Abstract
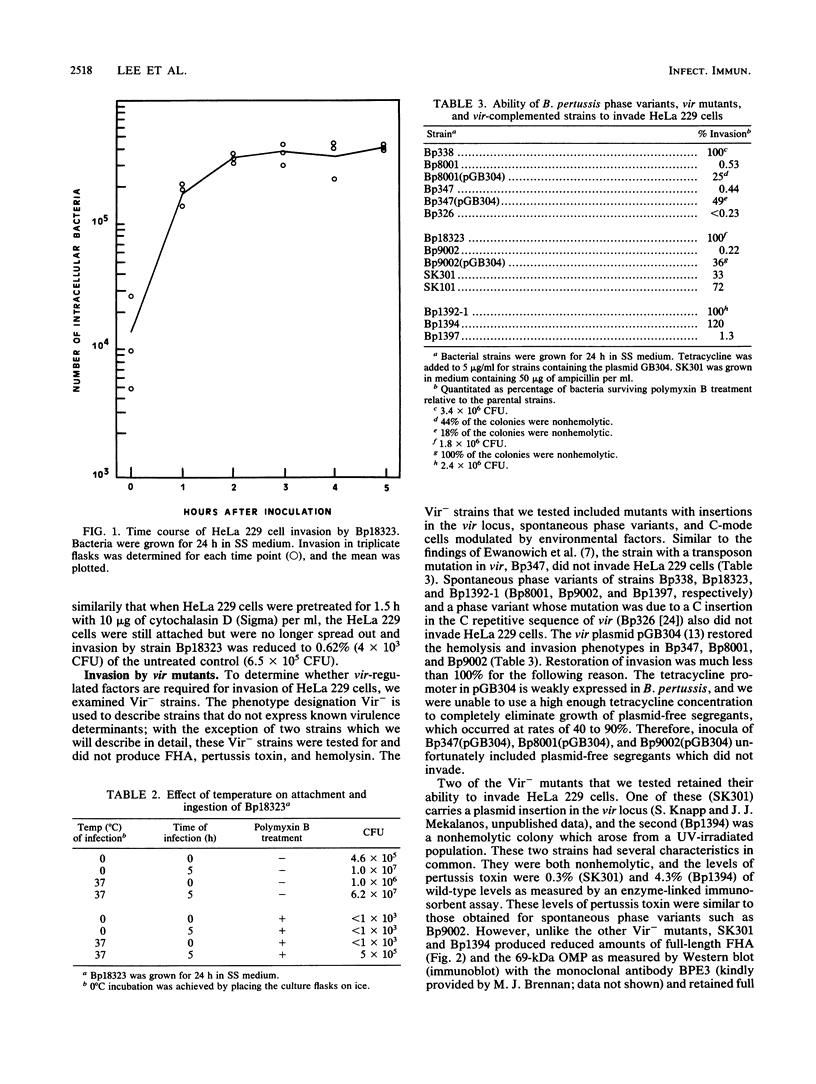
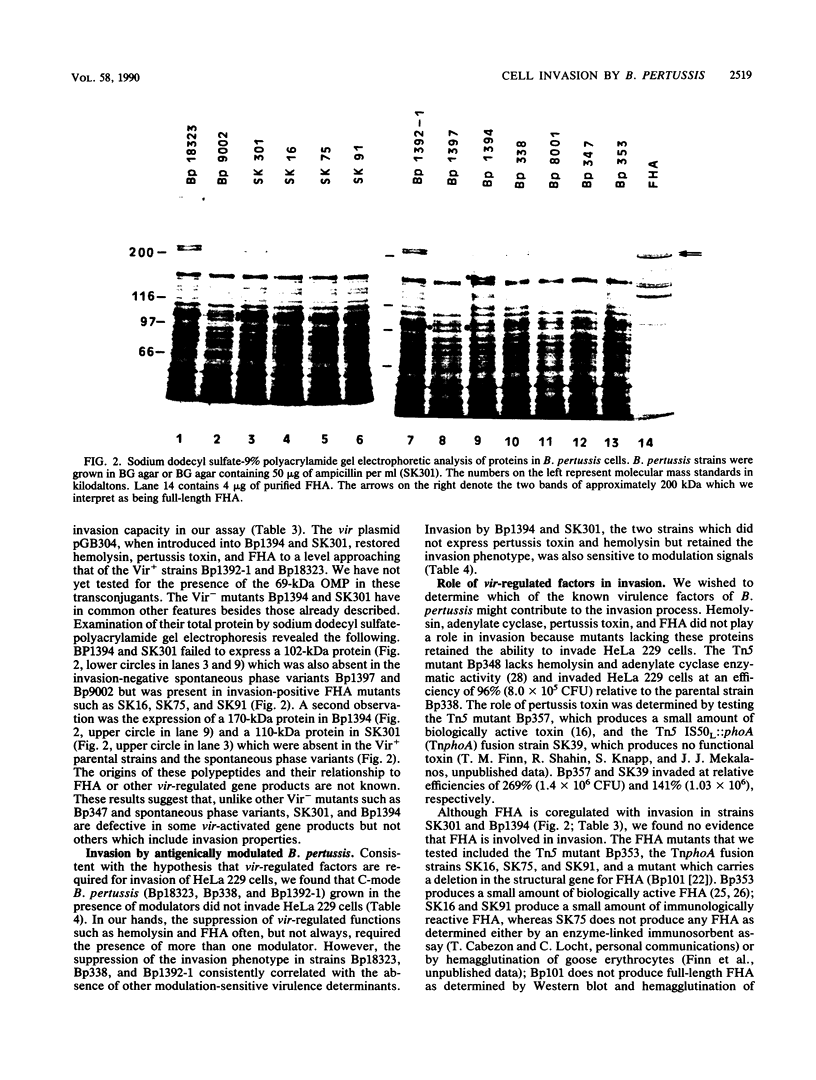
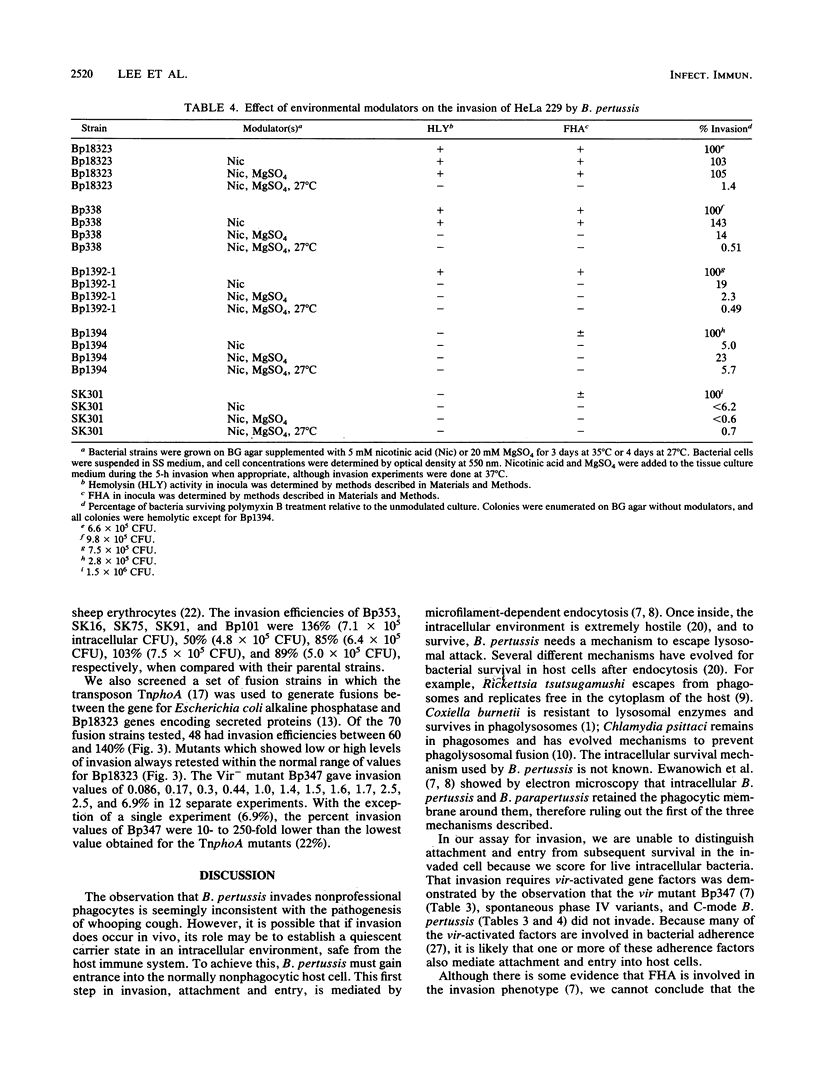
Invasion and intracellular survival of Bordetella pertussis in HeLa 229 cells was studied by a new assay that utilizes polymyxin B instead of gentamicin to rapidly kill extracellular organisms. Invasion measured by this assay was time and temperature dependent and was inhibited by the microfilament drug cytochalasin D. The invasion process was also dependent on a functional vir locus (also known as bvg), the positive regulator of virulence gene expression in B. pertussis. Four spontaneous Vir- phase variants of B. pertussis and a mutant with a transposon insertion mutation in the vir locus did not invade. Cells that were environmentally modulated and thus did not express virulence determinants also did not invade. Two Vir- mutants, a vir-directed plasmid insertion mutant and a UV-light-induced mutant, were capable of invasion, although they did not produce other known virulence factors such as pertussis toxin and hemolysin but did produce small amounts of filamentous hemagglutinin (FHA) and the 69-kilodalton outer membrane protein. None of 70 Tn5 IS50L::phoA (TnphoA) insertion mutants of strain Bp18323 (including three mutants defective in FHA) tested showed any reproducible defect in invasion. A mutant carrying a site-directed deletion mutation in FHA was also capable of invasion in our assay. These data suggest that there is redundancy in the invasion functions of B. pertussis and that one or more of these are coordinately regulated with FHA and the 69-kilodalton outer membrane protein more tightly than with other vir-activated gene products.
Full text
PDF

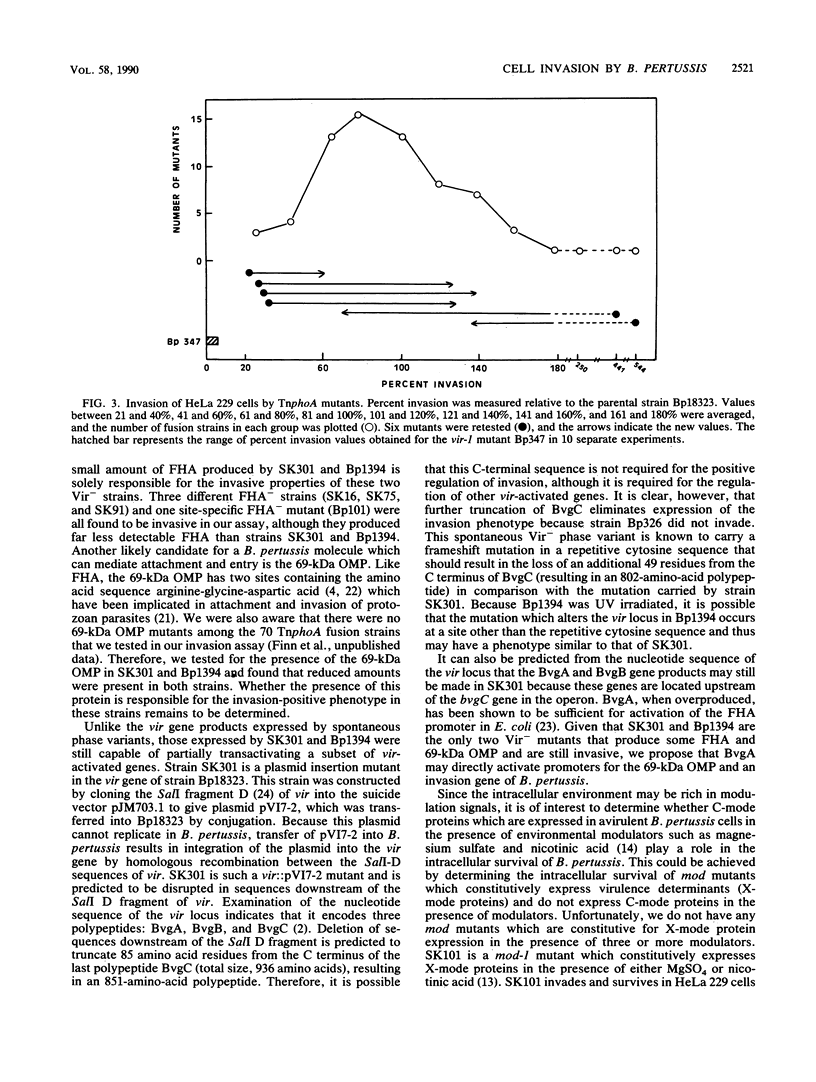

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akporiaye E. T., Rowatt J. D., Aragon A. A., Baca O. G. Lysosomal response of a murine macrophage-like cell line persistently infected with Coxiella burnetii. Infect Immun. 1983 Jun;40(3):1155–1162. doi: 10.1128/iai.40.3.1155-1162.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aricó B., Miller J. F., Roy C., Stibitz S., Monack D., Falkow S., Gross R., Rappuoli R. Sequences required for expression of Bordetella pertussis virulence factors share homology with prokaryotic signal transduction proteins. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6671–6675. doi: 10.1073/pnas.86.17.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan M. J., Li Z. M., Cowell J. L., Bisher M. E., Steven A. C., Novotny P., Manclark C. R. Identification of a 69-kilodalton nonfimbrial protein as an agglutinogen of Bordetella pertussis. Infect Immun. 1988 Dec;56(12):3189–3195. doi: 10.1128/iai.56.12.3189-3195.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles I. G., Dougan G., Pickard D., Chatfield S., Smith M., Novotny P., Morrissey P., Fairweather N. F. Molecular cloning and characterization of protective outer membrane protein P.69 from Bordetella pertussis. Proc Natl Acad Sci U S A. 1989 May;86(10):3554–3558. doi: 10.1073/pnas.86.10.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheers C., Gray D. F. Macrophage behaviour during the complaisant phase of murine pertussis. Immunology. 1969 Dec;17(6):875–887. [PMC free article] [PubMed] [Google Scholar]
- Coleman K. D., Wetterlow L. H. Use of implantable intraperitoneal diffusion chambers to study Bordetella pertussis pathogenesis: growth and toxin production in vivo. J Infect Dis. 1986 Jul;154(1):33–39. doi: 10.1093/infdis/154.1.33. [DOI] [PubMed] [Google Scholar]
- Ewanowich C. A., Melton A. R., Weiss A. A., Sherburne R. K., Peppler M. S. Invasion of HeLa 229 cells by virulent Bordetella pertussis. Infect Immun. 1989 Sep;57(9):2698–2704. doi: 10.1128/iai.57.9.2698-2704.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewanowich C. A., Sherburne R. K., Man S. F., Peppler M. S. Bordetella parapertussis invasion of HeLa 229 cells and human respiratory epithelial cells in primary culture. Infect Immun. 1989 Apr;57(4):1240–1247. doi: 10.1128/iai.57.4.1240-1247.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing E. P., Jr, Takeuchi A., Shirai A., Osterman J. V. Experimental infection of mouse peritoneal mesothelium with scrub typhus rickettsiae: an ultrastructural study. Infect Immun. 1978 Mar;19(3):1068–1075. doi: 10.1128/iai.19.3.1068-1075.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi A., Suzuki Y., Ono S., Sato H., Sato Y. Effect of heptakis (2,6-O-dimethyl) beta-cyclodextrin on the production of pertussis toxin by Bordetella pertussis. Infect Immun. 1983 Sep;41(3):1138–1143. doi: 10.1128/iai.41.3.1138-1143.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R. R. Determinants for thermoinducible cell binding and plasmid-encoded cellular penetration detected in the absence of the Yersinia pseudotuberculosis invasin protein. Infect Immun. 1989 Jul;57(7):1998–2005. doi: 10.1128/iai.57.7.1998-2005.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp S., Mekalanos J. J. Two trans-acting regulatory genes (vir and mod) control antigenic modulation in Bordetella pertussis. J Bacteriol. 1988 Nov;170(11):5059–5066. doi: 10.1128/jb.170.11.5059-5066.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACEY B. W. Antigenic modulation of Bordetella pertussis. J Hyg (Lond) 1960 Mar;58:57–93. doi: 10.1017/s0022172400038134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee C. K., Roberts A., Perrin S. Expression of pertussis toxin in Bordetella bronchiseptica and Bordetella parapertussis carrying recombinant plasmids. Infect Immun. 1989 May;57(5):1413–1418. doi: 10.1128/iai.57.5.1413-1418.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton A. R., Weiss A. A. Environmental regulation of expression of virulence determinants in Bordetella pertussis. J Bacteriol. 1989 Nov;171(11):6206–6212. doi: 10.1128/jb.171.11.6206-6212.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Falkow S. Evidence for two genetic loci in Yersinia enterocolitica that can promote invasion of epithelial cells. Infect Immun. 1988 May;56(5):1242–1248. doi: 10.1128/iai.56.5.1242-1248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W. Comparative biology of intracellular parasitism. Microbiol Rev. 1985 Sep;49(3):298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouaissi A., Capron A. Some aspects of protozoan parasite-host cell interactions with special reference to RGD-mediated recognition process. Microb Pathog. 1989 Jan;6(1):1–5. doi: 10.1016/0882-4010(89)90002-8. [DOI] [PubMed] [Google Scholar]
- Relman D. A., Domenighini M., Tuomanen E., Rappuoli R., Falkow S. Filamentous hemagglutinin of Bordetella pertussis: nucleotide sequence and crucial role in adherence. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2637–2641. doi: 10.1073/pnas.86.8.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy C. R., Miller J. F., Falkow S. The bvgA gene of Bordetella pertussis encodes a transcriptional activator required for coordinate regulation of several virulence genes. J Bacteriol. 1989 Nov;171(11):6338–6344. doi: 10.1128/jb.171.11.6338-6344.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stibitz S., Aaronson W., Monack D., Falkow S. Phase variation in Bordetella pertussis by frameshift mutation in a gene for a novel two-component system. Nature. 1989 Mar 16;338(6212):266–269. doi: 10.1038/338266a0. [DOI] [PubMed] [Google Scholar]
- Stibitz S., Weiss A. A., Falkow S. Genetic analysis of a region of the Bordetella pertussis chromosome encoding filamentous hemagglutinin and the pleiotropic regulatory locus vir. J Bacteriol. 1988 Jul;170(7):2904–2913. doi: 10.1128/jb.170.7.2904-2913.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urisu A., Cowell J. L., Manclark C. R. Filamentous hemagglutinin has a major role in mediating adherence of Bordetella pertussis to human WiDr cells. Infect Immun. 1986 Jun;52(3):695–701. doi: 10.1128/iai.52.3.695-701.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L., Myers G. A., Falkow S. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect Immun. 1983 Oct;42(1):33–41. doi: 10.1128/iai.42.1.33-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L. Virulence factors of Bordetella pertussis. Annu Rev Microbiol. 1986;40:661–686. doi: 10.1146/annurev.mi.40.100186.003305. [DOI] [PubMed] [Google Scholar]