Abstract
Background
Adolescent rats are less sensitive to the sedative effects of ethanol than older animals. They also seem to perceive the reinforcing properties of ethanol. However, unlike neonates or infants, ethanol-mediated appetitive behavior has yet to be clearly shown in adolescents. Appetitive ethanol reinforcement was assessed in adolescent (postnatal day 33, P33) and adult rats (P71) through second-order conditioning (SOC).
Methods
On P32 or P70 animals were intragastrically administered ethanol (0.5 or 2.0 g/kg) paired with intraoral pulses of sucrose (CS1, first-order conditioning phase). CS1 delivery took place either 5-20 (Early pairing) or 30-45 (Late pairing) min following ethanol. CS1 exposure and ethanol administration were separated by 240 min in unpaired controls. On P33 or P71, animals were presented the CS1 (second-order conditioning phase) while in a distinctive chamber (CS2). Then, they were tested for CS2 preference.
Results
Early and late paired adolescents, but not adults, had greater preference for the CS2 than controls, a result indicative of ontogenetic variation in ethanol-mediated reinforcement. During the CS1 - CS2 associative phase, paired adolescents given 2.0 g/kg ethanol wall-climbed more than controls. Blood and brain ethanol levels associated with the 0.5 and 2.0 g/kg doses at the onset of each conditioning phase did not differ substantially across age, with mean BECs of 38 and 112 mg %.
Conclusions
These data indicate age-related differences between adolescent and adult rats in terms of sensitivity to ethanol’s motivational effects. Adolescents exhibit high sensitivity for ethanol’s appetitive effects. These animals also showed EtOH-mediated behavioral activation during the second-order conditioning phase. The SOC preparation provides a valuable conditioning model for assessing ethanol’s motivational effects across ontogeny.
Keywords: adolescence, adulthood, ethanol, reinforcement, second-order conditioning
Introduction
Age of first alcohol exposure is strongly associated with later patterns of alcohol consumption; i.e., the earlier the contact with the drug, the higher the possibility of later alcohol abuse. Yet, it remains to be determined whether this association is causal (Hawkins et al., 1997; Pedersen & Skrondal, 1998). Alcohol intake in humans usually starts during adolescence, with recent data indicating that approximately 28% of underage drinkers started around age 13 (Faden, 2006). Beyond casual use, heavy drinking is also widespread, with 30% of 12th graders reporting they had been drunk at least once in the last 30-days (Johnston et al., 2007). Why do adolescents consume alcohol and why are they so predisposed to binge drinking? Answering this question has been troublesome, as long as experimental behavioral research in humans is limited by obvious ethical and legal constraints (Witt, 1994). However, substantial progress was made during the last decade, thanks to a surge in the employment of experimental animal models (Spear & Molina, 2005; Spear & Varlinskaya, 2005).
Adolescence has been conservatively defined as the period between postnatal day 28 and postnatal day 42 (P28 – P42; e.g., Spear, 2000), although some adolescent typical characteristics may last, particularly in males, until P60 or so (Smith, 2003; Spear, 2000). During adolescence animals exhibit enhanced levels of basal behavioral activation as well as greater expression of conspecific-directed behaviors, including play behavior (Panksepp, 1981; Spear & Brake, 1983). They also show age-specific patterns of response to several drugs, notably psychostimulants and ethanol (Adriani & Laviola, 2000; Spear, 2000; Spear & Varlinskaya, 2005). For instance, expression of amphetamine, nicotine, cocaine, and ethanol-mediated conditioned taste aversions has been reported to be not as strong in adolescent rats as in older animals (Infurna & Spear, 1979; Schramm-Sapyta et al., 2006; Varlinskaya et al., 2006). Conditioned aversion induced by lithium chloride, an emetic agent, is also reduced in adolescents relative to older subjects (Schramm-Sapyta et al., 2006). Taken together, these results suggest that adolescents may exhibit an increased threshold for the acquisition of aversive motivational learning.
Cognitive impairment induced by ethanol has been found to be greater in adolescence than in adulthood in learning tasks involving aversive conditioning and spatial abilities (Land & Spear, 2004a; Markiwiese et al., 1998). On the other hand, detrimental effects of ethanol on appetitive conditioning were greater in adult rats than among adolescents (Land & Spear, 2004b). In general terms, adolescent rats are less sensitive than mature animals to a wide array of ethanol’s effects; these effects include ethanol-induced motor impairment, narcosis, sedation and social inhibition (Little et al., 1996; Spear & Varlinskaya, 2005). This phenomenon may relate to an increased propensity of the adolescent rat for developing acute tolerance to ethanol (Silveri & Spear, 2004). Given that the sedative and motor impairing effects of ethanol are thought to limit intake of the drug, these age-specific predispositions in terms of ethanol sensitivity and tolerance may put adolescents at risk for ethanol-related problems.
Recent experiments have suggested that adolescents may be particularly sensitive to ethanol’s positive motivational reinforcing effects. Philpot et al. (2003) found ethanol-mediated conditioned place preference in rats at the transition between late adolescence and adulthood (P45) but not in adults (P60). Also, Fernández Vidal et al. (2003) trained adolescent rats (P30-33) to discriminate a moderate ethanol toxic state from a non-drug state in a procedure that involved rats having access to sucrose after ethanol (0.5 g/kg) or sham intubations. Unexpectedly, sucrose seeking and intake behaviors increased in those animals where sucrose availability had been signaled by ethanol. These experiments indicate that adolescents may perceive the postabsorptive consequences of ethanol as reinforcing. However, ethanol-mediated conditioned preferences in adolescents (i.e., P28-42) comparable to those found in neonates (Cheslock et al., 2001) and infant rats (Molina et al., 2006; 2007) have yet to be clearly demonstrated.
Adult (> P60) and preweanling (i.e., < P21 days) rats generally do not express preferences for visual, tactile or taste cues (conditional stimuli, CSs) that had been earlier paired with the postingestive effects of alcohol (unconditional stimulus, US). On the contrary, the predominant motivational effects of this association are aversive, as indexed by rats avoiding the ethanol-paired texture or rejecting the taste previously paired with ethanol (Cunningham et al., 1993; Pautassi et al., 2002). However, tactile conditioned preferences mediated by postabsorptive ethanol have been found recently in preweanling infant rats by means of a relatively novel experimental preparation that makes use of second-order conditioning (SOC; Molina et al., 2006; 2007).
In a SOC preparation, conditioning induced by CS1-US pairings is not assessed through re-exposure at test to the CS1. Instead, an additional set of pairings is conducted. These pairings comprise the original CS1 and a second, different neutral stimulus (CS2). This second stage (also known as second-order conditioning phase) is meant to allow the CS1 to transfer behavioral control to the CS2. If the learning experience is successful, the CS2 should now elicit a conditional response (Rescorla, 1980). The SOC procedure has been observed to permit expression of otherwise seemingly “silent” associations in developing organisms (Miller et al., 1990). This procedure might be particularly suited to assess ethanol conditioning in developing organisms as it involves functional capabilities known to be expressed early in life (see Molina et al., 2006). Specifically, developing rats encode stimuli of different sensory modality by taking into account their amodal properties, including their common affective properties (e.g., Spear & Molina, 1987). In other words, developing animals integrate, associate and functionally equate CSs of different sensory modalities and transfer motivational information between them (Kraebel & Spear, 2000; Molina et al., 1991).
Molina et al. (2006, 2007) adapted the SOC procedure to scrutinize the preweanling’s perception of ethanol’s hedonic effects. Specifically, 14 day old rats were given intraoral infusions of a tastant (CS1) after administration of 0.5 or 2.0 g/kg ethanol. Pups were subsequently exposed to the CS1 while over sandpaper (CS2), and then preference for sandpaper was tested. Intraoral CSs paired with low dose ethanol (0.5 g/kg) or the early effects (5-15 min postadministration, PAT) of the higher dose induced preference for the sandpaper texture. That is, the ethanol-paired tastant (CS1) endowed the tactile cue (CS2) with positive, second-order reinforcing capabilities (Molina et al., 2006). Interestingly, second-order tactile aversions emerged when the pairing between the higher dose and the CS1 was delayed until 30-45 min PAT, an interval characterized by peak blood ethanol concentrations (Molina et al., 2007). In other words, the SOC preparation can detect differential (i.e., appetitive vs. aversive) motivational properties of ethanol as a function of dose and postadministration time. Similar biphasic motivational effects of ethanol have been observed in adult mice (Risinger & Cunningham, 1992; Cunningham et al., 2002).
The present study assessed rewarding properties of ethanol in adolescent and adult rats using a second order conditioning procedure similar to that used previously to detect differential motivational effects of ethanol in preweanlings (Molina et al., 2006; 2007). In this preparation, a first-order association between ethanol and a taste (CS1) provides the basis for second order place-preference conditioning (using a tactile/visual cue as the CS2). In other words, ethanol-mediated learning was tested in terms of the capability of the taste CS1 to act as a reinforcer when paired with a novel tactile CS2. Particular attention was given to the possibility of ethanol exerting differential hedonic effects as a function of dose and postadministration time (Pautassi et al., 2002; Molina et al., 2007). Hence, the initial pairing of ethanol and the taste CS was performed using a relatively low (0.5 g/kg) and a relatively high (2.0 g/kg) ethanol dose. Within each dose there was systematic variation of postadministration time interval (either 5-20 min or 30-45 min after ethanol administration). These post-administration times were chosen on the basis of previous research indicating that, in preweanling rats, a 5 min delay between intragastric ethanol administration and the taste CS results in second-order conditioned place preference, while place aversions are more likely to be observed when exposure to the CS is delayed until 30 min following drug intubation (Molina et al., 2006; 2007).
The explicit intention of this study was to test, in adolescent and adult rats, ethanol reinforcement (preference or aversion) using a similar SOC procedure. To provide supplementary evidence relevant to possible first-order conditioned responses, behavioral reactivity to CS1 (locomotor activity, wall climbing and head shaking) was assessed during the second-order conditioning phase. Brain and blood ethanol levels (BrELs and BELs, respectively) associated with the behavioral expression of ethanol’s effects in both age groups were examined in a separate group of animals. Levels attained in blood and brain were measured at postadministration intervals representing the onset of each conditioning phase (7.5 and 32.5 min).
General Methods
Subjects
A total of one hundred and ninety-one Sprague-Dawley rats were employed. Seventy-four adolescent and 61 adult rats, representative of 31 litters, were used during second-order conditioning procedures. Blood and brain ethanol measurements employed 34 adolescent and 22 adults, derived from 10 litters. Animals were born and reared at the Center for Developmental Psychobiology (Binghamton University, USA). The colony was kept at 22 – 24 °C and a 12-hour light-dark cycle was used, with lights on at 8:00 AM. Births were examined daily and the day of parturition was considered as P0. On P1, litters were culled to 10 animals, 5 males and 5 females, whenever possible. Pups were housed with the dam in standard maternity cages with free access to water and food. Weaning was performed at P21. At that day, 8 animals from each litter (4 males and 4 females) were transferred to clean tubs lined with pine shavings. Any remaining animal in the litters was not used in these experiments. At P28, males and females were transferred, in same-sex groups of 4, to clean tubs. To prevent the stress associated with overcrowding, animals tested at adulthood were further separated in pairs at P60. Adolescents and adult animals had a mean body weight of 129.06 ± 12.5 g and 325.4 ± 80.23 g, respectively. Experimental procedures complied with the Guide for Care and Use of Laboratory Animals (NIH, Institute of Laboratory Animal Resources, 1996) and were also approved by the Institutional Animal Care and Use Committee within an AAALAC-accredited facility.
Experimental Design
A 2 (age: adolescents or adults) × 2 (ethanol dosage: 0.5 or 2.0 g/kg) × 3 (conditioning procedure) factorial design defined the assessment of ethanol-mediated second-order conditioning. Subjects were exposed to the CS1 during an early (5-20 min; Early pairing, EP) or a later (30-45 min; Late pairing, LP) postadministration time, or the CS1 was explicitly unpaired with ethanol’s postabsorptive effects (Unpaired controls, UP). Groups had a minimum of 9 and a maximum of 12 animals.
For the analysis of blood and brain ethanol concentrations, a 2 (age: adolescents or adults) × 2 (ethanol dose: 0.5 or 2.0 g/kg) × 2 (postadministration time: early or late) factorial design was employed. Each of the 8 groups was composed of 8-9 animals.
Across procedures and to avoid overrepresentation of litters within each specific group, no more than two animals per litter (one male and one female) were assigned to any particular experimental condition. Each conditioning group was composed of approximately 5 males and 5 females. For blood and brain ethanol measurements, each experimental condition had an average of 6 males and 6 females.
Surgery and Cannulation Procedures
Procedures conducted during conditioning and CS1 testing required animals to be intraorally implanted with cannulae made out of polyethylene tube (Kiefer, 1995; Kiefer et al., 2005). This procedure allowed control over the amount and timing of the intraoral stimulation provided by sucrose infusion (CS1). Surgery took place on P30 for adolescents and P67 for adults. Animals were food deprived for 2 hrs prior to be anesthetized with isoflurane (via vapor 2.5 %, carrier: oxygen, 55 psi). Level of muscle tone and pupil reflexes were assessed to ensure that animals were under the effects of the anesthetic. A small square of fur was shaved at the back of the neck above the scapula and also in the right cheek. Betaiodine and ethanol were rubbed in the skin. An incision was then made in the cheek by means of a thin-walled 14-gauge disposable needle (Harvard Instruments, Columbus, OH). A 10-cm section of PE 10 polyethylene tube (Clay-Adams, Parsippany, NJ) was run through the needle. Following this procedure the needle was removed. Then, a small flange was created in one end of the polyethylene tube. The tube was gently pulled through the medial internal surface of the cheek. Consequently, the flanged end of the cannula rested over the oral mucosae while the remainder exited from the mouth. During the second phase of the surgery, another needle was inserted at the back of the neck and guided subcutaneously to exit close to the site of the tube. Next, the tube was run through the needle, hence exiting on the top of the neck where it was secured with a fast-action adhesive (SuperGlue, Santa Anita, CA). The procedure took approximately 10 m per animal and was conducted under an air-sealed hood. After surgery, rats were treated with a topical antibiotic (Neosporin) and placed in individual holding cages with free access to food and water. These cages were lined initially with paper towels (i.e., first 24 hrs post-surgery) and then with regular pine shavings. When not subjected to conditioning procedures, animals were housed in these individual holding cages. No infections or surgery-related complications were observed. During conditioning, delivery of fluids was conducted by slipping the free end of the cannula inside a second polyethylene tube (PE 10), which in turn was connected to a Gilmont syringe (Barnant Co., Barrington, IL) mounted in a rotary microsyringe infusion pump (Kashinsky et al., 1990).
Apparatus
The experiment was conducted under bright room illumination (four 25-w fluorescent lamps located in the wall opposite to the chambers). The habituation phase and CS1 exposure period were conducted in square–shaped chambers made of wood (sides and height: 23 cm). These chambers were different (in shape, color and material) from the cages where animals were kept whenever not undergoing experimental procedures. The apparatus employed for the assessment of tactile preferences consisted of three interconnected chambers (28 × 20 × 21 cm each): a neutral central area made of white Plexiglas and lined with Formica and two end compartments. One of these compartments was also employed as CS2 during Phase 2: it contained a vertical striped pattern on the walls with a sandpaper floor (Gatorgrit, USA, coarse: 60). The other compartment had a horizontal striped wall pattern with a smooth floor (the reverse side of a sandpaper sheet). Sandpaper was replaced after each animal underwent phase 2 conditioning and after testing. A preliminary experiment conducted in our lab indicated the absence of age-related differences in bias for the distinctive chamber.
Conditioning and Testing Procedures
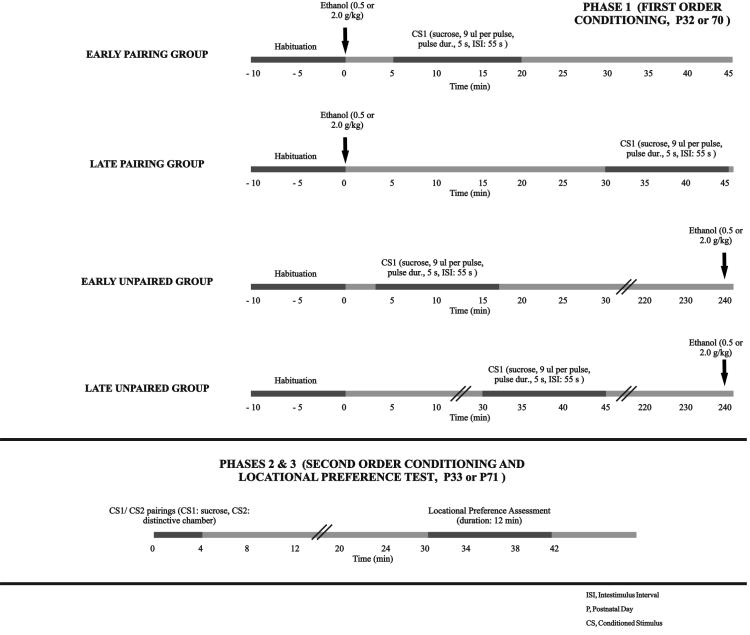
The experiment was divided into 3 phases (see Figure 1): first-order conditioning, second-order conditioning and locational preference assessment. On P32 or P70 (first-order conditioning phase) animals were given intraoral sucrose infusion (CS1) either 5-20 or 30-45 min after ethanol administration. Ethanol-sucrose pairings were followed 24 hrs later by a brief exposure to the CS1 while subjects were placed in a distinctive chamber lined with sandpaper (CS2, second order conditioning phase). Thirty min later, subjects were evaluated in a 12 min place preference assessment (P33 or 71). A detailed account of conditioning and testing procedures follows:
Figure 1.
Methods for the analysis of motivational properties of ethanol in adolescent and adult rats. Phase 1, first-order conditioning, postnatal day 32 or 70, P32 or 70: animals were administered ethanol (0.5 or 2.0 g/kg, intragastric) and then given a conditioned stimulus (CS1) consisting of intraoral pulses of sucrose. CS1 delivery took place either 5-20 or 30-45 min after EtOH (groups Early pairing and Late pairing, respectively). In Unpaired controls, CS1 exposure and EtOH administration were separated by 240 min. Unpaired adolescents were assigned to two conditions (early or late), as a function of the interval between habituation and CS1 exposure (5 or 30 min, respectively). These conditions did not differed in terms of either CS1 responsiveness or CS2 preference and, for the purpose of the statistical analyses, have been collapased in a single condition. At adulthood, only early unpaired groups were employed. All groups underwent an initial, non-reinforced habituation phase (duration: 10 min). Phase 2, second-order conditioning, P33 or P71: animals were briefly stimulated with 10% sucrose (trial duration: 4 min) while placed in a visually and tactile distinctive chamber (CS2). Duration of wall climbing, frequency of head-shakings and general locomotion were registered. Phase 3, locational preference test, P33 or P71: time spent on the CS2 chamber was recorded in a 12 min, place preference test.
Phase 1 (first-order conditioning, postnatal day 32 or 70, P32 or P70,)
Conditioning procedures closely followed those used by Molina et al. (2006; 2007). Animals were food and fluid deprived 120 min before commencement of the conditioning session. This deprivation aimed at eliminating the confounding factor of differential stomach loading, that could affect absorption and distribution of ethanol. Next, they were individually placed in a square-shaped chamber lined with cotton. A 10 min habituation phase was conducted to familiarize subjects with the chambers. Immediately following habituation, animals in the paired group were weighed to the nearest 0.01 g (Sartorius, Gottingen, Germany) and intragastrically (i.g.) administered 0.5 or 2.0 g/kg ethanol. The rats were then returned to their individual holding chambers. Conditioning took place after animals were returned to the square-shaped chambers. In these chambers animals were given a conditioned stimulus (CS1) consisting of intraoral pulses of sucrose (10% v/v, 9 μl per pulse, pulse duration: 5 sec, interval between pulses: 55 sec). CS1 delivery took place either 5-20 or 30-45 min after ethanol (groups Early pairing and Late pairing, respectively) and was performed by connecting the intraoral cannula to the infusion pump.
The experiment employed explicitly unpaired controls (UP). These animals were also subjected to the 10 min habituation and weighing procedure. Adolescents unpaired controls were exposed to the CS1 either 5 (Early unpaired group) or 30 min (Late unpaired group) after having been weighted. This procedure aimed at equating the interval between habituation and conditioning across groups. Since interval between habituation and conditioning failed to affect responsiveness at test in adolescent controls (see data analysis section), only early unpaired controls were employed when examining adult animals. These animals were habituated to the chambers, weighed and 5 min later exposed to the intraoral CS1. All unpaired animals were returned to their holding chambers immediately after CS1 exposure. Ethanol administration (0.5 or 2.0 g/kg) in UP animals took place in these holding chambers 240 min following CS1 exposure. Volume of EtOH administration was calculated using the weight measurement obtained earlier during the day (i.e., immediately after the habituation phase).
Phase 2 (second-order conditioning, P33 or P71)
On the day following Phase 1 conditioning, animals were food and fluid deprived for 120 minutes. Subsequently, they were confined, by means of an acrylic barrier, to the sandpaper-lined side of the place preference apparatus, as described earlier. During confinement to this compartment, animals were intermittently stimulated with 10% sucrose (four 9-μl pulses, interstimulus interval: 55 s). During the 4 min second order conditioning phase, animals were videotaped to allow later examination of behavioral responsiveness. The following variables were registered: duration of wall climbing, frequency of head-shakings and general locomotion. Wall climbing duration was registered when animals stood on their rear limbs with the forepaws placed on and treading the walls of the chamber. Head shaking was measured when observing rapid side-to-side movements of the head. A head movement of approximately 90° off midline to each side was necessary to generate a positive count, with these head shakes individually counted in real-time. Finally, time spent in forward locomotion (i.e., the combined movement of the four paws in a horizontal plane) was also registered in real-time. A researcher blind to the experimental conditions measured these behaviors.
Phase 3 (locational preference test, P33 or P71)
Thirty min following phase 2, animals were tested in a 12 min locational preference test. This procedure represents a variant of the widely-employed conditioned place preference test. During this phase, barriers separating the compartments were absent, hence animals could freely explore the three-chamber test box. Position of the test box during testing was the same as in phase 2 of conditioning, so as to keep constant potential distal spatial cues that could have signaled CS1 delivery. Spatial location of the target compartment affects expression of ethanol-mediated place preference when the procedure involves visual CSs (Cunningham et al., 2006). Preference assessment started by placing the animal in the central portion of the neutral compartment. Time spent over each end compartment of the apparatus was recorded in real time by two experimenters who were blind in regard to the training conditions of the animals. A subject was considered to be in a particular compartment when two paws and the head were over that section. The middle section of the apparatus (i.e., the start box) was considered as a neutral area. In this as well as in the previous experimental phases, the apparatus was cleaned with distilled water after each animal was tested.
Sucrose and Drug preparation procedures
Ethanol doses (0.5 and 2.0 g/kg) were achieved by intragastrically administering 0.015 ml of a 4.2 or 16.8 % v/v ethanol solution per gram of body weight (190-proof Ethanol, Pharmaco, Brookfield; vehicle: tap water). The administration was conducted in less than 10 sec by gently introducing a 12-cm section of PE polyethylene tubing (Clay-Adams) into the animal’s oral cavity. The tubing (diameter: 10” or 50”, for adolescents and adults, respectively) was connected to a syringe (5 or 12 cc, adolescents and adults, respectively) mounted with a 27 or 25 G ½ needle (Becton Dickinson & Co., Rutheford, N.J). About 6 cm of tubing was guided into the subjects’ stomach prior to the delivery of EtOH. Sucrose (Sigma-Aldrich, St. Louis, MO) was prepared daily employing distilled water as a vehicle.
Determination of Blood and Brain Ethanol Concentration
Adolescent and adult animals used for determination of ethanol concentrations were naive to any experimental manipulation until being food and fluid deprived for 120 min prior to injection of 0.5 or 2.0 g/kg ethanol, on P32 or P70, respectively. Animals were individually placed in pine-shaving lined containers, with brain and blood ethanol samples taken at 7.5 or 32.5 min after ethanol administration. Trunk blood (2 μl samples) was obtained through decapitation, employing a heparinized capillary tube, and centrifuged at high speed (15 min / 3000 rpm; Micro-Haematocrit Centrifuge, Hawksley & Sons LTD, Sussex, England). Blood samples were processed by means of an AM1 Alcohol Analyzer (Analox Instruments, Lunenburg, MA). The apparatus estimates BELs by oxidating ethanol to acetaldehyde in the presence of ethanol oxidase. This process is oxygen-dependent and the quantity of O2 is proportional to ethanol concentration. Whole brains were collected, maintained in a -80° C freezer and later sonicated in a water solution as previously described by Silveri & Spear (2000). These samples were analyzed for ethanol content by means of a head-space gas-chromatograph method (Hewlett Packard 5890 series II, Wilmington, DE). BELs and BrELs values were expressed as milligrams of ethanol per deciliter of body fluid (mg/dl = mg %).
Data Analysis
The SOC procedure was executed in experimental replications composed of either adolescent or adult animals. That is, adults and adolescent rats were run separately, in groups of approximately 8 animals. Adolescent experimental replicas were run sequentially in a 3 month period. A second phase was devoted to the adult experimental replications. A period of approximately 3 months elapsed between these two studies, hence the data for adolescents and adults were analyzed separately. Pharmacokinetic measurements, on the other hand, were conducted simultaneously at both ages, and hence the data were analyzed via between-age analyzes.
Preliminary analyses conducted at each age indicated no significant main effects of sex or significant interactions involving sex in terms of either CS2 preference scores or behavioral responsiveness during phase 2. Likewise, sex was not found to affect blood and brain ethanol levels (BELs and BrELs, respectively). Hence, descriptive and inferential analysis of the data was performed after collapsing across gender.
Preliminary statistical analysis indicated that adolescent unpaired groups spent similar amounts of time (indexed both in seconds and percent time) on the CS2 at test regardless of the interval between habituation and CS1 exposure (i.e., Early or Late adolescent unpaired groups) [F (1, 34) = 0.15, for 0.5 g/kg unpaired animals; F (1, 34) = 0.36, for 2.0 g/kg unpaired animals; both ps > 0.55]. Further analysis of the data based on behavioral responsiveness during the second-order phase also indicated that these unpaired conditions did not differ [F (1, 34) = 0.01; F (1, 34) = 0.04; F (1, 34) = 0.50; for locomotion, wall-climbing and head shaking, respectively; all ps > 0.45]. Hence, data corresponding to the unpaired adolescent controls were collapsed across timing of CS1 exposure. This manipulation, which does not preclude analysis of pertinent interactions, allowed simplification in analysis and presentation of the data set (see Pautassi et al., 2005; Roth et al., 2006: Thiele et al., 1998).
The main dependent variables under analysis were total amount of time (seconds and percentage) spent on the sandpaper-lined compartment (CS2) during the location preference test and behavioral responsiveness registered during phase 2 (wall-climbing, locomotion and head-shaking). Percent time spent on the rough-floor compartment measured time on CS2 compared to time spent on the smooth-floor compartment and was calculated by the formula: (total time spent over sandpaper × 100) / (total time spent over sandpaper + total time spent over smooth. Hence, time spent in the neutral compartment was not taken into account.
Behavioral variables were analyzed by separate two-way mixed analyses of variance (ANOVAs). The between-group factors were treatment during conditioning [unpaired (UP), early pairing (EP) or late pairing (LP)] and ethanol dose (0.5 or 2.0 g/kg). The loci of significant main effects or interactions were further examined by means of pairwise post-hoc comparisons (Fisher’s Least Mean Significant tests, with alpha level set at 0.05).
For each particular age, Pearson product-moment correlations were conducted between CS2 preference at test (%) and behavioral scores during the CS1-CS2 transfer phase. Correlations were processed for the overall sample of subjects as well as separately for each combination of ethanol dose and treatment group.
Blood and Brain ethanol levels were analyzed separately by means of three-way mixed ANOVAs [Age (P32 or P70) × postadministration time (PAT, 7.5 or 32.5 min) × dose: 0.5 or 2.0 g/kg].
Results
Texture Preferences at test (P 33 or P71)
Adolescent subjects (P33)
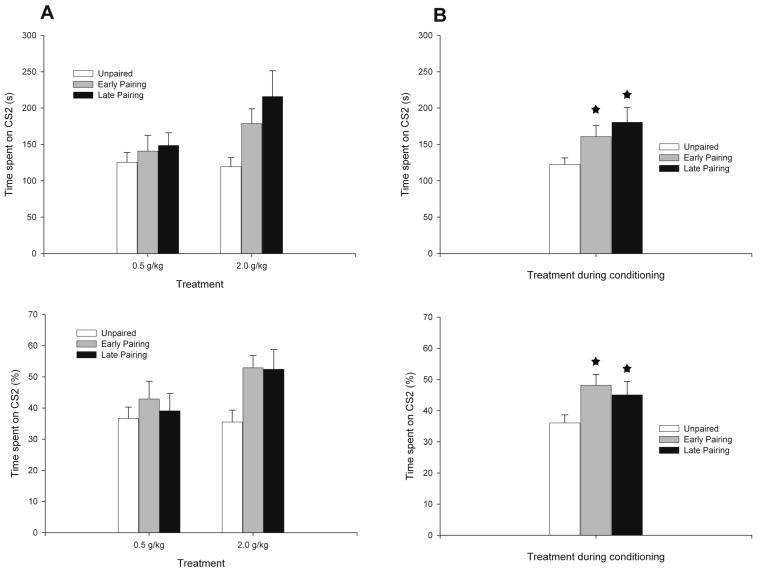
As depicted in Figure 2 (a and b), the initial pairing of intraoral sucrose (CS1) and ethanol increased preference for the sandpaper-lined compartment (CS2). The ANOVA yielded a significant main effect of conditioning group for absolute and percent time spent on CS2: F(2, 68) = 5.58; p< 0.005 and F (2,68) = 4.12; p < 0.05, respectively. Post-hoc tests indicated that adolescents in the EP and LP paired groups spent significantly more time in the CS2 compartment than the unpaired condition (p < 0.05 and p < 0.005, respectively). Inspection of Fig. 2a also suggests that the conditioning effect is driven largely by the 2.0 g/kg group, but this was not confirmed statistically; no significant main effect nor significant interaction comprising ethanol dose was detected by the ANOVA.
Figure 2.
Texture preferences at test in adolescent subjects (postnatal day 33).A. Total time (s) spent on the sandpaper lined chamber (top panel) and its corresponding percentage preference for the chamber (bottom panel) during the 12-minute test session as a function of treatment during conditioning (Unpaired, Early pairing and Late Pairing) and Ethanol Dose (0.5 and 2.0 g/kg, intragastric). The statistical analysis for either dependent variable indicated significant main effects of treatment during conditioning. B. Total time (s) spent on the sandpaper lined chamber (top panel) and its corresponding percentage preference for the chamber (bottom panel) during the 12-minute test session as a function of treatment during conditioning. In Fig. 2B, asterisks indicate significant differences from the Unpaired group (p < 0.05). Vertical bars indicate the standard error of the mean.
Adult subjects (P71)
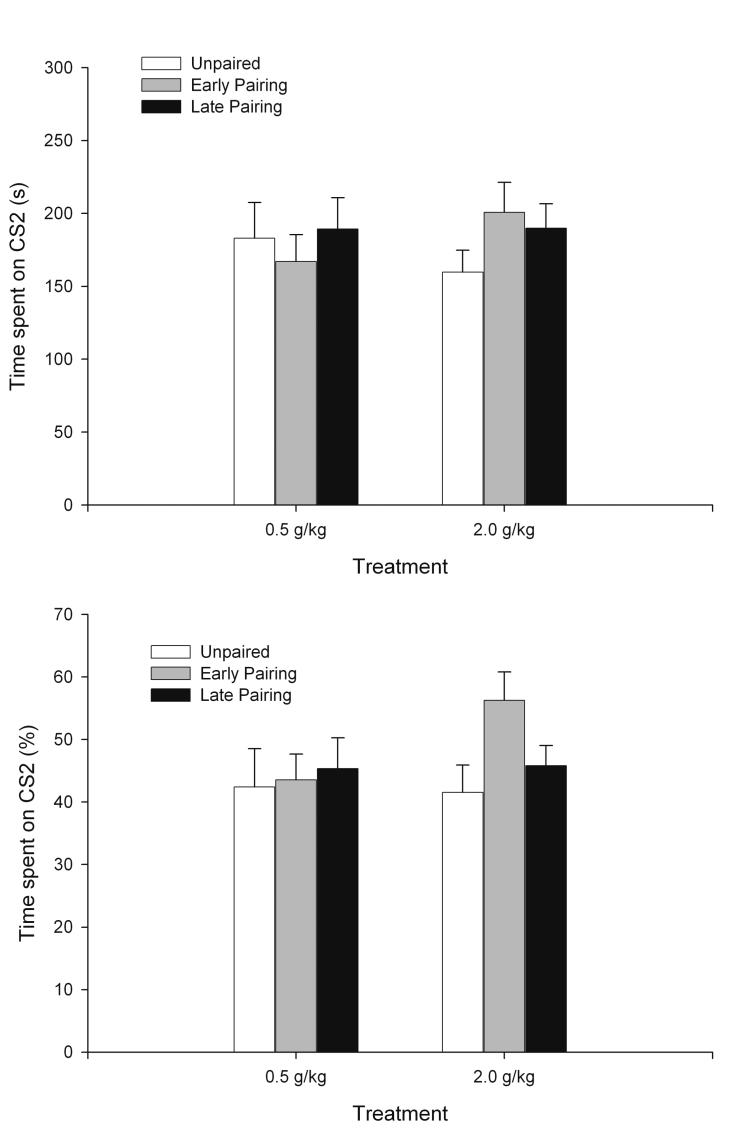
The 2 × 3 ANOVAs (ethanol dosage: 0.5 or 2.0 g/kg × conditioning procedure: EP, LP or UP) for both absolute and percent time spent over the sandpaper CS2 indicated that neither the main factors nor the interaction between them had significant effects on these dependent variables. These results are shown in Figure 3.
Figure 3.
Texture preferences at test in adult subjects (postnatal day 71). Total time (s) spent on the sandpaper-lined floor (top panel) and its corresponding percentage preference for the chamber (bottom panel) during the 12-minute test session as a function of treatment during conditioning (Unpaired, Early pairing and Late Pairing) and Ethanol Dose (0.5 and 2.0 g/kg, intragastric). The statistical analysis revealed that absolute and percent preference for sandpaper were not affected by ethanol dose or treatment during conditioning. Vertical bars indicate the standard error of the mean.
Behavioral Responsiveness during CS1-CS2 pairings (P33 or P71)
Adolescent subjects (P33)
The ANOVA for wall-climbing scores in adolescent subjects yielded a significant interaction between treatment during conditioning and ethanol dose, F(2, 68) = 3,79; p< 0.05. Post-hoc tests indicated similar levels of wall-climbing in P33 animals given 0.5 g/kg, regardless of nature of the contingency between sucrose and ethanol in Phase 1. In contrast, adolescents in groups EP and LP previously given 2.0 g/kg ethanol during Phase 1 exhibited more wall climbing during the transfer phase (in which no ethanol was given) than their unpaired controls (both ps < 0.05). Descriptive data (i.e., means and standard errors) relative to locomotion, head-shaking and wall-climbing can be found in Table 1.
Table 1.
Behavioral responsiveness during CS1-CS2 pairings (second-order conditioning phase).
| Adolescents | ||||||
|---|---|---|---|---|---|---|
| Wall-Climbing (sec) | Locomotion (sec) | Head Shaking (freq) | ||||
| 0.5 g/kg | 2.0 g/kg | 0.5 g/kg | 2.0 g/kg | 0.5 g/kg | 2.0 g/kg | |
| Unpaired | 37.94 +/- 3.57 |
27.30 +/- 3.29 |
10.97 +/- 1.40 |
9.76 +/- 0.79 |
2.66 +/- 0.58 |
1.66 +/- 0.47 |
| Paired Early |
29.72 +/- 5.80 |
44.65 +/- 7.84 (*) |
11.41 +/- 1.63 |
12.06 +/- 1.24 |
1.88 +/- 0. 71 |
2.5 +/- 0.89 |
| Paired Late |
34.85 +/- 8.31 |
47.01 +/- 6.11 (*) |
10.65 +/- 1.86 |
12.64 +/- 1.91 |
2.30 +/- 0.97 |
3.33 +/- 0.78 |
| Adults | ||||||
| Unpaired | 45.85 +/- 8.50 |
38.75 +/- 5.87 |
9.22 +/- 1.14 |
11.00 +/- 1.10 |
0.80 +/- 0.39 |
2.66 +/- 1.09 |
| Paired Early |
48.43 +/- 6.12 |
41.25 +/- 5.46 |
11.53 +/- 1.14 |
10.09 +/- 1.22 |
2.40 +/- 0. 84 |
1.9 +/- 0.31 |
| Paired Late |
51.76 +/- 11.47 |
46.76 +/- 6.47 |
13.84 +/- 2.29 |
12.72 +/- 2.09 |
0.78 +/- 0.33 |
1.60 +/- 0.54 |
Values represent mean +/- SEMs.
Asterisks indicate significant differences from the respective Unpaired group (p < 0.05).
Adult subjects (P71)
The 2 × 3 ANOVAs for the behavioral data from adult animals during the second order conditioning phase revealed no significant main effects or significant interactions. Thus, locomotion, wall-climbing and head-shaking of adults during the CS1-CS2 transfer phase revealed no effects of ethanol dosage or nature of the conditioning procedure during phase 1. Table 1 depicts mean and SEM for each of these variables.
Pearson product-moment correlations between CS2 preference and behavioral responsiveness at phase 2
Adolescent subjects (P33)
there was a modest but significant positive correlation between sandpaper preference scores at test (%) and frequency of wall-climbing during the second-order conditioning phase (r = 0.28, p = 0.015). Wall-climbing also correlated significantly with head-shaking and locomotion scores (r’s = 0.48 and 0.40, respectively; both ps < 0.001). Overall correlation scores can be examined in Table 2. An individual analysis for each treatment group revealed that the association between sandpaper preference and wall-climbing was largely driven by those animals assigned to the late pairing condition. Specifically, the correlation achieved significance in the latter group (r = 0 .48, p < 0.05) but not in the UP or EP groups (r’s = 0.12 and 0.15, respectively, both ps > .30). Within the PL condition, ethanol dosage seemed to affect the magnitude of the association, with animals given 0.5 g/kg achieving an even greater correlation (r = 0.67, p < 0.05).
Table 2.
Overall pearson product-moment correlations between sandpaper preference scores and behavioral responsiveness during the second-order conditioning phase.
| Experiment 1 | ||||
|---|---|---|---|---|
| Sandpaper preference (%) |
Locomotion (s) |
Wall-Climbing (s) |
Head Shaking (freq) |
|
| Sandpaper preference (%) |
-------------------- | |||
| Locomotion (s) |
0.20 | -------------------- | ||
| Wall-Climbing (s) |
0.28 | 0.48 | -------------------- | |
| Head Shaking (freq) |
0.20 | 0.42 | 0.40 | -------------------- |
| Experiment 2 | ||||
| Sandpaper preference (%) |
Locomotion (s) |
Wall-Climbing (s) |
Head Shaking (freq) |
|
| Sandpaper preference (%) |
-------------------- | |||
| Locomotion (s) |
0.02 | -------------------- | ||
| Wall-Climbing (s) |
-0.34 | 0.04 | -------------------- | |
| Head Shaking (freq) |
-0.09 | 0.03 | 0.25 | -------------------- |
Correlations significant at p < 0.05 are marked in bold. Mirrored correlations coefficients were deleted.
Adult subjects (P71)
When data across all adult groups were examined, a significant negative correlation was found between sandpaper preference and wall-climbing scores (r = - 0.34, p < 0.01, see Table 2). A negative and significant correlation comprising these variables was also observed in the PL condition (r = - 0.54, p < 0.05). No significant correlations were detected when focusing on the remaining conditioning groups. Ethanol dosage did not significantly affect the magnitude or direction of the correlations.
Blood and brain ethanol concentrations
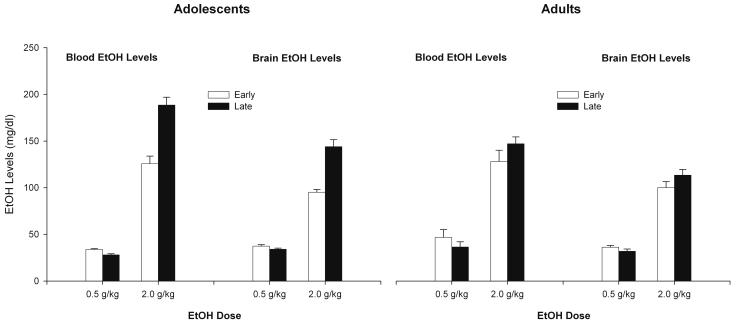
Significant main effects of dose and postadministration time (PAT) [F (1,58) = 456.91; F (1,58) = 9.86; respectively, both ps < 0.002], and their interaction, [F (1,58) = 22.03; p < 0.001] emerged in the analysis of BELs. Post-hoc analyses revealed that ethanol levels derived from the 2.0 g/kg dose were significantly higher than those obtained with 0.5 g/kg ethanol, particularly at the later PAT. BELs were similar across age at the lower ethanol dose and at the early PAT associated with the 2.0 g/kg dose. However, BELs induced by 2.0 g/kg were higher in adolescents than in adults at 32.5 min postadministration (p < 0.0001). This ANOVA also yielded a significant dose x age interaction as well as a significant PAT x age interaction [F (1,58) = 8.41; F (1,58) = 5.40; respectively, both ps < 0.05], with the three-way interaction between dose, age and PAT approaching significance, F (1, 58) = 3.56, p = 0.06.
Similar results were found when analyzing BrELs. The ANOVA indicated significant main effects of dose and PAT [F (1, 58) = 5599.35, F (1, 58) = 15.50; both ps < 0.001], along with significant interactions of dose x PAT [F (1, 58) = 29.11, p < 0.001], PAT X age [F (1, 58) = 6.67, p < 0.05], and dose x PAT x age [F (1, 58) = 7.64, p < 0.01]. Post-hoc tests indicated that animals administered 2.0 g/kg showed greater BrELs than those receiving 0.5 g/kg, particularly during the second time interval (all ps < 0.0001). Also, brain ethanol levels induced by the 2.0 g/kg dose were higher in adolescents than in adults during the second time interval, as indicated by the pertinent post-hoc test (p < 0.0001). The descriptive profile of BECs and BrELs is shown in Fig. 4.
Figure 4.
Blood and brain ethanol levels (mg %) in adolescent and adult rats (P32 and P 70, respectively) given 0.5 or 2.0 g/kg ethanol (intragastric). Blood and brain samples were collected either at 7.5 or 32.5 min postadministration (early and late postadministration time, PAT; respectively). A detailed account of the statistical findings and signficant differences across groups can be found in the Results section. Vertical bars indicate the standard error of the mean.
Discussion
Adolescent and adult rats appear to exhibit differential sensitivity to the motivational effects of ethanol when assessed by means of a second order conditioning (SOC) procedure. In adolescents, the association between an intraoral CS1 and ethanol’s postabsorptive consequences successfully endowed the CS1 with reinforcing properties. Specifically, CS1 later acted as an appetitive second-order reinforcer, mediating the expression of conditioned place preferences. Adult animals, in contrast, did not show significant changes in tactile preferences as a function of the preceding associations between ethanol (0.5 or 2.0 g/kg, yielding BELs between 47 and 150 mg%; Fig. 4) and the CS1. Adolescent and adult subjects had similar ethanol levels in blood and brain across doses and postadministration time, except in the case of the higher ethanol dose at the later sampling interval, where BELs and BrELs were found to be lower in adult rats. The effects obtained at each age were neither dose-dependent nor did they vary significantly as a function of postadministration time.
Age-dependent effects of ethanol have been observed frequently. Adolescent subjects show less sensitivity than adult animals to the hypnotic and sedative effects of ethanol but are sometimes more affected than adult conspecifics by the cognitive-impairing effects of the drug (Spear & Varlinskaya, 2005). In terms of ethanol’s motivational effects, Philpot et al. (2003) reported ethanol-induced place preference in 45-day old but not in 35-day old rats given 0.5 or 1.0 g/kg. In fact, the latter dose induced tactile aversion in the P35 adolescent animals. Ethanol (0.2 – 2.0 g/kg) failed to affect preference scores in older animals (P60). Philpot et al. (2003) reported conditioned aversion at P35, whereas, at similar age and dose, conditioned preference was found in the present study. This difference might be explained by procedural differences. Whereas Philpot et al. (2003) employed traditional first order conditioned procedures, the present study used a second-order conditioning paradigm. This brief and relatively simple preparation seems to be highly sensitive for detecting appetitive motivational effects of ethanol in developing rats (Molina et al., 2006, 2007).
Motivational properties of ethanol have been long considered an important factor in the modulation of ethanol acceptance patterns (Cunningham et al., 2000). If adolescent humans perceive the drug as more rewarding than adults do, they could be at enhanced risk for engaging in ethanol seeking and consumption and for progressing from drug use to abuse and dependence. Accordingly, Deas et al. (2000) found that appearance of the first symptoms of alcohol dependence in human adolescents requires only 7 months of regular drinking, whereas adults needed a much longer period (approximately, 3 years).
Adolescents expressed ethanol-induced conditioning not only in terms of CS2 preference but also via conditioned behavioral activation, expressed in response to CS1 during the second-order conditioning phase. After pairings of 2.0 g/kg ethanol and CS1, adolescents exhibited more wall-climbing than unpaired controls when briefly re-exposed to CS1. Conditioned wall-climbing is often observed in response to tastants previously paired with the aversive postingestive effects of emetic agents (Pautassi et al., 2008) or drugs of abuse, including ethanol (Arias & Chotro, 2006). Hence, the increased wall-climbing observed in paired animals might reflect aversive effects of ethanol. However, this finding seems to be better explained in terms of an ethanol-mediated conditioned motor response, as reported in 14-day olds by Molina et al. (2006), with conditioning procedures similar to those of the present study. Specifically, (Molina et al. (2006; 2007) also found greater wall-climbing during the second-order conditioning phase in paired animals than in unpaired controls, believed to represent an ethanol-mediated conditioned motor response that had become conditioned to CS1. Cunningham & Noble (1992) provided support for this hypothesis. These authors found that the progressive increase in general activity observed when animals are given daily exposures to ethanol (a phenomenon also known as behavioral sensitization, Kawakami et al., 2007) is mediated to a large extent by Pavlovian learning. The present study adds new evidence suggesting that, in adolescent rats, ethanol is capable of inducing first-order conditioned motor activation. Yet in terms of another measure of activation — general locomotion — similar conditioning was not apparent. Conditioned increases in locomotion can be considered a measure of learning when assessing motivational conditioning (Arias & Chotro, 2006; Brining et al., 1991). Nevertheless, overall locomotion does not seem to be as sensitive (Arias & Chotro, 2005; 2006) or as specific as wall climbing (Hoffman et al., 1991).
Expression of appetitive, ethanol-mediated second order conditioning and primary conditioned activation during the transfer phase seem to be related phenomena. Adults showed neither behavioral activation during the second order phase nor second-order ethanol-mediated tactile preferences. On the other hand, when conditioned place preference was evident in adolescents, behavioral activation (i.e., wall-climbing) emerged during CS1-CS2 pairings. Furthermore, adolescents exhibited a significant positive correlation between preference for CS2 (sandpaper) and wall climbing scores, particularly in the experimental condition (LP Group) that yielded ethanol-mediated place preference. Wall-climbing seems to decline as a function of age (Scalzo & Burgue, 1992). This raises the possibility that adults failed to exhibit ethanol-related changes in wall-climbing just because the latter is not a pertinent dependent variable for mature subjects. However, an association between wall climbing scores during the transfer and tactile preference scores was also found in adult subjects and, as in adolescents, it was exhibited by paired but not control subjects, indicating sensitivity of the wall-climbing measures for adults. The direction of these associations in adults was, however, opposite to that found in adolescents. For adults a negative relationship was observed: the greater the frequency of wall-climbing, the less their preference for CS2. Further research is needed to test the reliability of these apparent associations as well as to scrutinize neural and behavioral mechanisms underlying the expression of ethanol-mediated conditioned preferences and behavioral activation.
An alternative explanation for the age-related difference in locational preference observed in this study is that conditioning of one-trial ethanol-mediated SOC may simply have been more effective in adults than in adolescents The absence of conditioned motor responses in paired adult subjects might be taken as evidence of a lack of first-order conditioning at this particular age. Furthermore, degree of training was not explicitly varied in these studies. Yet, previous literature indicates that adult rats acquire and express second-order conditioning (e.g., Rescorla, 1980) and are also capable of detecting differences in the present tactile stimuli (i.e., sandpaper and smooth surfaces; Hughes, 2007). Adult rodents are also likely to exhibit better learning performance than adolescents in tasks in which hyperactivity is likely to detract from performance of the conditioning (Spear & Brake, 1983). The conditioned place preference task employed in the present work is probably one of those tasks. Adult subjects also showed an association between behavioral activation during the transfer phase and sandpaper scores. These facts suggest that adults are capable of encoding and acquiring information about the state of intoxication and its contingency with the CS, although we cannot discount the possibility that adolescents are more effective or show qualitative differences in this learning. Indeed, when ethanol-mediated conditioned place preference has been found in adult rats, extensive training procedures were employed (Bozarth, 1990; Bienkowsky et al., 1995), whereas one-trial, ethanol-mediated second-order conditioning seems to be a reliable phenomenon earlier in the ontogeny of the rat (Molina et al., 2006; 2007). It could also be postulated that late paired animals might have been trained via a backward rather than simultaneous or delay conditioning (i.e., with the CS following the US). Thus, the appearance of age differences might relate to differences in backward conditioning.
The age-related differences obtained in the late paired condition could also be influenced by the fact that, after 2.0 g/kg, adults exhibited lower blood and brain ethanol levels at 32.5 min post-intubation than did adolescents. Nevertheless, BECs/BrECs in paired adults given 2.0 g/kg were still within the range that exerted appetitive effects in the younger animals. Finally, it could also be that adults show specific sensitivity to the motivational properties of ethanol in the SOC preparation at a dosage not tested in the present work.
In the present study, we observed age-related differences in terms of baseline preference for the tactile cue employed as CS2. Specifically, UP adolescents exhibited less preference for the sandpaper-lined floor than their adult counterparts. This differential preference may reflect inherent age-related differences in predilection for rough textures. Stimuli of the same modality are organized on “natural preference scales” (Rakovee-Ratar & Weeler, 1997) and such preferences are known to change across ontogeny. At any rate, it should be noted that, in the present work, the claim for ethanol-mediated conditioned responses at each age is made by contrasting animals exposed to ethanol-CS pairings (Paired groups) against groups in which the CS had relatively little contingency with ethanol’s postabsorptive effects (i.e., Unpaired animals). In other words, differential sandpaper baseline preference across age should not obscure the fact that a single pairing between the latter cue and an ethanol-related flavor significantly affected tactile preferences in adolescent but not in adult subjects.
In summary, under the present experimental circumstances, adolescents, but not adults, acquired ethanol-mediated conditioning. These age-related differences may imply different degrees of vulnerability to ethanol abuse and dependence across age. Adolescence is characterized by behavioral traits such as novelty seeking, impulsivity, increased peer interaction and risk taking (Spear, 2000). It has been proposed that these traits represent “biological markers” for drug abuse or dependence (Kleaubur & Bardo, 1999; Zuckerman, 1994). However, the ontogenetic analysis of ethanol-related learning in phenotypes characterized by varying degrees of overlap between these traits has been scarce. In the rat, this void in the literature is likely to be related to a lack of behavioral techniques sensitive to ethanol’s motivational properties and amenable to being employed with minimal procedural changes across development. The second-order conditioning technique seems to provide a relatively simple and brief screening test to analyze sensitivity for motivational properties of ethanol or of other drugs across ontogeny. Further studies are needed to conclusively determine if the adolescent stage represents a sensitive period in terms of vulnerability to the associative effects of drugs of abuse.
Acknowledgements
This work was supported by grants from NIAAA (AA11960, AA013098, AA015992) and NIMH (MH035219) to NES, NIAAA grants AA12525 and AA16887 to LPS, and the Agencia Nacional de Promocion Cientifica y Tecnologica (PICT 05-14024) to JCM. The authors wish to express their gratitude to Teri Tanenhaus, Carlos Martínez, Judy Sharp and Carrie Wilmouth for their technical assistance.
References
- Adriani W, Chiarotti F, Laviola G. Elevated novelty seeking and peculiar D-amphetamine sensitization in periadolescent mice compared with adult mice. Behav Neurosci. 1998;112:1152–1166. doi: 10.1037//0735-7044.112.5.1152. [DOI] [PubMed] [Google Scholar]
- Adriani W, Laviola G. A unique hormonal and behavioral hyporesponsivity to both forced novelty and d-amphetamine in periadolescent mice. Neuropharmacology. 2000;39:334–346. doi: 10.1016/s0028-3908(99)00115-x. [DOI] [PubMed] [Google Scholar]
- Bienkowski P, Kuka P, Kowstowski W. Conditioned place preference after prolonged preexposure to ethanol. Pol J Pharmacol. 1995;47:185–187. [Google Scholar]
- Bozarth M. Evidence for the rewarding effects of ethanol using the Conditioned Place Preference method. Pharmachol Biochem Behav. 1990;35:485–487. doi: 10.1016/0091-3057(90)90191-j. [DOI] [PubMed] [Google Scholar]
- Cheslock S, Varlinskaya E, Petrov ES, Silveri MM, Spear LP, Spear NE. Ethanol as a reinforcer in the newborn’s first suckling experience. Alcohol Clin Exp Res. 2001;25:395–402. [PubMed] [Google Scholar]
- Cunningham CL. Drug Conditioning and seeking behavior. In: O’Donohue W, editor. Learning And Behavior Therapy. University of Nevada; Allyn and Bacon: 1998. pp. 518–540. [Google Scholar]
- Cunningham CL, Clemans JM, Fidler TL. Injection timing determines whether intragastric ethanol produces conditioned place preference or aversion in mice. Pharmacol Biochem Behav. 2002;72:659–68. doi: 10.1016/s0091-3057(02)00734-7. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Niehus JS, Noble D. Species difference in sensitivity to ethanol’s hedonic effects. Alcohol. 1993;10:97–102. doi: 10.1016/0741-8329(93)90087-5. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Patel P, Milner L. Spatial location is critical for conditioning place preference with visual but not tactile stimuli. Behav Neurosci. 2006;120:1115–1132. doi: 10.1037/0735-7044.120.5.1115. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Noble DC. Conditioned Activation induced by ethanol: Role in sensitization and Conditioned Place Preference. Pharmacol Biochem Behav. 1992;43:307–313. doi: 10.1016/0091-3057(92)90673-4. [DOI] [PubMed] [Google Scholar]
- Deas D, Riggs P, Langenbucher J, Goldman M, Brown S. Adolescents are not adults: developmental considerations in alcohol users. Alcohol Clin Exp Res. 2000;24:232–237. [PubMed] [Google Scholar]
- Ettenberg A. Opponent process properties of self-administered cocaine. Neurosci Biobehav Rev. 2004;27:721–728. doi: 10.1016/j.neubiorev.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Fernández-Vidal JM, Molina JC, Spear NE. Adolescent rats dicriminate a mild state of ethanol intoxication likely to act as an appetitive unconditional stimulus. Alcohol. 2003;30:45–60. doi: 10.1016/s0741-8329(03)00093-4. [DOI] [PubMed] [Google Scholar]
- Faden VB. Trends in Initiation of Alcohol Use in the United States 1975 to 2003. Alcohol Clin Exp Res. 2006;30:1011–1022. doi: 10.1111/j.1530-0277.2006.00115.x. [DOI] [PubMed] [Google Scholar]
- Grant B, Dawson DA. Age of alcohol onset and its association with DSM-IV alcohol abuse and dependence: Results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Hawkins JD, Graham JW, Maguin E, Abbott R, Hill KG, Catalano RF. Exploring the Effects of Age of Alcohol Use Initiation and Psychosocial Risk Factors on Subsequent Alcohol Misuse. J Stud Alcohol. 1997;58:280–290. doi: 10.15288/jsa.1997.58.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RN. Rats’ responsiveness to tactile changes encountered in the dark, and the role of mystacial vibrissae. Behav Brain Res. 2007;179:273–280. doi: 10.1016/j.bbr.2007.02.023. [DOI] [PubMed] [Google Scholar]
- Infurna RN, Spear LP. Developmental changes in amphetamine-induced taste aversions. Pharmacol Biochem Behav. 1979;11:31–35. doi: 10.1016/0091-3057(79)90293-4. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources, Commission on Life Sciences . Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, DC: 1996. National Research Council. [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE.Monitoring the Future National Survey Results on Drug Use, 1975-2006: Vol 1, Secondary School Students 2007. (NIH Pub. No. 07-6205). National Institute of Drug Abuse; Bethedesa, MD [Google Scholar]
- Kashinsky WM, Rozboril LW, Robinson SR, Smotherman WP. An inexpensive rotatory infusion pump for delivery microliter volumes of fluid to animal subjects. Physiol Behav. 1990;47:1279–1281. doi: 10.1016/0031-9384(90)90383-f. [DOI] [PubMed] [Google Scholar]
- Kawakami SE, Quadros IM, Takahashi S, Suchecki D. Long maternal separation accelerates behavioural sensitization to ethanol in female, but not in male mice. Behav Brain Res. 2007;184:109–116. doi: 10.1016/j.bbr.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Kiefer SW. Alcohol, palatability, and taste reactivity. Neurosci Biobehav Rev. 1995;19:133–141. doi: 10.1016/0149-7634(94)00027-x. [DOI] [PubMed] [Google Scholar]
- Kiefer SW, Hill KG, Coonfield DL, Ferraro FM., 3rd Ethanol familiarity and naltrexone treatment affect ethanol responses in rats. Alcohol. 2005;37:167–72. doi: 10.1016/j.alcohol.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Kleabaur JE, Bardo MT. Individual Differences in Novelty Seeking on the Playground Maze Predict Amphetamine Conditioned Place Preference. Pharmacol Biochem Behav. 1999;63:131–136. doi: 10.1016/s0091-3057(98)00258-5. [DOI] [PubMed] [Google Scholar]
- Kraebel KS, Spear NE. Infant rats are more likely than adolescents to orient differentially to amodal (intensity-based) features of single-element and compound stimuli. Dev Psychobiol. 2000;36:49–66. [PubMed] [Google Scholar]
- Land C, Spear NE. Ethanol impairs memory of a simple discrimination in adolescent rats at doses that leave adult memory unaffected. Neurobiol Learn Mem. 2004a;81:75–81. doi: 10.1016/j.nlm.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Land C, Spear NE. Fear conditioning is impaired in adult rats by ethanol doses that do not affect periadolescents. Int J Dev Neurosci. 2004b;22:355–362. doi: 10.1016/j.ijdevneu.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Lett BT. Enhancement of conditioned preference for a place paired with amphetamine produced by blocking the association between place and amphetamine-induced sickness. Psychopharmacol. 1988;95:390–394. doi: 10.1007/BF00181954. [DOI] [PubMed] [Google Scholar]
- Marglin SH, Mkinzie DK, Mattie M, Hui Y, Reid LD. Ethanol with small doses of morphine establishes a conditioned place preference. Alcohol. 1988;5:309–313. doi: 10.1016/0741-8329(88)90071-7. [DOI] [PubMed] [Google Scholar]
- Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res. 1998;22:16–21. [PubMed] [Google Scholar]
- Miller JS, Molina JC, Spear NE. Ontogenetic differences in the expression of odor-aversion learning in 4- and 8-day-old rats. Dev Psychobiol. 1990;23:319–330. doi: 10.1002/dev.420230404. [DOI] [PubMed] [Google Scholar]
- Molina JC, Pautassi RM, Truxell E, Spear NE. Differential motivational properties of ethanol during early ontogeny as a function of dose and postadministration time. Alcohol. 2007;41:41–45. doi: 10.1016/j.alcohol.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina JC, Ponce LF, Truxell E, Spear NE. Infantile sensitivity to ethanol’s motivational effects: Ethanol reinforcement during the third postnatal week. Alcohol Clin Exp Res. 2006;30:1506–1519. doi: 10.1111/j.1530-0277.2006.00182.x. [DOI] [PubMed] [Google Scholar]
- Molina JC, Hoffmann H, Serwatka J, Spear NE. Establishing intermodal equivalence in preweanling and adult rats. J Exp Psychol Anim Behav Process. 1991;17:433–47. doi: 10.1037//0097-7403.17.4.433. [DOI] [PubMed] [Google Scholar]
- Pautassi RM, Arias C, Molina JC, Spear NE. Domperidone interferes with conditioned disgust reactions but not taste avoidance evoked by a LiCl-paired taste in infant rats. Dev Psychobiol. 2008;50:343–52. doi: 10.1002/dev.20288. [DOI] [PubMed] [Google Scholar]
- Pautassi RM, Godoy JC, Spear NE, Molina JC. Early responsiveness to stimuli paired with different stages within the state of alcohol intoxication. Alcohol Clin Exp Res. 2002;26:644–654. [PubMed] [Google Scholar]
- Pautassi RM, Melloni C, Ponce LF, Molina JC. Acute ethanol counteracts the acquisition of aversive olfactory learning in infant rats. Alcohol. 2005;36:99–105. doi: 10.1016/j.alcohol.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Pautassi RM, Molina JC, Spear NE. Infant Rats Exhibit Aversive Learning Mediated By Ethanol’s Orosensory Effects But Are Positively Reinforced By Ethanol’s Postingestive Effects. Pharmacol Biochem Behav. 2008;88:393–402. doi: 10.1016/j.pbb.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Sanders S, Miller S, Spear NE, Molina JC. Early Ethanol’s Anxiolytic Effects Assessed Through an Unconditional Stimulus Revaluation Procedure. Alcohol Clin Exp Res. 2006;30:448–459. doi: 10.1111/j.1530-0277.2006.00049.x. [DOI] [PubMed] [Google Scholar]
- Panksepp J. The ontogeny of play in rats. Developmental Psychobiology. 1981;14:327–332. doi: 10.1002/dev.420140405. [DOI] [PubMed] [Google Scholar]
- Pedersen W, Skrondal A. Alcohol consumption debut: predictors and consequences. J Stud Alcohol. 1998;59:32–42. doi: 10.15288/jsa.1998.59.32. [DOI] [PubMed] [Google Scholar]
- Philpot RM, Badanich KA, Kirstein CL. Place conditioning: Age-related changes in the rewarding and aversive effects of alcohol. Alcohol Clin Exp Res. 2003;27:593–599. doi: 10.1097/01.ALC.0000060530.71596.D1. [DOI] [PubMed] [Google Scholar]
- Rakover-Atar S, Weller A. The influence of natural preference for tactile stimuli on appetitive learning in rat pups. Dev Psychobiol. 1997;30:29–39. doi: 10.1002/(sici)1098-2302(199701)30:1<29::aid-dev3>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Second-Order Conditioning. Erlbaum; Hillsdale, NJ: 1980. [Google Scholar]
- Risinger FO, Cunningham CL. Kalivas P, Samson H, editors. Ethanol produces rapid biphasic hedonic effects. in The Neurobiology of drug and alcohol addiction. Ann N Y Acad Sci. 1992;654:506–508. doi: 10.1111/j.1749-6632.1992.tb26014.x. [DOI] [PubMed] [Google Scholar]
- Roth TL, Moriceau S, Sullivan RM. Opioid modulation of Fos protein expression and olfactory circuitry plays a pivotal role in what neonates remember. Learn Mem. 2006;13:590–598. doi: 10.1101/lm.301206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalzo FM, Burge LJ. The ontogeny of phencyclidine-induced wall climbing and locomotor activity. Dev Psychobiol. 1992;25:597–612. doi: 10.1002/dev.420250806. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Morris R, Kuhn CM. Adolescents rats are protected from the conditioned aversive properties of cocaine and lithium chloride. Pharmacol Biochem Behav. 2006;84:344–352. doi: 10.1016/j.pbb.2006.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. The effects of NMDA and GABAA pharmacological manipulations on acute and rapid tolerance to ethanol during ontogeny. Alcohol Clin Exp Res. 1998;28:884–894. doi: 10.1097/01.alc.0000128221.68382.ba. [DOI] [PubMed] [Google Scholar]
- Smith RF. Animal Models of periadolescent substance. Neurotoxicol Teratol. 2003;25:291–301. doi: 10.1016/s0892-0362(02)00349-5. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Ontogeny of ethanol elimination and ethanol-induced hypothermia. Alcohol. 2000;20:45–53. doi: 10.1016/s0741-8329(99)00055-5. [DOI] [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Adolescence: Alcohol sensitivity, tolerance, and intake. In: Galanter M, editor. Recent Developments in Alcoholism, Volume 17: Alcohol Problems in Adolescents and Young Adults. Kluwer Academic Publishers; Higham, MA: 2005. pp. 143–159. [PubMed] [Google Scholar]
- Spear NE, Molina JC. The role of sensory modality in the ontogeny of stimulus selection. In: Krasgenor N, Blass ME, Hoffer MA, Smotherman WP, editors. Perinatal Development: A Psychobiological Perspective. Academic Press; Orlando, FL: 1987. pp. 83–110. [Google Scholar]
- Spear NE, Molina JC. Fetal or infantile exposure to ethanol promotes ethanol ingestion in adolescence and adulthood: A theoretical review. Alcohol Clin Exp Res. 2005;29:909–929. doi: 10.1097/01.alc.0000171046.78556.66. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Van Dijk G, Yagaloff KA, Fisher SL, Schwartz M, Burn P, Seeley RJ. Central infusion of melanocortin agonist MTII in rats: assessment of c-Fos expression and taste aversion. Am J Physiol. 1998;274:248–254. doi: 10.1152/ajpregu.1998.274.1.R248. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Falkowitz S, Spear LP. Adolescent-associated insensitivity to ethanol-induced taste aversions; Paper presented at the 36th Meeting of the Society for Neuroscience; 2006; Atlanta, GA: [Google Scholar]
- Witt ED. Mechanisms of Alcohol Abuse and Alcoholism in Adolescents: A Case for Developing Animal Models. Behav Neural Biol. 1994;62:168–177. doi: 10.1016/s0163-1047(05)80015-9. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. Behavioral expressions and biosocial bases of sensation seeking. Cambridge University Press; 1994. [Google Scholar]