Abstract
Background
Alcohol abuse among adolescents is a major health and developmental problem. The 2-[14C]deoxyglucose (2-DG) technique allows for the in vivo quantification of local cerebral glucose utilization (LCGU) as a measure of functional neuronal activity.
Methods
LCGU rates were examined after acute ethanol administration within selected brain regions of adolescent alcohol-preferring (P) and -nonpreferring (NP) rats. Postnatal day 45 male P and NP rats were injected with saline or 1.0 g/kg ethanol, i.p., 10 min prior to an intravenous bolus of [14C]-2-Deoxyglucose (125 μCi/kg). Image densities were determined using quantitative autoradiography and LCGU values calculated.
Results
Acute ethanol injection significantly decreased LCGU rates in select brain regions including the olfactory tubercles (OTU), the frontal cortex (Fr) and subregions of the posterior hippocampus (pCA1 and pCA3). Acute ethanol had no significant effects on LCGU rates in any region of the adolescent NP rats. Significant basal LCGU rate differences were apparent between the rat lines in a nearly global fashion with adolescent P rats having much higher basal LCGU rates compared to adolescent NP rats.
Conclusions
These findings suggest that the adolescent P and NP rats are less sensitive to the effects of acute ethanol than their adult counterparts. The adolescent P rat is relatively more sensitive to the initial effects of acute ethanol in select brain regions as compared to the adolescent NP rat. Additionally, the innate hyper-excited state of the adolescent P CNS is a likely factor in the development of their high alcohol drinking behaviors.
Keywords: local cerebral glucose utilization, adolescent, alcohol-preferring rats, ethanol, alcohol-nonpreferring rats
Introduction
In the United States, most teenagers, about 80%, have consumed alcohol by the time they graduate from high school and almost half, 44%, have done so by the 8th grade (Johnston et al., 2005). More than 30% of the 12th graders in the 2004 Monitoring the Future survey of the National Institute of Drug Abuse (Johnston et al., 2005) reported being drunk within the previous 30 days and to have participated in binge drinking behaviors (defined as consuming 5 or more drinks per occasion in the 2 weeks prior to survey). It has been found in numerous studies that an early age onset of alcohol drinking predicts a higher rate of alcohol abuse and alcoholism in adulthood (Chou and Pickering, 1992; Clapper et al., 1995; Ghodsian and Power, 1987; Grant and Dawson, 1998; Hawkins et al., 1997; Pitkanen et al., 2005). Understanding the determinants and neurobiological consequences of adolescent alcohol use and abuse is a critical public health issue.
The [14C]-2-deoxyglucose (2-DG) technique is a quantitative method of mapping neuronal activity patterns, both within and across regions. This can be a powerful tool to help delineate the brain regions affected by acute ethanol. Selectively bred rat lines, including the alcohol-preferring P and non-preferring NP rat lines, which exhibit divergent ethanol drinking behaviors, have been used for many years to help identify the neurobiological underpinnings of high alcohol consumption. The alcohol-preferring P rat line satisfies all of the proposed criteria for an animal model of alcoholism (Cicero, 1979; McMillen, 1997) and is a good model of adolescent alcohol drinking behaviors (McBride et al., 2005). These selected rat lines exhibit numerous neuroanatomical, neurochemical and electrophysiological differences in key limbic brain regions (for review, see McBride & Li, 1998; Murphy et al., 2002; McBride et al., 2005). P rat pups have been shown to rapidly acquire high alcohol drinking behavior by 3 to 4 weeks of age and attain adult levels of alcohol consumption by approximately 5 weeks (McKinzie et al., 1998). This suggests that the neural substrates underlying high alcohol drinking behavior are already present in the periadolescent animal. However, substantial evidence suggests that adolescent neural modification and maturation continues through approximately week 8 in rats (Spear, 2000). The quantification of neuronal activity changes following ethanol administration in adolescent selectively bred animals, particularly in brain regions implicated in ethanol reward, offers great potential for understanding the neural substrates that underlie adolescent alcohol drinking.
In a recent comparative study of adult male P and NP rats, administration of a low (0.25 g/kg) dose of ethanol resulted in few significant changes in LCGU rates in P rats and no significant changes in NP rats (Strother et al., 2005). Whereas a moderate dose (1.0 g/kg) of ethanol resulted in numerous significant LCGU reductions in a nearly global fashion in the P rats but not in the NP rats. Additionally, we found that there were significant basal LCGU rate differences between the two rat lines, with the P rat having higher LCGU rates after saline injection in numerous brain regions compared to the NP rat (Strother et al., 2005). This study demonstrated that the P rat line is more sensitive than the NP rat line to an acute administration of a moderate dose of ethanol, particularly in limbic and limbic-association areas. The current study was undertaken to determine the effects of a moderate dose of ethanol on LCGU rates in adolescent alcohol-preferring P and -nonpreferring NP rats. The hypothesis to be tested is that adolescent P rats will be more sensitive to acute ethanol administration than their non-preferring counterparts and will closely resemble the adult P rats in their acute ethanol responses as we believe that the neural mechanisms underlying alcohol preference are already well established in the adolescent animal.
Methods
Animals
For the purposes of this study we used the Spear and Brake (1983) criterion and designated postnatal days (PND) 28–42 as periadolescence and PND 43–55 as true adolescence. Male, ethanol-naïve P and NP rat pups, from the 51st generation were randomly assigned to treatment groups: saline or 1.0 g/kg ethanol (n = 6–7 rats per group/per line). Rats were individually housed on PND 30 in plastic tubs on a reversed 12:12 light cycle (lights off at 0900h) with food and water available ad libitum. Beginning one week before catheterization surgery (PND 36 – PND 42), animals were habituated to clear Plexiglas (22 × 45 × 39 cm) test cages in a separate room maintained on the same 12:12 light schedule. Every morning before lights out, animals were moved to their test cages for a period of 4 hr with access to water only. Food was removed during this time to control for the effects of differential feeding upon blood glucose levels. Post-surgery, rats were returned to the test cages daily though experiment completion. The animals used in these experiments were maintained in facilities fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). All research protocols were approved by the institutional care and use committee and are in accordance with the guidelines of the Institutional Care and Use Committee of the National Institute on Drug Abuse, National Institutes of Health, and the Guide for the Care and Use of Laboratory Animals, Institute of Laboratory Animal resources, Commission on Life Sciences, National Research Council, 1996.
Surgical procedure
Following the habituation period, adolescent rats (PND 43) underwent unilateral femoral vein and artery catheterization. Catheters were constructed from two pieces of Tygon (Norton Performance Plastics, Akron, OH) tubing held together by heat-fusing the tubing with a soldering iron. The smaller piece, or intravessel portion, had an OD = 1.5 mm and an ID = 0.50 mm; the larger piece, or extravessel portion, had an OD = 0.76 mm and an ID = 0.25 mm. Approximately 3–5 cm of the small tubing was inserted into the femoral artery and vein of each rat while under 1–3% isofluorane anesthesia. The catheters were flushed with heparinized saline, secured with surgical thread and fed subcutaneously to a small incision at the nape of the neck. The inner thigh incision, exposing the femoral vessels, was closed with surgical steel staples. Catheters were externalized and secured at the neck with Vetbond tissue adhesive (3M Animal Care Products, St. Paul, MN). After the adhesive was dry, catheters were cut short to prevent animal access, and capped with sewing pins. Rats were moved to the DG test cages after they had regained consciousness for the daily 4-hr habituation session.
Measurement of LCGU
LCGU rates were measured in accordance with the method described by Sokoloff et al. (1977), as adapted for use in freely moving animals (Crane & Porrino, 1989). On the second day post-surgery (PND 45), animals were placed in test cages and catheters checked for patency. Rats weighed approximately 160–195 grams on test day. At 1100 hr, animals were injected i.p. with saline or 1.0 g/kg 10 % (v/v) ethanol; 10 minutes later rats received a 0.5 ml bolus of [14C]2-DG (125 μCi/kg; sp. act. = 56 mCi/mmol; Amersham Corp., Arlington Heights, IL) injected via the venous catheter. The [14C]2-DG bolus was immediately followed by 0.3 ml heparinized saline to clear the catheter of [14C]2-DG. Arterial blood samples were collected via syringe in the arterial catheter 5 min before and immediately prior to [14C]2-DG injection, then at 0.5, 1, 1.5, 3.5, 7, 15, 30 and 45 min after injection. Each blood sample was approximately 200 μl. Arterial catheters were flushed with heparinized saline before and after each blood sample, and catheters were cleared of heparinized saline in the dead space prior to each sample. Blood samples were maintained on ice, and then centrifuged at 16,000 rpm to separate the plasma fraction. Plasma from each sample was assayed for glucose and ethanol (GL5 Analyzer, Analox Instruments, London, UK), as well as for [14C]2-DG content (Beckman LS 7500 Liquid Scintillation Counter, Irvine, CA).
Immediately following the 45-min blood sample, rats were decapitated; brains quickly removed, frozen in isopentane at −50°C, wrapped in cooled foil and stored at −70°C. Coronal serial sections (20 μm) were prepared at −25°C in a cryostat microtome (Reichert-Jung, Cambridge Instruments, Buffalo, NY). Sections were mounted on glass cover slips and quickly dried on a 60°C hotplate. Cover slips were taped to rigid poster board and apposed to Kodak Biomax MR film (Kodak, Rochester, NY) along with calibrated [14C]microscale standards (Amersham, Arlington Heights, IL) in X-ray cassettes. Cassettes were stored at room temperature for 48 hr and then developed using a Kodak M35A X-OMAT Processor (Kodak, Rochester, NY).
Image densities were analyzed using quantitative autoradiography (Scion Image, Version 1.59, NIH, USA). Film analysis was completed by lab personnel blind to rat line and treatment groups. Brain areas were delineated according to the rat brain atlas of Paxinos and Watson (1998). [14C]2-DG tissue concentrations were determined from 8–10 bilateral measurements of each brain region and a mean average was calculated for each animal. LCGU rates were calculated using the operational equation for the 2-DG method as defined by Sokoloff et al. (1977). The “lumped” constant of 0.464 for conscious, unanesthetized rats, was used (Sokoloff et al. 1977) and LCGU rates were determined for each rat within each brain region. These values were subsequently grouped according to rat line and treatment group.
Statistical analyses
Blood ethanol concentrations were analyzed with a mixed ANOVA (time x line with repeated measures on time). Brain regions were separated according to general neuroanatomical groups (e.g., thalamic nuclei were grouped, hippocampal regions were grouped, etc.). In addition, the reward-relevant limbic regions (Koob et al., 1998; McBride and Li., 1998): medial prefrontal cortex (MPFC), olfactory tubercle (OTU), nucleus accumbens core and shell (Acb-c, Acb-sh), ventral tegmental area (VTA), central nucleus (CeA) and basolateral nucleus (BLA) of the amygdala, lateral septum (LS), and ventral pallidum (VP) were also grouped together for analysis. Data were separated by line after significant line × treatment interactions and mixed ANOVAs (treatment × subregion with repeated measures on subregion) run on all grouped regions. Because there were only 2 groups (saline and 1.0 g/kg EtOH) within each line, traditional post-hoc tests could not be performed, therefore significant treatment × subregion interactions were followed up by one-way ANOVAs on each region to determine main effect of treatment. Brain regions that could not be subdivided or functionally grouped were analyzed with one-way ANOVAs. The alpha level was conservatively set at p < 0.01 due to the increased possibility of Type I errors due to multiple comparisons. While we believe this strategy is prudent, results of p≤ .05 will also be indicated for a broader understanding.
Results
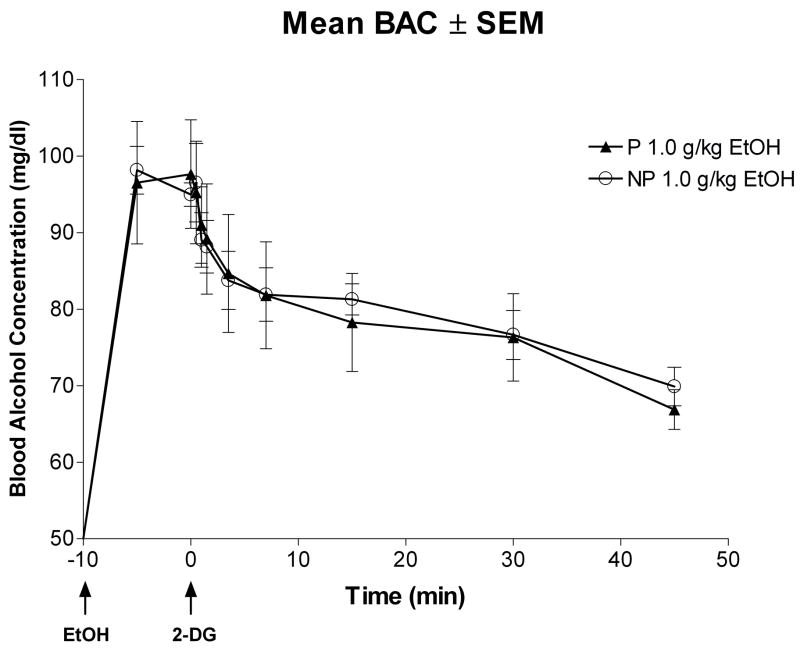
Figure 1 depicts the average blood alcohol concentration (BAC) curves across time for the P and NP rat lines after i.p. administration of 1.0 g/kg ethanol. A mixed ANOVA revealed that there was a main effect of time [F(9,81) = 29.02; p < 0.0001], but there was no main effect of line [F(1,9) = 0.16; p = 0.70] and no time × line [F(9,81) = 0.49; p = .88] interaction.
Fig. 1.
Time course of changes in blood alcohol concentrations following i.p. injection of 1.0 g/kg ethanol (EtOH) in adolescent P and NP rats. EtOH was injected 10 min prior to i.v. infusion of [14C]2-deoxyglucose (2-DG). There was no significant differences between the P and NP rats treated with 1.0 g/kg ethanol at any time point measured.
LCGU data were calculated in units of μmol/100g/min (means ± SEM). Significant differences after saline injection were detected between the P and NP lines in an overall two-way mixed ANOVA (line × region interaction: [F(42,966) = 1.92, p < 0.0001] and main effect of line: [F(1,23) = 7.03, p = 0.01]). This finding is consistent with a previous report from our laboratory demonstrating differential basal LCGU rates between P and NP rats (Smith et al., 2001a). Consequently, data was analyzed separately by line, but the results of the initial line comparisons are presented below by region or grouped regions.
Reward-Relevant Brain Regions
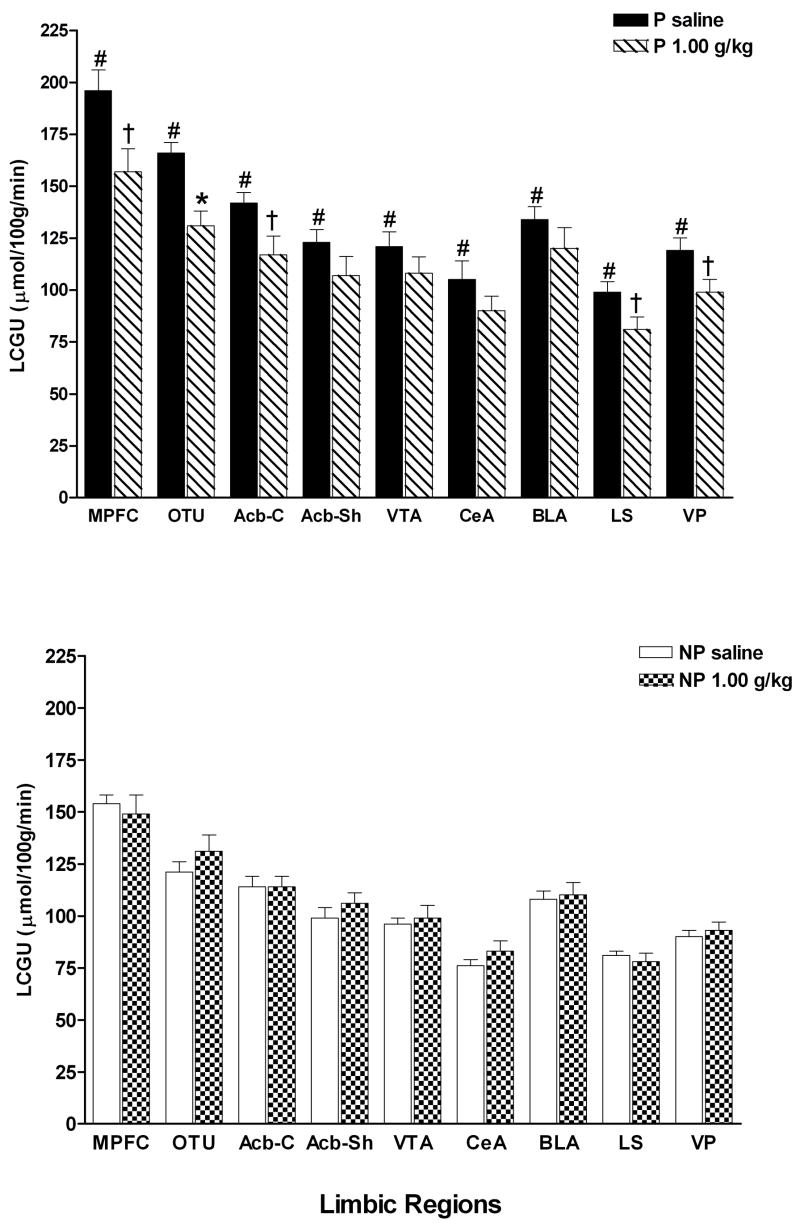
Figure 2 demonstrates the significant differences in 9 limbic regions between the P rats that received saline and the NP rats that received saline. A line × region mixed ANOVA with repeated measures on region revealed a significant effect of line [F(1,11) = 18.57; p = .001], region [F(8,88) = 155.07; p < .0001] and significant line × region interaction [F(8,88) = 2.81; p = .008]. All limbic regions in the P line had significantly higher LCGU rates compared to the NP line (MPFC [F(1,12) = 16.98; p = .002], OTU [F(1,12) = 20.01; p = .001], Acb-C [F(1,12) = 16.94; p = .002], Acb-Sh [F(1,12) = 9.60; p = .01], VTA [F(1,12) = 10.76; p = .007], CeA [F(1,12) = 9.81; p = .01], BLA [F(1,12) = 15.40; p = .002], LS [F(1,12) = 11.38; p = .006], VP [F(1,12) = 18.22; p = .001].
Fig. 2.
LCGU rates in limbic regions of adolescent P and NP rats following i.p. injection of saline or 1.0 g/kg ethanol. Values are for the following regions: medial prefrontal cortex (MPFC), olfactory tubercle (OTU), nucleus accumbens core and shell (Acb-C and Acb-Sh), ventral tegmental area (VTA), central nucleus of the amygdala (CeA), basolateral amygdala (BLA), lateral septum (LS) and ventral pallidum (VP). #Indicates that the saline value for the P rats are significantly different (p < 0.01) compared to the saline value for the NP rats. *Indicates that ethanol value is significantly lower (p < 0.01) than the respective saline value for that region within the same line. † Indicates p < 0.05 from corresponding saline LCGU rates. Data are the means ± SEM; n = 6 for all adolescent P rats and n = 7 for saline and n = 6 for ethanol for the adolescent NP rats.
The limbic, reward-relevant regions were analyzed by a treatment × region mixed ANOVA with repeated measures on region (Fig. 2). In the P rats, a treatment × region mixed ANOVA with repeated measures on region revealed a significant effect of region [F(8,80) = 88.78; p < .001] and a significant treatment × region interaction [F(8,80) = 2.59; p = .01], but no main effect of treatment [F(1,10) = 6.03; p = .03]. Follow up one-way ANOVAs on each region determined that acute ethanol significantly decreased LCGU rates only in the OTU [F(1,11) = 14.15; p = .004] but not in the MPFC [F(1,11) = 6.74; p = .02], Acb-C [F(1,11) = 6.99; p = .02], Acb-Sh [F(1,11) = 1.93; p = .19], VTA [F(1,11) = 1.26; p = .28], CeA [F(1,11) = 1.59; p = .23], BLA [F(1,11) = 1.66; p = .22], LS [F(1,11) = 7.59; p = .02], or VP [F(1,11) = 5.30; p = .04] although there was a strong trend in these several of these regions (MPFC, Acb-C, LS, VP).
In the NP rats (Fig. 2), this analysis resulted in no effect of treatment [F(1,11) = .47; p = .51], a significant effect of region [F(8,88) = 122.8; p < .0001] and no treatment × region interaction [F(8,88) = 2.08; p = .05].
Cerebral Cortex
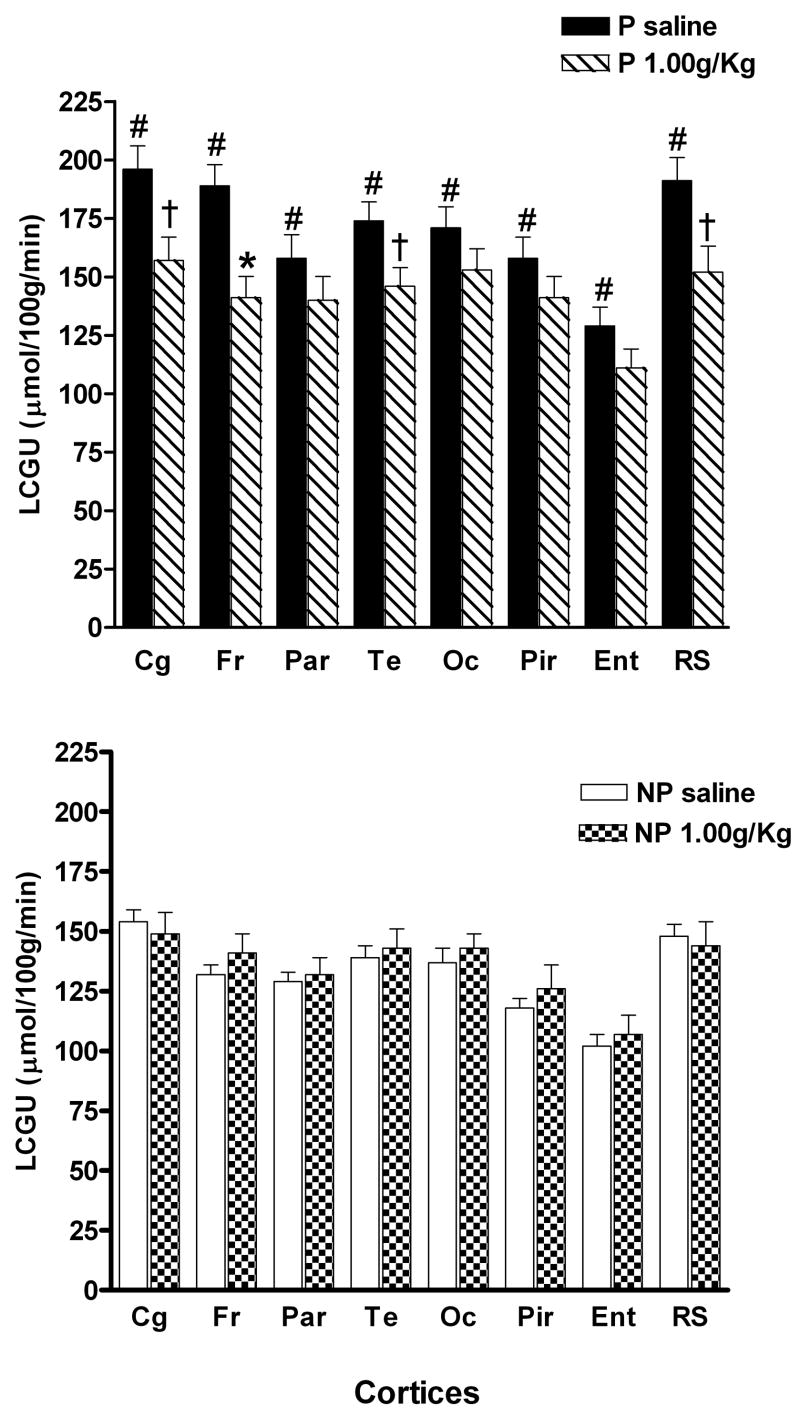
Figure 3 demonstrates that there were significant differences in the 8 cortical regions measured between the P rats that received saline and the NP rats that received saline. A line × region mixed ANOVA with repeated measures on region revealed a significant effect of line [F(1,11) = 25.10; p < .0001], region [F(6,66) = 41.40; p < .0001] and a significant line × region interaction [F(6,66) = 2.88; p = .01]. All cortical regions in the P line had significantly higher LCGU rates compared to rates for the NP line (Cg [F(1,12) = 16.98; p = .002], Fr [F(1,12) = 38.17; p < .0001], Par [F(1,12) = 11.96; p = .005], Te [F(1,12) = 13.63; p = .004], Oc [F(1,12) = 15.44; p = .002], Pir [F(1,12) = 16.05; p = .002], Ent [F(1,12) = 12.63; p = .005], and RS [F(1,12) = 14.32; p = .003].
Fig. 3.
LCGU rates in cortical regions of adolescent P and NP rats following i.p. injection of saline or 1.0 g/kg ethanol. Values are for the following regions: cingulate cortex (Cg), frontal (Fr), parietal (Par), temporal (Te), occipital (Oc), piriform (Pir), entorhinal (Ent) and retrosplenial (RS). #Indicates that the saline value for the P rats are significantly different (p < 0.01) compared to the saline value for the NP rats. *Indicates that ethanol value is significantly lower (p < 0.01) than the respective saline value for that region within the same line. † Indicates p < 0.05 from corresponding saline LCGU rates. Data are the means ± SEM; n = 6 for all adolescent P rats and n = 7 for saline and n = 6 for ethanol for the adolescent NP rats.
The cerebral cortex was divided into cingulate, frontal, parietal, temporal, occipital, pyriform, retrosplenial, and entorhinal cortices (Figure 3). In the P rats, there was a significant effect of region [F(14,140) = 31.43; p < .0001], region × treatment [F(14,140) = 2.35; p = .006], but no main effect of treatment [F(1,10) = 4.42; p = .06]. Each of these regions were then analyzed by one-way ANOVA to determine in which regions there were treatment effects. Significant differences between saline and ethanol treated P rats occurred only in the frontal [F(1,11) = 11.96; p = .006] cortex, with near significance found in the cingulate [F(1,11) = 6.78; p = .03], temporal [F(1,11) = 5.24; p = .04], and retrosplenial [F(1,11) = 6.44; p = .03] cortices, but not in the parietal [F(1,11) = 1.40; p = .26], occipital [F(1,11) = 1.79; p = .21], piriform [F(1,11) = 1.38; p = .27], or entorhinal [F(1,11) = 2.54; p = .14] cortices. In the NP rats, the two-way repeated measures mixed ANOVA yielded no significant treatment effect [F(1,11) = .56; p = .47] or treatment × region interaction [F(14,154) = 1.07; p = .39], but a main effect of region [F(14,154) = 49.7; p < .0001].
Hippocampus
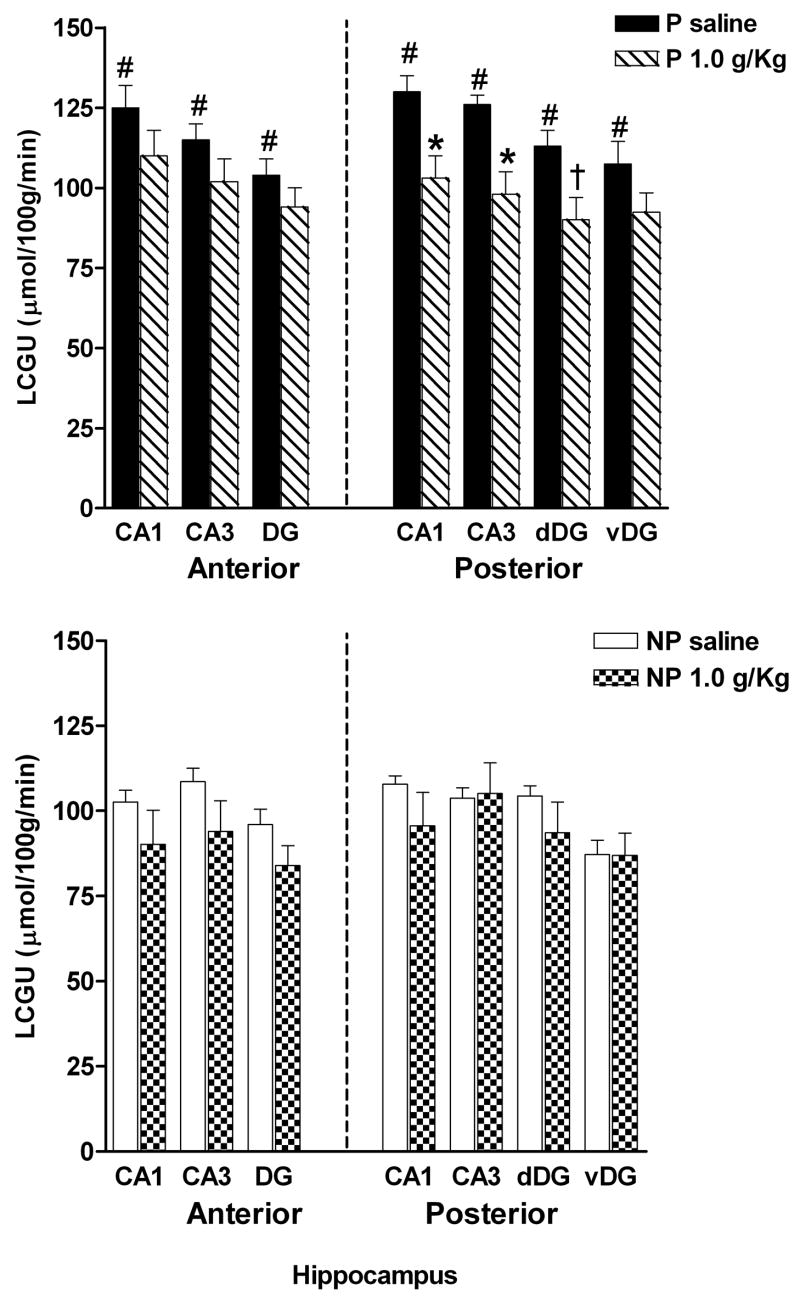
LCGU values were obtained for the CA1, CA3, and dentate gyrus subregions in both the anterior and posterior hippocampus (Fig. 4). There were significant differences in all regions measured between the P rats that received saline and the NP rats that received saline. A line × region mixed ANOVA with repeated measures on region revealed a significant effect of line [F(1,11) = 12.87; p = .004], region [F(6,66) = 39.88; p < .0001] and a nearly significant line × region interaction [F(6,66) = 2.31; p = .04]. All hippocampal regions of the P rat line had significantly higher LCGU rates than did the NP rat line (aCA1 [F(1,12) = 10.65; p = .008], aCA3 [F(1,12) = 11.17; p = .007], aDG [F(1,12) = 13.05; p = .004], pCA1 [F(1,12) = 12.02; p = .005], pCA3 [F(1,12) = 10.78; p = .007], pdDG [F(1,12) = 10.56; p = .008], and pvDG [F(1,12) = 10.45; p = .008]).
Fig. 4.
LCGU rates in the sub-regions of the anterior and posterior hippocampus of P and NP rats following i.p. injection of saline or 1.0 g/kg ethanol. Values were obtained for the following regions: CA1 and CA3 subregions, dentate gyrus (DG), dorsal dentate gyrus (dDG) and ventral dentate gyrus (vDG). #Indicates that the saline value for the P rats are significantly different (p < 0.01) compared to the saline value for the NP rats. *Indicates that ethanol value is significantly lower (p < 0.01) than the respective saline value for that region within the same line. † Indicates p < 0.05 from corresponding saline LCGU rates. Data are the means ± SEM; n = 6 for all adolescent P rats and n = 7 for saline and n = 6 for ethanol for the adolescent NP rats.
Anterior and posterior regions were subdivided and analyzed by a mixed treatment × region ANOVA. In the anterior hippocampus of the P rats, there was a significant effect of region [F(2,20) = 23.61; p < .0001], but no treatment [F(1,10) = 5.9; p = .03] or treatment × region interaction [F(2,20) = 3.07; p = .07]. In the anterior hippocampus of the NP rats, there was also a significant effect of region [F(2,22) = 18.82; p < .0001], but no treatment [F(1,11) = 1.29; p = .28] or treatment × region interaction [F(2,22) = .396; p = .68].
In the posterior hippocampus of P rats, there was not a significant effect of treatment [F(1,10) = 3.81; p = .08], but a significant effect of region [F(3,30) = 48.52; p < .0001] and a nearly significant treatment × region interaction [F(3,30) = 3.03; p = .04]. Individual one-way ANOVAs indicated a significant effect of acute EtOH in the CA1 [F(1,11) = 8.65; p = 0.01] and CA3 subfields [F(1,11) = 10.38; p = 0.009], but not in the dorsal dentate gyrus [F(1,11) = 6.42; p = .03], although there was a strong trend, nor in the ventral dentate gyrus [F(1,11) = 3.97; p = .07]. In the posterior hippocampus of the NP rats, there was no significant effect of treatment [F(1,11) = .005; p = .94] nor treatment × region interaction [F(3,33) = 1.22; p = .32], but there was a significant effect of region [F(3,33) = 53.75; p < .0001].
Basal Ganglia
The caudate putamen (CPU), the substantia nigra pars reticulata (SNpr) and pars compacta (SNpc) were functionally grouped and analyzed with a treatment × region ANOVA with repeated measures for region (Table 1). There were significant differences in the basal ganglion regions between the P rats that received saline and the NP rats that received saline. The mixed ANOVA revealed a significant effect of line [F(1,11) = 10.57; p = .008], region [F(2,22) = 168.5; p < .0001] but no significant line × region interaction [F(2,22) = 2.29; p = .12]. One way ANOVAs determined that the SNpr [F(1,12) = 8.55; p = .01] and SNpc [F(1,12) = 17.56; p = .002] were significantly different with higher LCGU rates in the saline P rats compared to the saline NP rats. The CPU [F(1,12) = 6.47; p = .02] was nearly significant between the two rat lines.
Table 1.
LCGU rates (μmol/100g/min) in adolescent P and NP rats following acute ethanol injection
| Line: | P | P | NP | NP |
|---|---|---|---|---|
| Dose: | saline (N = 6) | 1.0 g/kg (N = 6) | saline (N = 7) | 1.0 g/kg (N = 6) |
| Basal ganglia | ||||
| Caudate Putamen | 152 ± 9† | 137 ± 10 | 127 ± 5 | 127 ± 8 |
| Substantia Nigra | ||||
| -Pars reticulata | 103 ± 6# | 86 ± 6 | 86 ± 3 | 87 ± 4 |
| -Pars compacta | 131 ± 6# | 114 ± 7 | 104 ± 3 | 107 ± 6 |
| Subcortical regions | ||||
| Septum | ||||
| Medial | 130 ± 5# | 110 ± 10 | 100 ± 4 | 101 ± 5 |
| Thalamus | ||||
| -Dorsomedial | 173 ± 9† | 147 ± 11 | 149 ± 4 | 139 ± 9 |
| -Lateral Dorsal | 174 ± 7# | 146 ± 12 | 146 ± 5 | 140 ± 10 |
| -Ventral Medial | 167 ± 8# | 152 ± 10 | 139 ± 6 | 142 ± 10 |
| -Medial Geniculate | 186 ± 10 | 157 ± 10 | 161 ± 8 | 146 ± 10 |
| Habenula | 166 ± 8# | 140 ± 11 | 139 ± 4 | 133 ± 7 |
| Hypothalamic preoptic area | ||||
| Medial | 86 ± 4# | 83 ± 6 | 74 ± 2 | 75 ± 3 |
| Lateral | 114 ± 5# | 98 ± 8 | 91 ± 3 | 93 ± 2 |
| Superior Colliculus | ||||
| -Superficial gray | 155 ± 10# | 130 ± 10 | 118 ± 4 | 121 ± 6 |
Values are the means ± SEM.
indicates significantly different (p < 0.01) from corresponding NP saline LCGU rates
indicates p < 0.05 from corresponding NP saline LCGU rates
In the individual line analyses; P rats there was no significant effect of treatment [F(1,10) = 2.72; p = .13], no significant treatment × region interaction [F(2,20) = .02; p = .98], but a significant effect of region [F(2,20) = 113.65; p < .0001]. In the NP rats, there was a significant effect of region [F(2,22) = 118.01; p < .0001], but no effect of treatment [F(1,11) = .06; p = .81], and no significant treatment × region interaction [F(2,22) = .123; p = .88].
Subcortical Regions
The lateral septum was included in the limbic analyses, therefore the medial septum was analyzed alone with a one-way ANOVA (Table 1). The two saline groups were significantly different between the lines [F(1,13) = 28.014; p < .0001], with higher LCGU rates in the P rats. In both the P and NP rats, there were no significant effects of treatment [F(1,12) = 3.24; p = .12] and [F(1,13) = .083; p = .78], respectively.
LCGU rates were calculated in the thalamus, which was subdivided into the dorsomedial, lateral dorsal, ventral medial, and medial geniculate nuclei (Table 1). These regions were grouped for analysis with a treatment × region mixed ANOVA. In the P rats this analysis found a significant effect of region [F(3,30) = 5.12; p = .006], but no effect of treatment [F(1,10) = 3.72; p = .08] nor a treatment × region interaction [F(3,30) = 1.45; p = .25]. In the NP rats, there was a main effect of region [F(3,33) = 8.07; p < .0001] and a significant treatment × region interaction [F(3,33) = 3.74; p = .02], but no main effect of treatment [F(1,11) = .414; p = .53]. The two saline groups significantly differed between the lines (higher LCGU rates in the P rats compared to the NP rats) in the lateral dorsal [F(1,13) = 11.31; p = .006] and ventral medial nuclei [F(1,13) = 8.84; p = .01], but not in the dorsomedial [F(1,13) = 6.64; p = .03] or medial geniculate [F(1,13) = 3.77; p = .08].
LCGU rates in the habenula were analyzed by one-way ANOVA. In both the P and NP rats, there were no significant effects of treatment [F(1,12) = 3.48; p = .09] and [F(1,13) = .51; p = .49], respectively. The two saline groups differed significantly between the lines [F(1,13) = 10.06; p = .009] (Table 1), with higher LCGU rates in the P rat line compared to the NP rat line.
The hypothalamus was subdivided into the medial and lateral preoptic areas (POA). For the P rats, there was no significant effect of treatment [F(1,10) = 1.46; p = .25], a significant effect of region [F(1,10) = 78.79; p < .0001], and a nearly significant treatment × region interaction [F(1,10) = 6.71; p = .03]. For the NP rats, a treatment × region mixed ANOVA revealed a significant effect of region [F(1,11) = 166.3; p < .0001], but no treatment effect [F(1,11) = .26; p = .62] and no treatment × region interaction [F(1,11) = .008; p = .93]. The two saline groups were significantly different between the lines in both regions, medial POA [F(1,13) = 9.08; p = .01] and lateral POA [F(1,13) = 17.08; p = .002], with higher LCGU rates in the P rat line compared to the NP rat line.
LCGU rates in the superior colliculus were analyzed by one-way ANOVA. In both the P and NP rats, there were no significant effects of treatment [F(1,12) = 2.92; p = .12] and [F(1,13) = .190; p = .67], respectively. The two saline groups were significantly different between the lines [F(1,13) = 12.77; p = .004], again with higher LCGU rates in the P rats compared to the NP rats.
Discussion
Adolescent alcohol use and abuse is a world-wide concern. Understanding why some adolescents quickly escalate to alcohol abuse and full blown alcoholism as opposed to those adolescents who intermittently experiment with alcohol would greatly facilitate education, diagnosis and treatments aimed at adolescents. The current study demonstrates that genetically predisposed rats (adolescent P rats) are significantly more sensitive to a moderate dose of acute ethanol as measured by local cerebral glucose utilization compared to their genetically non-predisposed counterparts (adolescent NP rats). Significant decreases in LCGU rates in the adolescent P rats were found in select limbic, cortical and hippocampal brain regions. LCGU rates were significantly decreased by approximately 15 – 25% in the adolescent P rats. Importantly, the differences in LCGU rates between the adolescent P and adolescent NP rats cannot be attributed to differences in ethanol levels, because BACs were not significantly different at any time point between the two lines (Fig. 1).
The current results also nicely replicate previous findings of basal LCGU rate differences between adult P, NP and stock Wistar rats (Smith et al., 2001; Strother et al., 2005) and suggest that the neural mechanisms responsible for the high basal LCGU rates in the P rat are already well-established in the adolescent animal. Interestingly, this difference appears to be even more pronounced in the adolescent P rat compared to the adult P rat with nearly every region examined in the current study displaying significantly higher basal LCGU rates compared to basal adolescent NP LCGU rates (Figs. 2 –4 and Table 1). Basal differences in functional activity have been suggested to be due, in part, to differences in monoamine receptor levels and innervation differences in the serotonergic and dopaminergic neurotransmitter systems between the rat lines, many of which are apparent in the adolescent or periadolescent animal (reviewed in McBride & Li, 1998; and McBride et al., 2005). Furthermore, a hyper-excited CNS has been proposed to be a hallmark of individuals with a genetic predisposition to alcoholism. It is hypothesized that predisposed individuals self-medicate with alcohol to reduce their hyperexcitable CNS to a more ‘normal’ level (Begleiter and Porjesz, 1999; Zhang et al., 2001; Muralidharan et al., 2008). This theory would be supported by the current study; adolescent P rats had significantly higher LCGU rates before a moderate dose of ethanol reduced their LCGU levels to more normative values, i.e., indistinguishable from LCGU rates observed in adolescent NP rats (data not shown).
Significant (p < .01) LCGU reduction after 1.0 g/kg i.p. ethanol was found in the OTU region of adolescent P rats. Reductions in LCGU rates were also seen in the MPFC, Acb-core, LS, and VP (all p < .05) in the adolescent P rats. Reductions in LCGU rates were in the 15–20% range, which are consistent with, although slightly less than, reductions found in adult P rats following 1.0 g/kg ethanol (Strother et al., 2005). Most of the limbic regions affected in this study were also significantly altered in the adult P rat study. This may suggest that the Acb-core is more sensitive to moderate ethanol in the adolescent P rat compared to the adult P rat. However, LCGU rates for other limbic regions, e.g. the VTA and BLA, were found to be significantly different following ethanol in the adult P rats but were not significantly different in the current study (Fig. 2), although there was a trend toward LCGU reduction in all limbic regions of both the adolescent and adult P rats. Taken together, it would suggest that the limbic regions respond to acute alcohol similarly in both the adolescent and adult P rat, but adolescent P rats may be slightly less sensitive to moderate ethanol compared to adult P rats. This would be consistent with much of the adolescent literature that has found adolescent animals to be less sensitive to many ethanol effects as compared to adult animals (see Spear and Varlinskaya, 2005, for review).
Similar to the limbic regions above, there was only one cortical region (Fig. 3) significantly altered by acute ethanol in the adolescent P rats; the frontal cortex (p < .05). The cingulate, temporal, and retrosplenial cortices were also decreased by acute ethanol in the P rats (p < .05). The largest decrease (25%) was found in the frontal cortex, with the other regions exhibiting LCGU reductions in the 15 –20% range. The frontal cortex has been the focus of much attention in the adolescent literature with the demonstration of significant developmental reorganization during the transition from adolescence to adulthood and the functional and behavioral consequences of that reorganization (see Spear, 2000 and 2002 for review). With regard to ethanol, the frontal cortex and its role in working memory has been shown to be particularly sensitive in adolescents compared to adults, in both humans and rodents (Crews et al., 2000; Brown et al., 2000; Monti et al., 2005; Tapert et al., 2004; Sircar and Sircar, 2005). Supporting this theory, acute ethanol did not significantly alter LCGU rates in the frontal cortex of adult P rats (Strother et al., 2005) but had the largest effect in adolescent P rats. Similar to the current study, the cingulate and temporal cortices were significantly decreased in the adult P rats. The retrosplenial cortex was not examined in the adult study. Additionally, there were several other cortical regions demonstrating significant LCGU reductions in the adult P rat that were not altered in the current adolescent study. All of this would suggest that while cortical regions taken as a whole would appear to be less sensitive in the adolescent P rat, the standout difference of the frontal cortex would suggest that it may be a region of particular relevance for the development of high alcohol drinking behaviors.
It has been clearly demonstrated that acute ethanol administration alters neuronal activity in the hippocampus, believed to be primarily through GABAergic mechanisms (Ariwodola and Weiner, 2004; Ariwodola et al., 2003; Lovinger et al., 1990; Sanna et al., 2004; Tokunaga et al., 2003; Tokunaga et al., 2006; Weiner et al., 1997). Our laboratory has previously demonstrated LCGU changes in the hippocampus of adult P rats following both acute ethanol injection and chronic ethanol drinking (Smith et al., 2001b; 2002; Strother et al., 2005). The current study reflects very similar changes in the adolescent P rat after acute ethanol as those seen in the adult P rat. Significant LCGU reductions following ethanol were limited to the posterior subregions of the hippocampus. Previous observations in the P rat have revealed decreased serotonergic fibers (Zhou et al., 1994), increased 5-HT1A receptors (McBride et al., 1994; Strother et al., 2003), decreased mu (McBride et al., 1998) and delta opioid receptors (Strother et al., 2001), and decreased GABA-A coupled benzodiazepine binding sites (Thielen et al., 1997), all in the posterior hippocampus.
The results of the current study also point out that there is a significant interaction between the effects of ethanol on functional activity and genetic background, the adolescent P rat being more sensitive than the adolescent NP rat to the effects of a moderate dose of ethanol on LCGU rates. Moreover, the present results indicate that the CNS functional activity of adolescent NP rats is insensitive to the acute effects of 1.0 g/kg ethanol, since no significant differences were observed in LCGU rates between the saline and ethanol treated groups. This lack of response to the initial effects of ethanol in the adolescent NP rats may reflect their genetic predisposition for low alcohol consumption, i.e., ethanol needs to have significant CNS effects on neuronal activity to promote alcohol drinking. For the NP rat, it may be necessary to have additional exposures to ethanol and/or exposures to other ethanol concentrations in order to observe effects on neuronal activity. Alternatively, this may be another example of the adolescent animal being less sensitive to acute ethanol because the adult NP rat has been shown to have small but significant LCGU rate changes to acute ethanol (Strother et al., 2005).
In summary, the present results indicate that many differences observed for basal functional activities and effects of ethanol on LCGU rates between adult P and NP rats (Strother et al., 2005) are also evident in the adolescent P and NP rats. The sensitivity of the CNS of P rats and the insensitivity of the CNS of NP rats to the initial effects of acute ethanol are likely factors that contribute to their disparate alcohol drinking behaviors.
Acknowledgments
The authors would like to thank Colleen C. Merrill for her excellent technical assistance. This work was supported in part by grants from the National Institute on Alcohol Abuse and Alcoholism: AA10256, AA07611.
References
- Ariwodola OJ, Crowder TL, Grant KA, Daunais JB, Friedman DP, Weiner JL. Ethanol modulation of excitatory and inhibitory synaptic transmission in rat and monkey dentate granule neurons. Alcohol Clin Exp Res. 2003;27:1632–9. doi: 10.1097/01.ALC.0000089956.43262.17. [DOI] [PubMed] [Google Scholar]
- Ariwodola OJ, Weiner JL. Ethanol potentiation of GABAergic synaptic transmission may be self-limiting: role of presynaptic GABA(B) receptors. J Neurosci. 2004;24:10679–86. doi: 10.1523/JNEUROSCI.1768-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. What is inherited in the predisposition toward alcoholism? A proposed model. Alcohol Clin Exp Res. 1999;23:1125–35. doi: 10.1111/j.1530-0277.1999.tb04269.x. [DOI] [PubMed] [Google Scholar]
- Brown SA, Tapert SF, Granholm E, Delis DC. Neurocognitive functioning of adolescents: effects of protracted alcohol use. Alcohol Clin Exp Res. 2000;24:164–71. [PubMed] [Google Scholar]
- Chou SP, Pickering RP. Early onset of drinking as a risk factor for lifetime alcohol-related problems. Brit J Addict. 1992;87:1199–1204. doi: 10.1111/j.1360-0443.1992.tb02008.x. [DOI] [PubMed] [Google Scholar]
- Cicero TJ. A critique of animal analogues of alcoholism. In: Majchrowicz E, Noble EP, editors. Biochemistry and Pharmacology of Alcohol. Vol. 2. Plenium Press; New York: 1979. pp. 533–560. [Google Scholar]
- Clapper RL, Buka SL, Goldfield EC, Lipsitt LL, Tsuang MT. Adolescent problem behaviors as predictors of adult alcohol diagnoses. Intl J Addict. 1995;30:507–523. doi: 10.3109/10826089509048741. [DOI] [PubMed] [Google Scholar]
- Crane AM, Porrino LJ. Adaptation of the quantitative 2-[14C]deoxyglucose method for use in freely moving rats. Brain Res. 1989;499:87–92. doi: 10.1016/0006-8993(89)91137-2. [DOI] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, 3rd, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24:1712–23. [PubMed] [Google Scholar]
- Ghodsian M, Power C. Alcohol consumption between the ages of 16 and 23 in Britain: a longitudinal study. Br J Addict. 1987;82:175–80. doi: 10.1111/j.1360-0443.1987.tb01457.x. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age of onset of drug use and its association with DSM-IV drug abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1998;10:163–73. doi: 10.1016/s0899-3289(99)80131-x. [DOI] [PubMed] [Google Scholar]
- Hawkins JD, Graham JW, Maguin E, Abbott R, Hill KG, Catalano RF. Exploring the effects of age of alcohol use initiation and psychosocial risk factors on subsequent alcohol misuse. J Stud Alcohol. 1997;58:280–90. doi: 10.15288/jsa.1997.58.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council. Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, DC: 1996. [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. NIH Publication No. 05–5726. Department of Health and Human Services; Bethesda, MD: 2005. National Results on Adolescent Drug Use from Monitoring the Future Study, overview of key findings 2004. [Google Scholar]
- Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytia P, Merlo-Pich E, Weiss F. Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res. 1998;22:3–9. [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. NMDA receptor-mediated synaptic excitation selectively inhibited by ethanol in hippocampal slice from adult rat. J Neurosci. 1990;10:1372–9. doi: 10.1523/JNEUROSCI.10-04-01372.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Bell RL, Rodd ZA, Strother WN, Murphy JM. Adolescent alcohol drinking and its long-range consequences. Studies with animal models. Recent Dev Alcohol. 2005;17:123–42. doi: 10.1007/0-306-48626-1_6. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Chernet E, McKinzie DL, Lumeng L, Li TK. Quantitative autoradiography of mu-opioid receptors in the CNS of alcohol-naive alcohol-preferring P and -nonpreferring NP rats. Alcohol. 1998;16:317–23. doi: 10.1016/s0741-8329(98)00021-4. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Guan XM, Chernet E, Lumeng L, Li TK. Regional serotonin1A receptors in the CNS of alcohol-preferring and -nonpreferring rats. Pharmacol Biochem Behav. 1994;49:7–12. doi: 10.1016/0091-3057(94)90449-9. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Li TK. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- McKinzie DL, Nowak KL, Murphy JM, Li T-K, Lumeng L, McBride WJ. Development of alcohol drinking behavior in rat lines selectively bred for divergent alcohol preference. Alcohol Clin Exp Res. 1998;22:1584–90. [PubMed] [Google Scholar]
- McMillen BA. Toward a definition of a valid animal model of alcoholism: multiple animal models for multiple diseases. Alcohol. 1997;14:409–419. doi: 10.1016/s0741-8329(97)90012-4. [DOI] [PubMed] [Google Scholar]
- Monti PM, Miranda R, Jr, Nixon K, Sher KJ, Swartzwelder HS, Tapert SF, White A, Crews FT. Adolescence: booze, brains, and behavior. Alcohol Clin Exp Res. 2005;29:207–20. doi: 10.1097/01.alc.0000153551.11000.f3. [DOI] [PubMed] [Google Scholar]
- Muralidharan K, Venkatasubramanian G, Pal PK, Benegal V. Abnormalities in cortical and transcallosal inhibitory mechanisms in subjects at high risk for alcohol dependence: a TMS study. Addict Biol. 2008 2008 Apr 16; doi: 10.1111/j.1369-1600.2007.00093.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, Lumeng L, Li TK. Phenotypic and genotypic characterization on the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genetics. 2002;32:363–388. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; New York: 1998. [Google Scholar]
- Pitkanen T, Lyyra AL, Pulkkinen L. Age of onset of drinking and the use of alcohol in adulthood: a follow-up study from age 8–42 for females and males. Addiction. 2005;100:652–61. doi: 10.1111/j.1360-0443.2005.01053.x. [DOI] [PubMed] [Google Scholar]
- Sanna E, Talani G, Busonero F, Pisu MG, Purdy RH, Serra M, Biggio G. Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. J Neurosci. 2004;24:6521–30. doi: 10.1523/JNEUROSCI.0075-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sircar R, Sircar D. Adolescent rats exposed to repeated ethanol treatment show lingering behavioral impairments. Alcohol Clin Exp Res. 2005;29:1402–10. doi: 10.1097/01.alc.0000175012.77756.d9. [DOI] [PubMed] [Google Scholar]
- Smith DG, Learn JE, McBride WJ, Lumeng L, Li TK, Murphy JM. Alcohol-naïve alcohol-preferring (P) rats exhibit higher local cerebral glucose utilization than alcohol non-preferring (NP) and Wistar rats. Alcohol Clin Exp Res. 2001a;25:1309–1316. [PubMed] [Google Scholar]
- Smith DG, Learn JE, McBride WJ, Lumeng L, Li TK, Murphy JM. Long-term effects of alcohol drinking on cerebral glucose utilization in alcohol-preferring rats. Pharmacol Biochem Behav. 2001b;69:543–53. doi: 10.1016/s0091-3057(01)00553-6. [DOI] [PubMed] [Google Scholar]
- Smith DG, Learn JE, McBride WJ, Lumeng L, Li TK, Murphy JM. Local cerebral glucose utilization after relapse in ethanol drinking in alcohol-preferring (P) rats. Alcohol. 2002;27:115–26. doi: 10.1016/s0741-8329(02)00216-1. [DOI] [PubMed] [Google Scholar]
- Sokoloff L, Reivich M, Kennedy C, DesRosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M. The 2-[14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence: Age-dependent behavior and psychopharmalogical responsivity in rats. Devel Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Adolescence. Alcohol sensitivity, tolerance, and intake. Recent Dev Alcohol. 2005;17:143–59. [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and the college drinker: biological basis of propensity to use and misuse alcohol. J Stud Alcohol Suppl. 2002;14:71–81. doi: 10.15288/jsas.2002.s14.71. [DOI] [PubMed] [Google Scholar]
- Strother WN, Chernet EJ, Lumeng L, Li TK, McBride WJ. Regional central nervous system densities of delta-opioid receptors in alcohol-preferring P, alcohol-nonpreferring NP, and unselected Wistar rats. Alcohol. 2001;25:31–8. doi: 10.1016/s0741-8329(01)00162-8. [DOI] [PubMed] [Google Scholar]
- Strother WN, Lumeng L, Li TK, McBride WJ. Regional CNS densities of serotonin 1A and dopamine D2 receptors in periadolescent alcohol-preferring P and alcohol-nonpreferring NP rat pups. Pharmacol Biochem Behav. 2003;74:335–42. doi: 10.1016/s0091-3057(02)01001-8. [DOI] [PubMed] [Google Scholar]
- Strother WN, Lumeng L, Li TK, McBride WJ. Dopamine and serotonin content in select brain regions of weanling and adult alcohol drinking rat lines. Pharmacol Biochem Behav. 2005;80:229–37. doi: 10.1016/j.pbb.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Strother WN, McBride WJ, Lumeng L, Li TK. Effects of acute administration of ethanol on cerebral glucose utilization in adult alcohol-preferring and alcohol-nonpreferring rats. Alcohol. 2005;35:119–28. doi: 10.1016/j.alcohol.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Pulido C, Paulus MP, Schuckit MA, Burke C. Level of response to alcohol and brain response during visual working memory. J Stud Alcohol. 2004;65:692–700. doi: 10.15288/jsa.2004.65.692. [DOI] [PubMed] [Google Scholar]
- Thielen RJ, McBride WJ, Chernet E, Lumeng L, Li TK. Regional densities of benzodiazepine sites in the CNS of alcohol-naive P and NP rats. Pharmacol Biochem Behav. 1997;57:875–82. doi: 10.1016/s0091-3057(96)00464-9. [DOI] [PubMed] [Google Scholar]
- Tokunaga S, McDaniel JR, Morrow AL, Matthews DB. Effect of acute ethanol administration and acute allopregnanolone administration on spontaneous hippocampal pyramidal cell neural activity. Brain Res. 2003;967:273–80. doi: 10.1016/s0006-8993(02)04266-x. [DOI] [PubMed] [Google Scholar]
- Tokunaga S, Silvers JM, Matthews DB. Chronic intermittent ethanol exposure during adolescence blocks ethanol-induced inhibition of spontaneously active hippocampal pyramidal neurons. Alcohol Clin Exp Res. 2006;30:1–6. doi: 10.1111/j.1530-0277.2006.00020.x. [DOI] [PubMed] [Google Scholar]
- Weiner JL, Gu C, Dunwiddie TV. Differential ethanol sensitivity of subpopulations of GABAA synapses onto rat hippocampal CA1 pyramidal neurons. J Neurophysiol. 1997;77:1306–12. doi: 10.1152/jn.1997.77.3.1306. [DOI] [PubMed] [Google Scholar]
- Zhang XL, Cohen HL, Porjesz B, Begleiter H. Mismatch negativity in subjects at high risk for alcoholism. Alcohol Clin Exp Res. 2001;25:330–7. doi: 10.1097/00000374-200103000-00003. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Pu CF, Murphy J, Lumeng L, Li TK. Serotonergic neurons in the alcohol preferring rats. Alcohol. 1994;11:397–403. doi: 10.1016/0741-8329(94)90024-8. [DOI] [PubMed] [Google Scholar]