| Parent DBD rating of attention | 1.17 | (1.87) | .52 | (1.44) | F(1, 80) = 2.77 | .100 |
| Parent DBD rating of hyperactivity/impulsivity | 1.00 | (1.27) | .91 | (1.85) | F(1, 80) = 0.04 | .836 |
Also presented in Table 3 are the results from the Parent/Teacher DBD rating scale. The DBD teacher ratings of behavioral problems for children with FASD indicate significantly more inattention characteristics than controls (p = .005), illustrating the difficulties that children with FASD face in regard to inattentive problem behaviors in the classroom. Interestingly, teacher ratings of hyperactivity/impulsivity were not significantly different between the FASD group and controls (p = .691). Similarly, parent ratings of disruptive behaviors among children with FASD were not shown to be significantly different from control children for inattention characteristics (p = .100), or hyperactive/impulsive behaviors (p = .836).
Additional statistical analyses
In order to better predict group membership from a set of behavioral predictors, a direct discriminant function analysis was performed using two behavioral symptoms (i.e., teacher ratings of attention and hyperactivity) as predictors of group membership. A discriminant function was calculated, χ2(2) = 12.26, p = .002, revealing a strong association between groups and one of the predictors. The discriminant function analysis accounted for 15% of the between group variability and maximally separated FASD-diagnosed children from controls (i.e., 75% of original grouped cases were correctly classified). And, using the count of attentional problems as a predictor, the analysis correctly classified 73.9% of the FASD-diagnosed children. The loading matrix of correlations between predictors and the discriminant function suggests that the best predictor for distinguishing between children with FASD and controls is the count of attentional problems and not the count of hyperactive problems.
While an essential feature of Attention Deficit/Hyperactivity Disorder (ADHD) is a persistent pattern of either inattention and/or hyperactivity-impulsivity, most children exhibit a predominant pattern (e.g., Inattentive Type, Hyperactive-Impulsive Type, or Combined Type) (DSM-IV, 1994). The association between group membership and the DSM-IV diagnostic criteria for ADHD was explored by analyzing the frequency of children who met the DSM-IV diagnosis in the two groups. Interestingly, two control children (3.5%) were rated by their teachers as meeting the DSM-IV criteria for ADHD hyperactive-impulsive type, and three (5.3%) met the DSM-IV criteria for ADHD combined type. Contrasted with the FASD sample, four children (17.4%) were rated by their teachers as meeting the DSM-IV diagnosis for ADHD inattentive type, and two children (8.7%) met the diagnosis for ADHD combined type. When compared with the control group, a significantly greater proportion of children with FASD met the DSM-IV criteria with inattention symptoms as reported by teachers (5% controls versus 26% FASD) [χ2(1, N = 80) = 7.11, p = .008]. In contrast, the prevalence rate of hyperactive symptoms among children with FASD was equivalent with that observed in the control group (8% FASD versus 8% control) [χ2(1, N = 80) = .000, p = .991]. These results provide evidence that a predominant feature of FASD, as reported by teachers, is inattention rather than hyperactivity-impulsivity. Conversely, when examining the parents’ report of child behavior within the FASD sample, only one child (4.3%) met the diagnosis of ADHD inattentive type, and no children from either the control or FASD groups, were rated by their parents as meeting the criteria for ADHD hyperactive type or ADHD combined type.
Pearson product-moment correlation coefficients between height, weight, and head circumference centiles, Total Dysmorphology Raw Score, Full Scale, Verbal and Performance IQ scores and the Raven CPM percentile scores are presented in Table 4. Head circumference was highly correlated with all WISC-R summary scores, as was the total child dysmorphology raw score. As expected, the Raven CPM and Performance IQ scores from the WISC-R were also highly correlated.
Additional analyses were performed examining the possibility of having some undiagnosed ARND children in our control sample. The data from the control children were further analyzed comparing the control children with no prenatal alcohol exposure (n = 38), with control children whose mothers reported consuming some alcohol during their pregnancy (n = 12). A summary of mean ratings on dysmorphology, WISC-R summary scores, and measures of language comprehension, academic achievement, and problem behaviors are presented in Table 5. The data indicate that the 2 groups of control children are comparable on all measures (p’s > .05) with only the IPDA scores approaching significance. These results highlight the unlikely possibility that some children who have ARND may have been inadvertently included in the control sample, as they were comparable on all dysmorphological and neuropsychological measures. Moreover, each and every child was examined by dsymorphologists and did not meet the criteria for any diagnosis within the continuum of FASD.
Discussion
These results support the prediction that children from an Italian school population who are diagnosed with FAS or PFAS would have impairment or lower scores on standard tests of intelligence, nonverbal reasoning, and language comprehension, as well as demonstrate more behavioral problems when compared to control children.
The decision to utilize the WISC-R with this cohort was influenced by our desire to link what is known by past research about neuropsychological functioning of children with FASD to potential intervention methods in schools. Therefore, we have used this opportunity to explore the types of deficits that are known to exist in children with FASD and how those deficits can be assessed using a commonly accepted, standardized testing instrument such as the WISC-R.
Our results reveal that prenatal exposure to alcohol was significantly related to poorer performance on all standard measures of IQ, including a nonverbal measure of intellectual performance (i.e., Raven CPM). More specifically, children with a diagnosis within FASD were shown to have more difficulty than control children on three of the twelve subscales of the WISC-R, suggesting that children with FAS or PFAS, exhibit lower overall intellectual ability. However, performance on the Similarities and Vocabulary subscales of the WISC-R among the FASD group was comparable with controls, reflecting some unaffected intellectual capacities in these children. Similar to Mattson and colleagues (1998), our within-group comparison results suggest no differences between Verbal and Performance IQ with FASD children. However, unlike previous findings (Mattson et al., 1997; Streissguth et al., 1991), the IQ scores from the children with FASD fell within the average range across Verbal, Performance, and Full Scale IQ scores, suggesting a relatively high functioning group of children with FASD.
The literature on neuropsychological functioning of children with FASD reveals consistent results, especially regarding memory and arithmetic difficulties (Mattson et al, 1996, Mattson and Roebuck, 2002, and Streissguth, 1989). While it is noted that the FASD-grouped children’s WISC-R profile was significantly different from controls, their performance for most of the subtests were well within the average range, while only two of the subtests mean scores (i.e., arithmetic and object assembly) were within the low average range. One possible explanation for having a relatively high functioning FASD group, is that the post natal environment for these children is stable, with a high level of familial education, adequate nutrition, and low unemployment rates. Furthermore, this sample is an FASD aggregate, comprised of a majority of children with PFAS and 4 with FAS. Perhaps with a pure FAS sample, the children’s intellectual functioning would be more impaired. Finally, these differences also may be a result of having a school-based epidemiological study rather than a clinically-ascertained population study. Children who are referred to and diagnosed in hospitals and behavioral clinics are generally more severely affected than are those identified by outreach in schools.
As predicted, the FASD group exhibited significantly lower scores than the control group on the Raven CPM, revealing difficulties of nonverbal intellectual ability and abstract reasoning. Consistent with previous findings in a different Italian cohort (Kodituwakku et al., 2006), affected children displayed lower mean scores on the Rustioni test of language comprehension, and on an Italian test of achievement (IPDA) suggesting that children with FASD demonstrate poorer linguistic comprehension and achievement. Success on the Rustioni test requires the integration of many thought processes including working memory and language competence. Moreover, language difficulties have been shown to be a primary distinguishing characteristic of children with FAS in a group of children from an epidemiological study in South Africa (Adnams et al., 2001). Lower performance on the Rustioni test, as compared to controls, support the finding that impaired language performance could be a key characteristic of FASD.
Previous studies have questioned the ability of the Raven CPM to discriminate or measure nonverbal reasoning for the Italian population (Kodituwakku, et al., 2006). Significant correlations between the Raven CPM and the WISC-R measures of IQ in the present study were consistent with previous correlational studies suggesting that the Raven is a useful measure of general ability in school children (Llabre, 1984). These significant correlations from the present study support the use of the Raven CPM as a measure of nonverbal reasoning and general intelligence in Italian school-age children.
On measures designed to assess the potential problem behaviors associated with FASD (Pelham’s Parent/Teacher DBD Rating Scale), teachers rated the children with a FASD as having more inattentive behaviors, but not more hyperactive/impulsive behaviors. This finding replicates previous research which indicates that children with FASD differ from controls on measures of inattention, but not hyperactivity/impulsivity (Kodituwakku et al., 2006). Moreover, attentional problems in the classroom were a better predictor of correct classification of FASD children than was hyperactivity. Effectively, inattentiveness may be one of the defining characteristics of the cognitive-behavioral phenotype of FASD. However, because parent-rated behaviors were not found to be significantly different from controls, it is possible that symptoms such as hyperactivity, impulsivity, and inattention are viewed differently in the average Italian home than they are in school. Also, a previous study investigating Italian children’s parent-rated problem behaviors has shown potential cultural differences, as compared to American children, in parent-tolerated behavior (Kodituwakku et al., 2006). The Italian parents of FASD-diagnosed children rated their behavior as better or more normal than did their teachers.
There are certain limitations associated with the present study. The first is the limited generalizability due to the small sample size. Also, due to the time constraints of this study, carried out in the context of a larger epidemiological inquiry, the range of neuropsychological tests utilized were somewhat limited. This limitation could have implications for children diagnosed with ARND, where some children may inadvertently be misdiagnosed and included in the control group using this particular population-based screening. Moreover, IQ was not used as a covariate in any of the analyses. As such, this may have had an impact upon our findings, especially with attention and hyperactivity. Finally, the rate of consent to participate (approximately 50% of those enrolled in the classes) makes it unknown to the researchers whether or not a systematic bias entered into subject selection.
Despite the above limitations, the present study has supported previous findings that demonstrate population-based neuropsychological difficulties in Italian children diagnosed with FAS or PFAS, the two most severe forms of an FASD. The results indicate that children diagnosed with a FASD, recruited through the use of a population-based study initiated by screening first on dysmorphology, differed significantly from a random sample of unaffected peers on almost all neuropsychological and behavioral measures utilized. Also, this research is the second known study to examine the effects of prenatal alcohol exposure on IQ performance in a sample of Italian school-aged children. The present findings and further consistencies in replications have implications for the development of intervention and educational programs for children with FASD. If school psychologists understand the learning deficits that exist in the alcohol-exposed population, and can apply that knowledge to the learning profile revealed through individual evaluations, they will be better equipped to promote interventions that are targeted to each child’s specific needs and thereby, be able to design appropriate interventions for FASD-diagnosed children in schools.
Acknowledgments
This project was funded in part by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) (pilot project subcontract # 53257A-P1660-7802-211 CSM from San Diego State University) as part of the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD) - AA014811 and AA014828 and a grant from the health department of the regional government of the Lazio Region, Assessorato alla Sanità della Regione Lazio.
We gratefully acknowledge the support and assistance of the many people who have made this research possible. Drs. Faye Calhoun, Kenneth Warren, and Ting Kai Li of NIAAA were instrumental in bringing the bi-national research team together and funded the initial collaboration to make this study possible. NIAAA also funded travel for the Italian team to journey to South Africa for training and to the United States to prepare for and carry out the final case conference, data consolidation, and analyses. In Italy many people assisted in the project. Luca Deiana, B.A., Luciana Chessa, M.D., Michele Stegagno, M.D., and Luigi Tarani, M.D., were all instrumental in hosting members of the project and participating in the early training and screening of children. Psychological testing to finalize the diagnoses of the children was carried out by two of the authors of the paper (Giovanna Coriale, and Daniela Fiorentino) with assistance from Francesca De Rosa and Corinna Ceoldo. We are also grateful for the support and assistance of various managers, school physicians, and psychologists from ASL RMG and RMH: dott. P. Trecca, dott. Carapellese, dott. Di Giovanni, dott. G. Versace, dott. De Carolis, dott. N. Roma, dott. C. D’Anna, dott.ssa L. Asci, G. Gironda, S. Gagliardi, and A. Pontecorvi. Finally, we thank various individuals from the school office of the Lazio Region and Rome Province: dott.ssa M.T. Silani, and dott.ssa R. Massacesi, and particular gratitude to Sifip and Sitac Onlus for the project support. We would also like to acknowledge the expertise of Gene Hoyme, M.D., Luther Robinson, M.D., and Melanie Manning, M.D., as well as the assistance from Chandra Stellevato.
All research methods, procedures, and consent forms were approved by the Ethics Committee of the regional Italian health department and the Human Research Review Committee (HRRC) of The University of New Mexico Health Sciences Center, approval # 03-389.
References
- Abkarian GG. Communication effects of prenatal alcohol exposure. J Commun Disord. 1992;25:221–240. doi: 10.1016/0021-9924(92)90017-q. [DOI] [PubMed] [Google Scholar]
- Adnams CM, Kodituwakku PW, Hay A, Molteno CD, Viljoen D, May PA. Patterns of cognitive-motor development in children with fetal alcohol syndrome from a community in South Africa. Alcohol Clin Exp Res. 2001;25:557–562. [PubMed] [Google Scholar]
- Alderton DL, Larson GE. Dimensionality of Raven’s advanced progressive matrices items. Educ Psychol Measurement. 1990;50:887–900. [Google Scholar]
- Alvik A, Haldorsen T, Groholt B, Lindemann R. Alcohol consumption before and during pregnancy comparing concurrent and retrospective reports. Alcohol Clin Exp Res. 2006;30:510–515. doi: 10.1111/j.1530-0277.2006.00055.x. [DOI] [PubMed] [Google Scholar]
- Aronson M, Hagberg B. Neuropsychological disorders in children exposed to alcohol during pregnancy: A follow-up study of 24 children to alcoholic mothers in Goteborg, Sweden. Alcohol Clin Exp Res. 1998;22:321–324. doi: 10.1111/j.1530-0277.1998.tb03655.x. [DOI] [PubMed] [Google Scholar]
- Autti-Rämö I, Fagerlund A, Ervalahti N, Loimu L, Korkman M, Hoyme HE. Fetal alcohol spectrum disorders in Finland: Clinical delineation of 77 older children and adolescents. American Journal of Medical Genetics. 2006;140(2):137–143. doi: 10.1002/ajmg.a.31037. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Wilson BA, Kopelman M. Handbook of Memory Disorders. 2nd Ed. Psychology Press; Hove: 2002. [Google Scholar]
- Bailey BN, Delaney-Black V, Covington CY, Ager J, Janisse J, Hannigan JH, Sokol RJ. Prenatal exposure to binge drinking and cognitive and behavioral outcomes at age 7 years. Am J Obstet Gynecol. 2004;191:1037–1043. doi: 10.1016/j.ajog.2004.05.048. [DOI] [PubMed] [Google Scholar]
- Bishop DVM. Test for the reception of grammar. Medical Research Council; London: 1989. [Google Scholar]
- Carmichael Olson H, Feldman JJ, Streissguth AP, Sampson PD, Bookstein FL. Neuropsychological deficits in adolescents with fetal alcohol syndrome: clinical findings. Alcohol Clin Exp Res. 1998;22:1998–2012. [PubMed] [Google Scholar]
- Clarren SK, Randels SP, Sanderson M, Fineman RM. Screening for fetal alcohol syndrome in primary schools: a feasibility study. Teratology. 2001;63:3–10. doi: 10.1002/1096-9926(200101)63:1<3::AID-TERA1001>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Raskind-Hood CL, Brown RT, Falek A, Smith IE. A comparison of children affected by prenatal alcohol exposure and attention deficit, hyperactivity disorder. Alcohol Clin Exp Res. 1997;21:150–161. [PubMed] [Google Scholar]
- Czarnecki DM, Russell M, Cooper ML, Salter D. Five-year reliability of self-reported alcohol consumption. J Stud Alcohol. 1990;51:68–76. doi: 10.15288/jsa.1990.51.68. [DOI] [PubMed] [Google Scholar]
- Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition American Psychiatric Association; Washington DC: 1994. [Google Scholar]
- EUROMAC A European concerted action: Maternal alcohol consumption and its relation to the outcome of the pregnancy and child development at 18 months. Int J Epidemiol. 1992;2(Suppl.1):S72–S78. doi: 10.1093/ije/21.supplement_1.s72. [DOI] [PubMed] [Google Scholar]
- Graves K, Kaskutas LA. Beverage choice among Native American and African American urban women. Alcohol Clin Exp Res. 2002;26:218–222. [PubMed] [Google Scholar]
- Grimaldi A, Lisi F. Wecshler Intelligence Scale for Children: A test of the reliability of the Italian version. Bollettino di Psicologia Applicata. 1983;166:41–45. [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragón AS, Khaole N, Viljoen DL, Jones KL, Robinson LK. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 institute of medicine criteria. Pediatrics. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW. Specificity of neurobehavioral outcomes associated with prenatal alcohol exposure. Alcohol Clin Exp Res. 1998;22:313–320. doi: 10.1111/j.1530-0277.1998.tb03654.x. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, Martier SS, Ager JW, Kaplan MG. Maternal recall of alcohol, cocaine, and marijuana use during pregnancy. Neurotoxicol Teratol. 1991;13:535–540. doi: 10.1016/0892-0362(91)90062-2. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Chiodo LM, Sokol RJ, Jacobson JL. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics. 2002;109:815–825. doi: 10.1542/peds.109.5.815. [DOI] [PubMed] [Google Scholar]
- Kalberg WO, Provost B, Tollison SJ, Tabachnick BG, Robinson LK, Hoyme HE, Trujillo PM, Buckley D, Aragon AS, May PA. Comparison of motor delays in young children with fetal alcohol syndrome to those with prenatal alcohol exposure and with no prenatal alcohol exposure. Alcoholism: Clinical and Experimental Research. 2006;30(12) doi: 10.1111/j.1530-0277.2006.00250.x. [DOI] [PubMed] [Google Scholar]
- Kaskutas LA, Graves K. An alternative to standard drinks as a measure of alcohol consumption. J Subst Abuse. 2000;12:67–78. doi: 10.1016/s0899-3289(00)00042-0. [DOI] [PubMed] [Google Scholar]
- Kaskutas LA, Graves K. Pre-pregnancy drinking: how drink size affects risk assessment. Addiction. 2001;96:1199–1209. doi: 10.1046/j.1360-0443.2001.968119912.x. [DOI] [PubMed] [Google Scholar]
- Kelly SA, Day N, Streissguth AP. Effects of prenatal alcohol exposure on social behavior in humans and other species. Neurotox Teratol. 2000;22:143–149. doi: 10.1016/s0892-0362(99)00073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodituwakku P, Coriale G, Fiorentino D, Aragon AS, Kalberg WO, Buckley D, Gossage JP, Ceccanti M, May PA. Neurobehavioral characteristics of children with fetal alcohol spectrum disorders in communities from Italy: Preliminary results. Alcohol Clin Exp Res. 2006;30:1551–1561. doi: 10.1111/j.1530-0277.2006.00187.x. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW, Kalberg W, May PA. The effects of prenatal alcohol exposure on executive functioning. Alcohol Res Health. 2001;25:192–198. [PMC free article] [PubMed] [Google Scholar]
- Kopera-Frye K, Dehaene S, Streissguth AP. Impairments of number processing induced by prenatal alcohol exposure. Neuropsychologia. 1996;34:1187–1196. doi: 10.1016/0028-3932(96)00043-7. [DOI] [PubMed] [Google Scholar]
- Llabre MM. Standard Progressive Matrices. In: Keyser DJ, Sweetland RC, editors. Test Critiques. Vol. 5. Test Corporation of America; Kansas City, MO: 1984. pp. 95–602. [Google Scholar]
- Lemoine P, Harosusseau H, Borteyru JP, Menuet JC. Les enfants des parents alcooliques: Anomolies observees a propos de 127 cas. Quest Medical. 1968;21:476–482. [Google Scholar]
- Lemoine P, Lemoine P. Outcome of children of alcoholic mothers (study of 105 cases followed to adult age) and various prophylactic findings. Ann Pediatr (Paris) 1992;39:226–235. [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Delis DC, Stern C, Jones KL. Verbal learning and memory in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 1996;20:810–816. doi: 10.1111/j.1530-0277.1996.tb05256.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling L, Delis DC, Jones KL. Heavy prenatal alcohol exposure with or without physical features of fetal alcohol syndrome leads to IQ deficits. J Pediatr. 1997;131:718–721. doi: 10.1016/s0022-3476(97)70099-4. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP. A review of the neurobehavioral deficits in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcohol Clin Exp Res. 1998;22:279–294. doi: 10.1111/j.1530-0277.1998.tb03651.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Roebuck TM. Acquisition and retention of verbal and nonverbal information in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 2002;26:875–882. [PubMed] [Google Scholar]
- May PA, Brooke L, Gossage JP, Croxford J, Adnams C, Jones KL, Robinson L, Viljoen D. Epidemiology of fetal alcohol syndrome in a South African community in the Western Cape Province. Am J Public Health. 2000;90:1905–1912. doi: 10.2105/ajph.90.12.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Brooke LE, Gossage JP, Snell C, Hendricks L, Croxford J, Marais A-S, Viljoen DL. Maternal Risk Factors for Fetal Alcohol Syndrome in the Western Cape Province of South Africa: A Population-Based Study. Am J Public Health. 2005;95:1190–1199. doi: 10.2105/AJPH.2003.037093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Fiorentino D, Gossage JP, Kalberg W, Hoyme HE, Robinson LK, Coriale G, Jones KL, del Campo M, Tarani L, Romeo M, Kodituwakku PW, Deiana L, Buckley D, Ceccanti M. Epidemiology of FASD in a province in Italy: Prevalence and characteristics of children in a random sample of schools. Alcoholism: Clinical and Experimental Research. 2006;30:1562–1575. doi: 10.1111/j.1530-0277.2006.00188.x. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP. Estimating the prevalence of fetal alcohol syndrome: A summary. Alcohol Research & Health. 2001;25:59–167. [PMC free article] [PubMed] [Google Scholar]
- Pelham WE, Jr, Gnagy EM, Greenslade KE, Milich R. Teacher ratings of DSM-III-R symptoms for the disruptive behavior disorders. J Am Acad Child Adolesc Psychiatry. 1992;31:210–218. doi: 10.1097/00004583-199203000-00006. [DOI] [PubMed] [Google Scholar]
- Raven JC, Court JH, Raven J. Manual for Raven’s Progressive Matrices and Vocabulary Scales section 1, general overview and section 2, Coloured Progressive Matrices. H.K. Lewis and Co. Ltd; London: 1947. [Google Scholar]
- Raven JC, Court JH, Raven J. Manual for Raven’s Progressive Matrices and Vocabulary Scales. H. K. Lewis; London: 1985. [Google Scholar]
- Riley EP, Mattson SN, Li TK, Jacobson SW, Coles CD, Kodituwakku PW, Adnams CM, Korkman MI. Neurobehavioral consequences of prenatal alcohol exposure: an international perspective. Alcohol Clin Exp Res. 2003;27:362–373. doi: 10.1097/01.ALC.0000052703.38558.B2. [DOI] [PubMed] [Google Scholar]
- Roccella M, Testa D. Fetal alcohol syndrome in developmental age: Neuropsychiatric aspects. Minerva Pediatr. 2003;55:63–74. [PubMed] [Google Scholar]
- Roebuck TM, Simmons RW, Richardson C, Mattson SN, Riley EP. Neuromuscular responses to disturbance of balance in children with prenatal exposure to alcohol. Alcohol Clin Exp Res. 1998;22:1992–1997. [PubMed] [Google Scholar]
- Room R. Public health policy on alcohol: an international perspective. Addiction. 2005;100:1562–1563. [Google Scholar]
- Rubini V, Padovani F. WISC-R Scala di Intelligenza Wechsler per bambini riveduta. Organizzazioni Speciali. (In Italian); Florence: 1986. [Google Scholar]
- Rustioni DML. Prove di valutazione della comprensione linguistica. Organizzazione Speciali; Firenze (Italy): 1994. [Google Scholar]
- Sobell LC, Sobell MB. Alcohol consumption measures. In: Allen JP, Columbus M, editors. Assessing alcohol problems. NIAAA; Bethesda, MD: 1995. [Google Scholar]
- Sobell LC, Agrawal S, Annis H, Ayala-Velazquez H, Echeverria L, Leo GI, Rybakowski JK, Sandahl C, Saunders B, Thomas S, Zioikowski M. Cross-cultural evaluation of two drinking assessment instruments: alcohol timeline followback and inventory of drinking situations. Subst Use Misuse. 2001;36:313–331. doi: 10.1081/ja-100102628. [DOI] [PubMed] [Google Scholar]
- Spohr HL, Willms J, Steinhausen HC. Prenatal alcohol exposure and long-term developmental consequences. Lancet. 1993;341:907–910. doi: 10.1016/0140-6736(93)91207-3. [DOI] [PubMed] [Google Scholar]
- SPSS Inc. SPSS Base 14.0 for Windows User’s Guide. SPSS Inc.; Chicago IL: 2005. [Google Scholar]
- Steinhausen HC. Children of alcoholic mothers: A review. Eur Child Adolesc Psychol. 1995;4:143–145. doi: 10.1007/BF01980453. [DOI] [PubMed] [Google Scholar]
- Steinhausen HC, Spohr HL. Long-term outcome of children with fetal alcohol syndrome: psychopathology, behavior, and intelligence. Alcohol Clin Exp Res. 1998;22:334–338. doi: 10.1111/j.1530-0277.1998.tb03657.x. [DOI] [PubMed] [Google Scholar]
- Stratton K, Howe C, Battaglia F. Fetal alcohol syndrome: Diagnosis, epidemiology, prevention, and treatment. National Academy Press; Washington, D.C.: 1996. [Google Scholar]
- Streissguth AP, Barr HM, Sampson PD. Moderate prenatal alcohol exposure: effects on child IQ and learning problems at age 7 1/2 years. Alcohol Clin Exp Res. 1990;14:662–669. doi: 10.1111/j.1530-0277.1990.tb01224.x. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Olson HC, Sampson PD, Bookstein FL, Burgess DM. Drinking during pregnancy decreases word attack and arithmetic scores on standardized tests: adolescent data from a population-based prospective study. Alcohol Clin Exp Res. 1994;18:248–254. doi: 10.1111/j.1530-0277.1994.tb00009.x. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Press S, Sampson PD. A fetal alcohol behavior scale. Alcohol Clin Exp Res. 1998;22:325–333. doi: 10.1111/j.1530-0277.1998.tb03656.x. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Sampson PD, Barr HM. Neurobehavioral effects of prenatal alcohol: III. PLS analyses of neuropsychologic tests. Neurotoxicology and Teratology. 1989 Sep-Oct;11(5):493–507. doi: 10.1016/0892-0362(89)90026-3. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Randels SP, Smith DF. A testetest study of intelligence in patients with fetal alcohol syndrome: Implications for care. Journal of the American Academy of Child & Adolescent Psychiatry. 1991;30(4):584–587. doi: 10.1097/00004583-199107000-00009. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics. Fourth Edition Allyn & Bacon; New York: 2007. [Google Scholar]
- Termine C, Balottin U, Nicoli F, Zopello M, Lanzi G. Verbal performance intelligence quotient discrepancies on the Wecshler scales: Are they still useful? Literature review and clinical implications. Minerva Psichiatrica. 2005;46:209–219. [Google Scholar]
- Terreni A, Tretti ML, Corcella PR, Cornoldi C, Tressoldi PE. Questionario osservativo per l’identificazione precoce delle difficoltà di apprendimento (IPDA) Erickson; Trento: 2002. [Google Scholar]
- Uecker A, Nadel L. Spatial locations gone awry: Object and spatial memory deficits in children with fetal alcohol syndrome. Neuropsychologia. 1996;34:209–223. doi: 10.1016/0028-3932(95)00096-8. [DOI] [PubMed] [Google Scholar]
- Viljoen D, Croxford J, Gossage JP, Kodituwakku PW, May PA. Characteristics of mothers of children with fetal alcohol syndrome in the Western Cape Province of South Africa: A case control study. J Stud Alcohol. 2002;63:6–17. [PubMed] [Google Scholar]
- Wilson BA, Alderman N, Burgess P, Emslie H, Evans J. Behavioral assessment of the dysexecutive syndrome. Thames Valley Test Company: Bury St. Edmunds; 1996. [Google Scholar]