Introduction
The functional domain of the cerebellum extends beyond its traditional role in motor control. Over the last few decades this brain structure has increasingly been seen as playing a part also in perceptual and cognitive processes (see review in Schmahmann, 1997). This “cerebellar cognitive revolution” has been driven by observations of cerebellar activation in many functional imaging studies in which motor demands are thought to be equated (Ivry & Fiez, 2000, Cabeza & Nyberg, 2000), as well as by findings showing that patients with cerebellar lesions are impaired on a range of perceptual (e.g., Ivry & Keele, 1989; Ackermann et al., 1997) and cognitive tasks (e.g., Botez-Marquard et al., 2001; Daum et al., 2001, Schmahmann & Sherman, 1998). Moreover, anatomical studies have revealed dense neuronal connections between the cerebellum and cortical areas involved in higher cognitive functions (Middleton & Strick, 2000; Ramnani et al., 2006).
These findings have inspired a wide variety of hypotheses regarding cerebellar function, including the coordination of attentional shifts (Akshoomoff et al., 1997, Allen et al., 1997) and mental imagery (Parsons & Fox, 1997), facilitating retrieval from working memory (Desmond & Fiez, 1998), and various aspects of response planning (Doyon, 1997; Hallett & Grafman, 1997) and automatization (Molinari et al., 1997; Nicholson et al., 2001; Thach, 1998). Despite the seemingly disparate nature of these ideas, a common feature of many of these hypotheses is an emphasis on “predictive functions – the ability to anticipate forthcoming information and ensure that actions correctly anticipate changes in the environment” (Ivry & Fiez, 2000, p. 1005).
Two alternative, but closely related, hypotheses focusing on such predictive functions are the sensory prediction hypothesis and the timing hypothesis. The sensory prediction hypothesis postulates that the cerebellum is critical in generating expectancies regarding forthcoming sensory information (Bower, 1997; Courchesne & Allen, 1997; Ito, 2005; Paulin, 1997; Ramnani, 2006; Wolpert et al., 1998). From this perspective, the cerebellum is seen as an adaptive predictor, capable of extracting and maintaining a short-term template for predictable sensory input. Consistent with this hypothesis, Tesche & Karhu (2000) reported a strong cerebellar response measured with magnetoencephalography (MEG) after the random omission of expected somatosensory stimulation. In contrast, the primary sensory cortex was only activated after actually delivered stimuli and showed no response to the unexpected omissions. Further support for the sensory prediction hypothesis comes from electrophysiological experiments in non-humans showing that electrical stimulation of the cerebellum modulates sensory responses in extracerebellar regions (e.g., superior colliculus, thalamus) to a range of stimuli (Crispino & Bullock, 1984).
A more specific variant of the prediction idea is offered by the timing hypothesis which postulates that the cerebellum is critical for representing the precise temporal relationship between task-relevant events (reviews in Ivry, 1997; Ivry et al., 2002). According to this hypothesis, the cerebellum generates temporal predictions in a manner analogous to an hourglass, set in motion by the onset of an event and terminating at its expected offset or at the expected onset of a subsequent event. Support for this hypothesis can be found in studies demonstrating that patients with cerebellar pathology have problems producing rhythmic movements (Ivry et al., 1988; Spencer et al., 2003) and judging the duration of intervals across different sensory modalities, including audition (Ivry & Keele, 1989; Mangels et al., 1998), vision (Ivry & Diener, 1991; Nawrot & Rizzo, 1995) and somatosensation (Grill et al., 1994). An especially compelling demonstration of a specific cerebellar role in temporal processing comes from a speech perception study by Ackermann et al. (1997). Patients with cerebellar dysarthria, a difficulty in speech production, were unable to discriminate words that differed solely in the duration of the intersyllabic silent period, yet showed no such deficit for words with different spectral cues. The well documented cerebellar role in eyeblink conditioning (Yeo & Hesslow, 1998) is also consistent with the timing hypothesis: animals with the cerebellar cortex removed can still acquire the association, but the conditioned response is poorly timed (Perret et al., 1993; Anderson & Keifer, 1997; Koekkoek et al., 2003).
Precise temporal regulation is necessary for a wide spectrum of motor, perceptual and cognitive processes. Indeed, some of the evidence marshaled in support of the sensory prediction hypothesis is also consistent with the timing hypothesis. For example, in the Tesche and Karhu (2000) study, the stimuli were presented periodically. Thus, the response to omitted stimuli can be seen as elicited by a violation of an expectancy that a stimulus will be presented after a specific interval. Consistent with this interpretation, cerebellar activity was observed just prior to an anticipated stimulus, whether or not the stimulus was actually delivered.
While timing in this manner constitutes a form of prediction, not all prediction involves precise timing (Ivry, 2000). For instance, when driving a car, predictions concerning the (sensory) consequences of turning the steering wheel or pushing the gas pedal would have to be temporally precise. In contrast, the expectation that a “stop” signal will eventually change to “go” need not have this precise temporal specificity. Thus, the timing hypothesis predicts cerebellar involvement only in temporal predictions, that is, conditions involving expectations about the duration of events or intervals between events. In contrast, the sensory prediction hypothesis through its emphasis on sensory prediction in general, does not explicitly distinguish between temporal and non-temporal predictions.
The aim of the present experiment was to contrast the sensory prediction and the timing hypotheses, as defined above, by examining the mismatch negativity (MMN) response in patients with cerebellar cortical atrophy and a neurologically healthy control group. The MMN is an electrophysiological response observed following the presentation of a discriminable change (deviant) in a sequence of regular (standard) stimuli (reviewed in Näätänen et al. 2007). The MMN has been most intensively studied with auditory stimuli. In these experiments the event-related potential (ERP) to the deviant sound shows a negative deflection compared to the ERP to the standard sound, peaking between 100 and 250 ms after the onset of the deviant stimulus feature.
Interestingly, the "standard" stimulus need not be fixed: an MMN is elicited when the pitch of a sound violates a predictable sequence of descending pitch changes, as in a musical scale (Tervaniemi et al., 1994). These results suggest that the structures generating the MMN not only retain the immediate auditory past, but may anticipate future events based on the extraction of a regularity in the past (Näätänen et al., 2001; Winkler, 2008). Thus, the MMN is assumed to index the detection of a mismatch between the auditory input and a memory-based expectation (or prediction) that evolves from the repeated presentation of the standard stimulus (Näätänen et al., 2005). Moreover, the amplitude and latency of the MMN has been shown to be related to the magnitude of deviation along a variety of dimensions (e.g. Tiitinen et al., 1994; Yago et al., 2001; Amenedo & Escera, 2000; Deouell et al., 2006), and can thus provide objective measures of the representation of a specific dimension in sensory memory. This makes the MMN a promising tool for investigating the integrity of sensory processing and prediction in clinical groups (Näätänen, 2003).
ERP source localization as well as fMRI studies indicate that the main generators of the MMN are associated with primary and secondary auditory cortex in the superior temporal gyrus (e.g. Alho, 1995; Halgren et al., 1995; Rosburg, 2003), along with secondary generators in the frontal (Giard et al., 1990; Deouell et al., 1998; Molholm et al., 2005; Tse, et al., 2006; for review see Deouell, in press) and possibly parietal cortex (Molholm et al., 2005). Involvement of the temporal and frontal cortical areas has received additional support from studies demonstrating reduced MMN amplitude in patients with lesions in frontal and temporal cortex (Alain et al., 1998; Alho et al., 1994).
The role of the cerebellum in the MMN has only been examined in a few studies. Rabbits show distinct electrical responses in the cerebellum in response to auditory (pitch), visual, and somatosensory deviants (Ruusuvirta, 1996, Astikainen et al., 2000, Astikainen, 2001), but whether these are similar to the properties of the MMN is not clear. In humans, two imaging studies have reported cerebellar activation in duration MMN paradigms (Dittman-Balçar et al., 2001; Schall et al., 2003). One patient study reported a diminished somatosensory mismatch response to deviant tactile stimuli applied to the affected (ipsilesional) hand of patients with unilateral cerebellar lesions compared to stimuli applied to the unaffected hand (Restuccia et al., 2007). In contrast, two of the patients showed normal MMN responses on an auditory task using a pitch deviant. However, as the authors discuss, the modality difference might be related to the fact that the auditory stimuli were presented bilaterally, possibly enabling the intact cerebellar hemisphere to compensate for the damaged one.
We extend this patient-based approach in the current study by testing patients with bilateral cerebellar atrophy on an MMN task in which we employed four types of stimulus deviation: duration, pitch, intensity, and location. We reasoned that the timing hypothesis would predict a selective impairment in the patients’ MMN to duration deviants, whereas the sensory prediction hypothesis would predict a more general form of impairment in the MMN responses to both temporal and non-temporal deviants.
While these differential predictions are straightforward, one complication needs to be considered. In a study of healthy individuals, Takegata and Morotomi (1999) reported an enhanced MMN amplitude to a pitch deviant when the interval separating successive stimuli was fixed compared to when this interval was variable. Thus, the sensory prediction was not only of a stimulus of a particular pitch, but also of one that would occur at a particular time. Should the patients have difficulty anticipating the timing of a stimulus, their MMN to non-temporal deviants might also be abnormal, leading to the erroneous conclusion of a general prediction deficit. To address this concern, the stimuli in the present experiment were either presented periodically with a fixed stimulus onset asynchrony (SOA) or aperiodically with a variable SOA. If temporal information contributes to the memory trace of the standard, then the timing hypothesis would predict that increasing the periodicity of the stimuli would enhance the MMNs to all deviants in the healthy control group, but less so in the patient group, reflecting an impaired ability to utilize the temporal regularity.
Methods
Participants
Seven patients with bilateral cerebellar degeneration and 10 age-, gender- and education-matched controls volunteered for this experiment. The data from three control participants were not included in the final analysis. There was a technical problem with one speaker for one of these participants. For the other two, the data were discarded because of excessively noisy data (less than 2/3 of trials remained after eliminating trials showing artifacts in the raw EEG traces). These three participants were replaced with three additional matched control participants, resulting in seven patients and seven controls in the final set of participants (6 male, 1 female in each group).
Table 1 provides a summary of clinical information regarding the patients. This is a heterogeneous group in terms of age, etiology, disease duration, and symptoms. While advanced cerebellar degeneration can be associated with atrophy in the brainstem or basal ganglia (Klockgether et al., 1998), the CT/MRI scans and radiological records did not show pronounced atrophy outside the cerebellum. Even in the one case of olivopontocerebellar atrophy (OPCA), the extracerebellar signs were minimal. At the time of testing, all of the patients were evaluated on a battery of tests to assess neurological function and neuropsychological status. As can be seen in the table, the mean ataxia rating on the International Cerebellar Ataxia Rating Scale (ICARS, Trouillas et al., 1997) was 34.5 with a range of 17.55 to 49.75, indicating that all of the patients were at least moderately ataxic and some exhibited more advanced symptoms. The mean full-scale IQ for the patients (WAIS-III) was 100.6. Control participants were selected to match the patients in terms of age (patients: 58.1, sd; 12.1; controls; 59.3, sd; 12.7) and education level (patients: 16.4, sd; 2.7; controls; 17.4, sd; 2.7).
Table 1.
Demographic and clinical data for the patients
| Patient ID (sex) | Age | Education | Ataxia type / etiology | Years since diagnosis | ICARS total | ICARS gait / posture | ICARS ataxiaa | ICARS speech | ICARS oculomotor | MMSE score | WAIS-III IQ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AC01 (F) | 59 | 18 | Unknown | 6 | 29.0 | 11.2 | 5.6 | 4.2 | 2.2 | 30 | 127 |
| AC04 (M) | 50 | 18 | SCA3 | 10 | 49.8 | 11.8 | 12.5 | 3.2 | 4.8 | 28 | 96 |
| AC06 (M) | 66 | 20 | Unknown | 14 | 42.8 | 12.8 | 11.0 | 5.5 | 2.5 | 27 | 80 |
| AC07 (M) | 39 | 16 | SCA2 | 15 | 36.5 | 11.8 | 10.0 | 3.2 | 4.8 | 29 | 97 |
| AC08 (M) | 52 | 14 | Unknown | 9 | 20.8 | 5.8 | 3.0 | 3.4 | 4.8 | 29 | 105 |
| AC09 (M) | 66 | 17 | OPCA | 7 | 17.8 | 4 | 4.0 | 3.2 | 4.8 | 29 | 108 |
| AC10 (M) | 75 | 12 | Unknown | 44 | 45.0 | 19.8 | 9.1 | 4.8 | 2.2 | 30 | 91 |
| Mean | 58.1 | 16.4 | - | 15.0 | 34.5 | 12.0 | 7.9 | 3.9 | 3.3 | 28.8 | 100.6 |
| (SD) | (12.1) | (2.7) | - | (13.2) | (12.3) | (5.6) | (3.7) | (.9) | (1.2) | (1.1) | (14.8) |
SCA: Spinocerebellar Ataxia (types 2 and 3); OPCA: Olivopontocerebellar Atrophy; ICARS: International Cerebellar Ataxia Rating Scale;
scores for left and right side are combined in this index; MMSE: Mini Mental Status Examination; WAIS-III: Full scale IQ score from the Wechsler Adult Intelligence Scale, 3rd edition.
The procedures employed in this study conformed to the Declaration of Helsinki, and were approved by the institutional review board at the University of California, Berkeley. Prior to the experiment, participants provided informed consent. The exact aims were explained at the end of the experimental session to avoid directing the participant’s attention to the sounds. Participants were paid for their participation.
Stimuli and procedures
Participants were seated comfortably in a sound-attenuated room and instructed to watch a subtitled movie for which the soundtrack was turned off. They were told that a continuous series of sounds would be presented during the movie and that they should ignore these.
The primary experiment consisted of 10 blocks of approximately 7 min each. Within each block, 515 sounds were presented. Standard stimuli were presented 60% of the time and had a fixed spectrum, intensity, duration, and location. The standard was a harmonic tone, composed of three sinusoidal partials of 500, 1000 and 1500 Hz. Compared to the first partial, the intensity of the second and third partials were reduced by 3 and 6 dB, respectively. Standard stimuli were presented from a speaker located 15 degrees to the right of the centre of the monitor and were 250 ms in duration (including 10 ms rise and fall times). Tone duration was chosen in order to avoid confounding the perception of intensity and duration. For durations up to about 200 ms, longer stimuli of equal physical intensity are judged as louder (Scharf, 1978; Cowan, 1984), and likewise, more intense stimuli of equal physical duration are judged as longer (Lifshitz, 1933). Since confounding the stimulus dimensions would be problematic in light of the questions motivating this experiment, we opted for stimuli of relatively long duration compared to most MMN experiments.
To ensure that the tones were clearly audible, the intensity of the standard was set on an individual basis to be 50 dB above the person’s detection threshold. This threshold was determined at the start of the experimental session using a simple ascending and descending staircase procedure. Thresholds, and correspondingly, the intensity of the standard used in the experimental session were not significantly different between patients (72.5 dB SPL, sd 6.6) and controls (67.8, sd 5.0 dB, t(12) = −1.51, p > .10).
The remaining 40% of the sounds differed from the standard on one of four dimensions: pitch (10% higher than the standard, i.e. composed of 550, 1100 and 1650 Hz partials), duration (150 ms longer than the standard), intensity (10 dB softer than the standard) or sound location (30 degrees to the right of the standard speaker). Each deviant differed on only one of these dimensions, with each occurring 10% of the time. This mixed-deviant procedure has been shown to produce robust MMNs (Deouell et al., 2000; Näätänen et al., 2004). The order of the tones within a block was randomized with two constraints. First, all blocks began with a series of 15 standard stimuli. Second, the same deviant was never presented twice in a row.
To investigate the effect of the temporal predictability of the stimuli, we used two different timing schemes. In the five periodic blocks, the stimulus onset asynchrony (SOA) was fixed at 800 ms. In the five aperiodic blocks, the SOA was randomly selected to be one of three equiprobable durations (650, 800 or 950 ms), with the constraint that a given SOA never occurred more than twice in a row.
In addition to the 10 primary blocks, we also included two duration control blocks, one at the beginning and one at the end of the experimental session. In these blocks, the duration of the standard and deviant were reversed, so that the duration of the standard was set to 400 ms and the duration of the deviant was set to 250 ms. These blocks were included to provide an alternative baseline (standard) ERP response to use in the analysis of the duration MMN; that is, a control duration MMN was obtained by comparing the ERP elicited by a 400 ms deviant sound in the main blocks to the ERP arising from a physically identical stimulus when used as a standard in the control blocks. This control was included given prior work showing that the MMN may be artificially inflated when a shorter standard is subtracted from a longer deviant (Jacobsen & Schröger, 2003). The SOA was fixed at 800 ms in the control blocks.
In pilot work, we also used similar “reversed” blocks to control for stimulus differences in the other dimensions. This work indicated that stimulus differences had negligible effects on the MMNs elicited in response to pitch, intensity, and location deviants. Consequently, in order to reduce the total duration of the recording session, these control blocks were not included in the final experimental design.
The entire session, including the preparation for the EEG recordings, lasted approximately 2 hours. Participants were provided with short breaks between the test blocks.
EEG recording and averaging
EEG was continuously recorded at 512 Hz by an Active 2 system (Biosemi), using 64 sintered Ag/AgCl electrodes in an electrode cap laid out according to the extended 10–20 system with three additions (nose, left and right mastoid). The electrooculogram (EOG) was monitored from electrodes near the outer canthi and below the left eye.
EEG was referenced to an average-reference computed offline, using the 64 cap electrodes and the mastoids, excluding occasional malfunctioning electrodes. During recording, a 128 Hz low pass filter was applied to avoid aliasing of high frequencies. Offline, the EEG was filtered with bandpass of 1–20 Hz (24 dB/octave) suitable for the frequency range of auditory late evoked potentials and the MMN. For ERP averaging, the EEG was divided into epochs of 750 ms starting 100 ms before stimulus onset, and the epochs were averaged separately for the responses to the standards and for the 4 types of deviant stimuli in each SOA condition. Epochs including an EEG or EOG voltage exceeding +/−75 µV, as well as those from the first 15 stimuli of each block were omitted from the averaging. The baseline was adjusted by subtracting the mean amplitude of the 100 ms pre-stimulus period of each ERP from all the data points in the epochs.
On average, the ERPs were based on 215 trials for each of the eight deviants (four dimensions × 2 SOA conditions), and this value did not differ between controls (range: 194 – 229) and patients (range: 185–242). The ERP for the standard is based on approximately six times as many trials as the deviants, except for the duration control ERP which is based on approximately twice as many trials as the duration deviant ERP.
Data analysis
In order to examine the possibility that any group differences in the MMN could be due to differences in auditory processing upstream from deviance detection, we analyzed the P1, N1, and P2 components of the ERP to standard sounds. Latencies and amplitudes of these components in the periodic and aperiodic condition were measured at electrode FCz. The P1-component was defined as the most positive peak occurring in the first 100 ms after stimulus onset, the N1-component as the most negative peak between 50 and 150 ms, and the P2 component as the most positive peak between 100 and 250 ms. Latencies and amplitudes were tested statistically by separate Group (2 levels: patients and controls) by Periodicity (2 levels: periodic and aperiodic SOA condition) ANOVAs for each component.
The MMN was identified by subtracting the waveform elicited by the standard from that of each deviant. In the case of the duration MMN, an additional control duration MMN was identified by subtracting the response to the 400 ms standard used in the two control blocks from the response to the duration deviant in the regular periodic blocks.
The distribution of the MMN across the 64 electrodes was used to verify that the response observed in the current study was similar to that reported in the literature. As expected, the MMN showed a distribution of frontal negativity and posterior-temporal positivity. Given that the response is maximal over frontal sites, the data from three anterior electrodes (F3, Fz and F4) were pre-selected for statistical analysis. MMN latencies were measured as the most negative peak occurring between 100 and 250 ms after the onset of deviance from the standard stimulus. Note that while the onset of deviance coincided with stimulus onset for the non-temporal deviants, in case of the duration deviant the deviance (delayed termination) occurred 250 ms after stimulus onset, and hence the expected MMN window was between 350 and 500 ms after stimulus onset. In order to facilitate comparison across deviant types, MMN latencies for duration changes were corrected in relation to the onset of deviation (e.g., the duration of the standard tone was subtracted from the peak latency). MMN amplitudes were integrated over 50 ms (± 25 ms around the individual peak latencies). Two-tailed t-tests were used to determine whether MMN amplitudes differed significantly from 0 µV.
In order to compare the MMN latencies and amplitudes between groups and SOA-conditions, we conducted three-way ANOVAs (Group × Periodicity × Electrode) separately for each deviant type. The Greenhouse-Geisser correction was applied when appropriate (the original degrees of freedom and corrected p-values are reported). Sources of significant interactions were examined with additional post hoc ANOVAs.
Since the control block 400 ms standard had only been recorded in a periodic SOA condition, we used the duration MMN made by subtracting the 250 ms standard from the 400 ms deviant for the initial analyses. However, all analyses on the duration MMN that did not involve the Periodicity factor (or were restricted to the Periodic SOA condition) were repeated with the control duration MMN.
Although our sample size is small, we also examined whether the degree of clinical severity was related to abnormalities in the ERP data. For these analyses, we computed Pearson product moment correlation coefficients between the total score on the ICARS evaluation with the MMN latency and amplitude data at electrode Fz.
Results
Auditory evoked potentials to standard tones
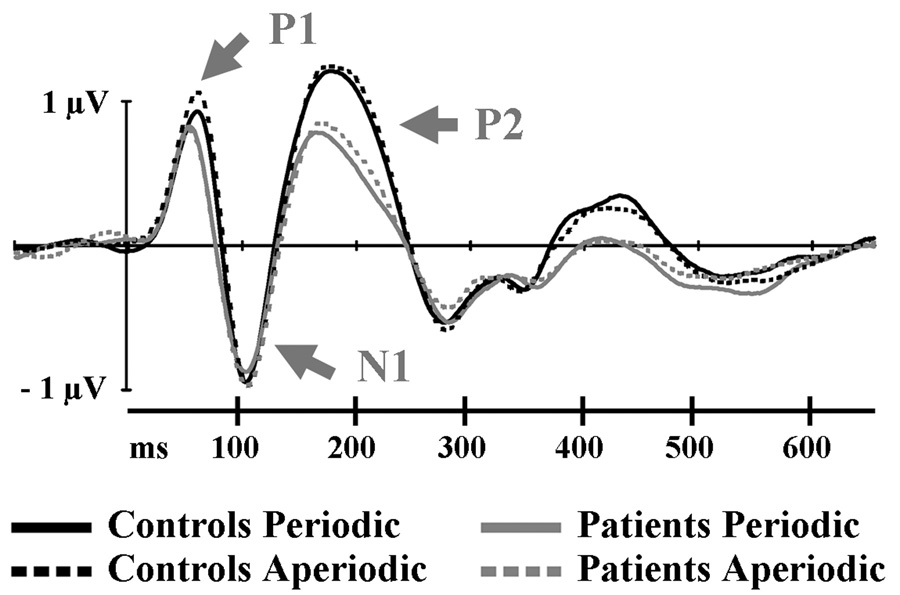
Standard stimuli elicited a waveform consisting of the P1, N1 and P2 components in both patients and controls (see Figure 1). There were no significant main effects or interactions involving Group on P1 latency. In contrast, the P1 amplitude yielded a significant Group × Periodicity interaction (F (1, 12) = 4.83; p < .05). Separate ANOVAs for each group revealed that the P1 amplitude was larger in the aperiodic compared to periodic condition for the controls (Periodicity: (F (1, 6) = 6.03; p < .05). There was no significant amplitude difference between these two conditions for the patient group (F < 1).
Figure 1.
Grand average ERPs to standard sounds recorded at the frontocentral FCz electrode.
Latencies and amplitudes of the N1 and P2 peaks did not differ between groups or periodicity conditions. While the waveforms suggest a somewhat reduced P2 amplitude in the patients relative to the controls (fig. 1), this difference failed to reach significance (F (1, 12) = 1.48; p = .25).
Mismatch negativity (MMN)
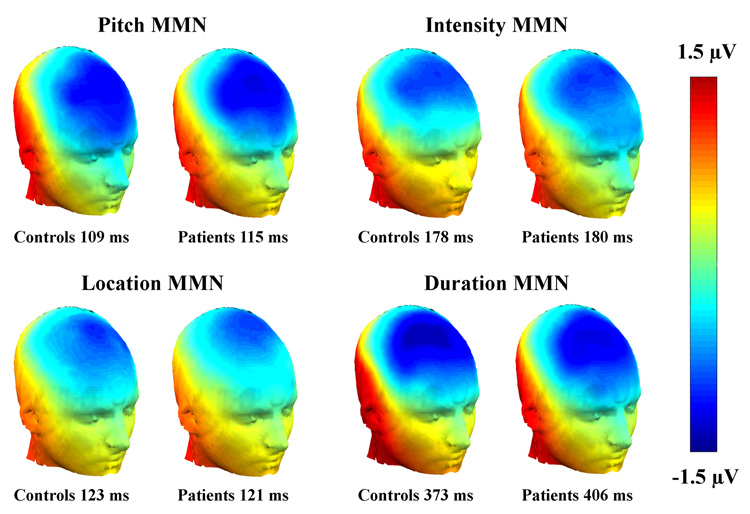
Both the control and the patient group produced identifiable MMNs to all deviant types in both the periodic and aperiodic conditions (Figure 2 and Figure 3). The MMN potentials showed the expected scalp distribution, with maximum negative amplitude over frontal electrodes and reversed polarity at posterior temporal electrodes (Figure 2).
Figure 2.
Scalp maps showing the spatial distribution of the grand average MMNs at peak latency (measured at Fz) in the periodic SOA-condition.
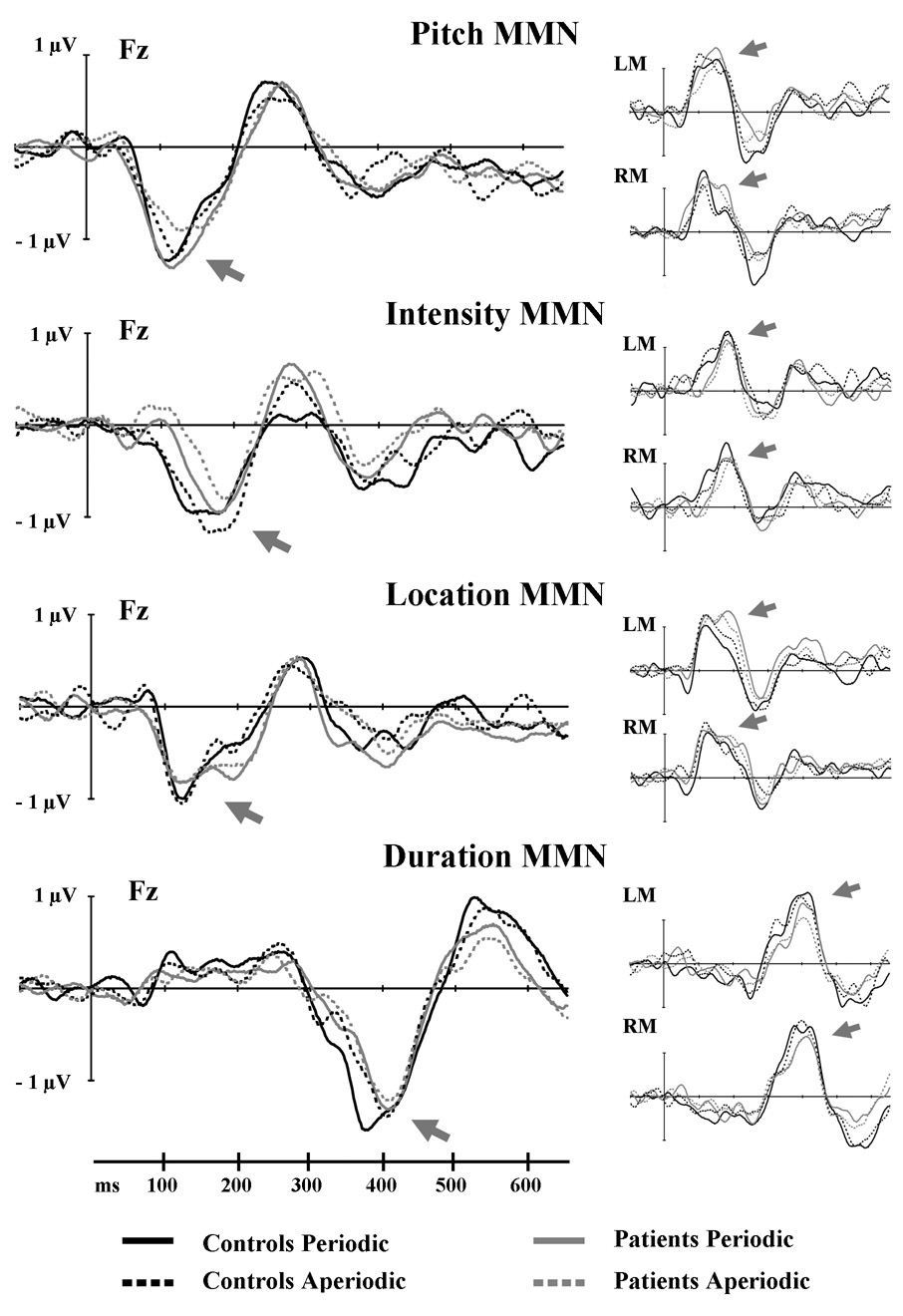
Figure 3.
Grand average difference waves showing MMN responses to the four different deviant types recorded at electrode Fz and the left (LM) and right (RM) mastoid. Responses for controls and patients in the periodic and aperiodic conditions are overlaid. Arrows indicate the MMN.
As can be seen in Figure 3, the MMN to location deviants had a clear double peak, consistent with previous reports (Tata & Ward, 2005; Deouell et al., 2006). The first peak reached maximum amplitude around 120 ms and the second around 195 ms. Given this double-peaked response, we manually determined the latencies and amplitudes of each peak and conducted our analyses on these measures.
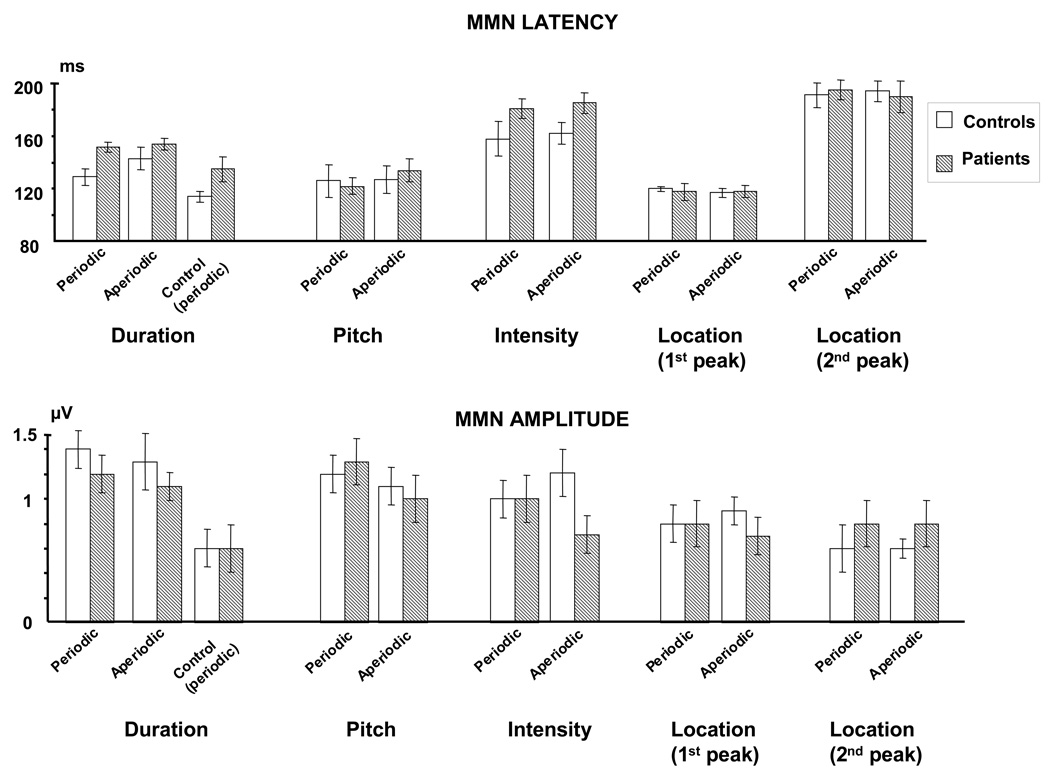
MMN-latencies and amplitudes for the controls and patients are provided in Figure 4. Two-tailed t-tests showed that for both patients and controls the MMN amplitude differed significantly from zero for all conditions and deviant types (p <.05). As noted in the Methods section, we also calculated a control duration MMN by subtracting the response to the 400 ms stimulus used as a standard in the control blocks from the response to the physically identical 400 ms duration deviant of the main block. Similar to previous findings (Jacobson and Schröger, 2003), this control duration MMN was reduced in amplitude compared to the duration MMN calculated by subtracting the 250 ms standard used in the main blocks from the 400 ms duration deviant (Figure 4). Importantly, however, the control duration MMN also differed significantly from zero for both groups (p < .05).
Figure 4.
Bar graphs showing mean MMN latencies and amplitudes. Error bars represent the standard error of the mean.
We conducted separate three-way (Group × Periodicity × Electrode) ANOVAs for each deviant type, using the latency data as dependent variable in one set of analyses and the amplitude data in a second set.
Latencies
Latencies of the pitch and location MMN did not differ between patients and controls (F-values < 1). In contrast, the MMN latency was delayed in the patients compared to the control group for the duration deviant (Group: F (1, 12) = 5.72, p < .05), and we observed a similar trend for the intensity MMN (Group: F (1, 12) = 4.32; p = .06). Consistent with the primary analysis of the duration MMN, a significant increase in the patients’ latency was also observed when the periodic control duration MMN was used in the analysis (Group: F (1, 12) = 5.80; p < .05).
There were no significant main effects or interactions involving periodicity for the latencies of the pitch, intensity and location MMNs. Importantly, however, the main effect of Group on duration MMN latency was qualified by a significant Group × Periodicity interaction (F (1, 12) = 6.26; p <.05). This interaction was due to a shortened latency for the control participants in the periodic relative to the aperiodic condition (F (1, 6) = 12.17; p < .05). This effect was not observed in the patient group (F (1, 6) < 1).
MMN latencies were similar across the three electrode sites for pitch, intensity, and location (no main effect of Electrode). However, there was a significant Electrode × Group interaction (F (2, 24) = 3.60; p < .05) for the latency of the duration MMN. This effect was due to a larger group latency difference over the right (F4) than over the central (Fz) and left (F3) electrodes (linear contrast: F (1, 12) = 5.40; p < .05).
Amplitudes
There were no significant main group effects on the MMN amplitudes to pitch (F (1, 12) < 1), duration (F (1, 12) < 1), intensity (F (1, 12) <1) or location (first peak: F (1, 12) < 1), second peak: F (1, 12) = 1.30; p = .28) deviants. Across groups, pitch MMN amplitude was increased in the periodic compared to the aperiodic condition (Periodicity: F (1, 12) = 13.07; p <.05), while periodicity did not affect the MMN amplitude to duration (Periodicity: F (1, 12) < 1), intensity (Periodicity: F (1, 12) < 1) or location (Periodicity: F (1, 12) < 1 for both the early and late peak) deviants. There were no significant interactions involving Group or Periodicity for any of the deviant types. Across groups, the pitch MMN was larger in amplitude at central (Fz) and right (F4) compared to the left (F3) electrode (Electrode: F (2, 24) = 4.93; p < .05). While the MMNs to the other deviant types also showed maximal amplitude at Fz or F4, these trends were not reliable.
Exploratory correlations
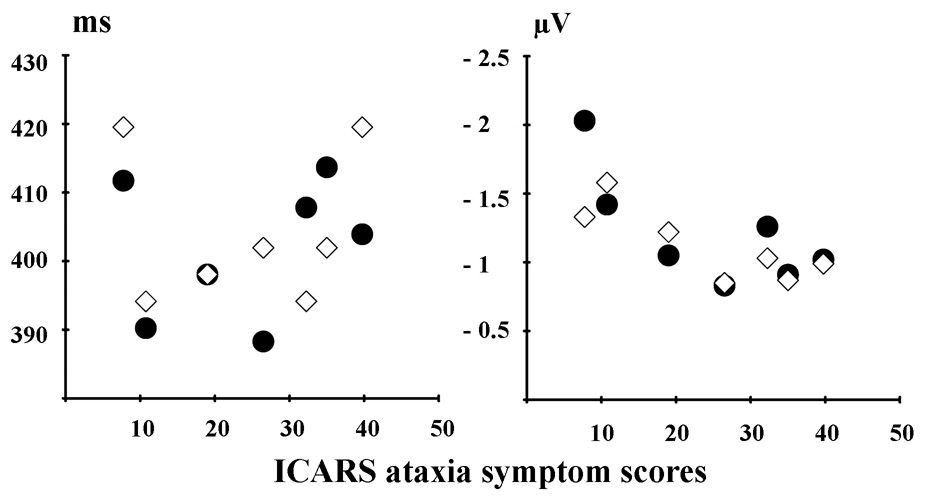
Correlations between MMN-measures and cerebellar symptom scores are given in Table 2. While ataxia scores were not significantly correlated with the MMN latencies to any of the deviants, we did observe a positive correlation between the amplitude of the duration MMN and the ataxia score: patients exhibiting the most severe signs of ataxia produced lower amplitude MMN responses to the duration deviant. This relationship was significant in the aperiodic condition (r = .83, p <.05), with a similar trend in the periodic condition (r = .73, p =.06) reflecting the fact that the MMN amplitude values were very similar within individuals for the two conditions. A marginally reliable correlation was also observed between the ataxia scores and the amplitude of the control duration MMN (r = .75, p =.05). Predominantly positive correlations between ataxia scores and MMN amplitudes to the other deviants failed to reach statistical significance. The scatter plots in Figure 5 show the relationships between ataxia scores and duration MMN latencies and amplitudes. While visual inspection of both panels suggest that degree of cerebellar pathology is related to the MMN measures, this failed to reach significance for the latency measures (also when removing the apparent outlier with the lowest ataxia score).
Table 2.
Correlations between ataxia scores and the MMN at Fz
| Deviant type | Latencies | Amplitudes |
|---|---|---|
| Duration Periodic | .26 | .73 (p = .06) |
| Duration Aperiodic | .05 | .83 (p < .05) |
| Duration Control | −.50 | .75 (p = .05) |
| Pitch Periodic | .11 | .58 |
| Pitch Aperiodic | −.54 | .60 |
| Intensity Periodic | −.45 | .68 (p =.09) |
| Intensity Aperiodic | −.48 | .58 |
| Location 1 Periodica | −.58 | .46 |
| Location 1 Aperiodica | −.50 | .18 |
| Location 2 Periodicb | −.36 | .12 |
| Location 2 Aperiodicb | −.32 | −.27 |
First peak of Location MMN;
Second peak of Location MMN.
Unless indicated, p > .1.
Figure 5.
Scatter plots showing the relationship between ICARS ataxia symptom scores and duration MMN latencies (left panel) and amplitudes (right panel). Filled circles indicate periodic and open diamonds indicate aperiodic SOA-condition.
Discussion
The goal of the present study was to contrast two related hypotheses of cerebellar function by investigating the MMN to temporal and non-temporal deviants in patients with cerebellar degeneration and a healthy control group. We reasoned that based on the sensory prediction hypothesis, the patients should show impaired MMNs to all deviant types. In contrast, the timing hypothesis predicts a selective impairment in the MMN to temporal duration deviants. In addition, the timing hypothesis would predict that controls should benefit when the stimuli are presented periodically, an effect that should be absent or reduced in the patient group. Common to both hypotheses, cerebellar degeneration was predicted to affect the MMN response, an early auditory evoked response that signifies the violation of a sensory expectancy.
Standard tones
In both groups standard stimuli elicited comparable auditory evoked potentials consisting of the P1, N1 and P2 peaks, suggesting intact initial cortical activation. Although the present experiment was focused on the MMN, we also observed a group difference in the effect of periodicity on P1 amplitude. The control group showed an enhanced P1 amplitude in the aperiodic compared to the periodic condition, an effect that was absent in the patients. While clearly speculative, it is tempting to relate this result to previous work on P1 suppression, which has been reported to be reduced in patients with Machado-Josephs disease (or SCA3), a form of cerebellar cortical degeneration (Ghisolfi et al., 2004). P1-suppression is a reduction in the P1 (or P50) potential evoked by the second tone when separated by a first tone with a fixed ISI (usually 500 ms; e.g. Fuerst et al., 2007), or in a periodic repetitive presentation (Erwin and Buchwald, 1986). This attenuation is commonly interpreted as a measure of sensory gating. A reduced P1 can also be demonstrated when a tone follows a somatosensory stimulus, suggesting that the gating process is not a passive refractory mechanism, but rather, an active (and potentially cross-modal) inhibitory process (Perlstein, Simons & Graham, 2001).
In the present experiment, the fixed SOA might have enabled the controls to suppress (or “gate out”) the irrelevant tones in the periodic condition, whereas such suppression would be more difficult in the less predictable aperiodic condition. The absence of a difference between conditions in the patient group may indicate that the cerebellum plays a role in filtering out predictable irrelevant stimuli, as has been suggested by researchers studying the cerebellar and cerebellar-like structures of lower animals (Devor, 2000; Bell, 2002). An interesting question for further research is whether difficulties in filtering out temporally predictable task-irrelevant stimuli might partly account for the attentional difficulties reported in patients with cerebellar lesions (Gottwald, Mihailovic, Wilde & Mehdorn, 2003).
While inspection of the waveforms (Figure 1) also suggested a group difference in P2-amplitude, this effect failed to reach significance. Nonetheless, since the time-window of the P2 partly overlaps that of the MMN, any group differences in MMN amplitudes might conceivably be related to this trend towards a P2 amplitude difference. Importantly, however, the observed group differences (discussed below) involved MMN latency rather than amplitude measures. Moreover, the duration MMN did not occur until 350–500 ms after stimulus onset, well after the time-window of the P2. Thus, the observed group differences in the MMN measures are unlikely to be due to differences in information processing prior to the generation of the MMN.
Mismatch negativity - deviant types
Our main finding was that cerebellar degeneration did not produce a common abnormality across the four types of deviants; rather, the abnormalities were selective, with the patients showing a delayed MMN to the duration deviant and a similar trend to the intensity deviant. While the small sample size warrants caution in interpreting the results of the exploratory correlation analyses, a further observation was that duration MMN amplitude was related to cerebellar symptom scores; patients with more advanced pathology produced more attenuated responses. Together, these results suggest that the cerebellum contributes to pre-attentive duration estimation. Importantly, the patients produced significant duration MMNs, indicating that they were not insensitive to the temporal deviation, despite their cerebellar pathology. Indeed, in spite of the correlation with cerebellar symptom scores, duration MMN amplitude did not differ significantly between the patient and the control group.
Taken together, the findings of preserved amplitude but delayed latency in the patients suggest that cerebellar degeneration results in coarser or less reliable temporal representations (Ivry et al., 2002). Given an unreliable temporal memory trace, a larger temporal difference between the standard and deviant would be required for a change to be detected. Since the duration deviance consisted of stimulus prolongation, a larger fraction of this prolongation would have to be presented before a violation of the standard could be detected, resulting in a delayed latency of the MMN.
Direct comparison of the present results with previous studies of overt duration discrimination in cerebellar patients is not straightforward since precise indexes of discriminatory ability cannot be calculated without overt responses. Nonetheless, the duration discrimination results are consistent with the hypothesis that the delayed MMN results from an increase in the variability of temporal processing. Temporal acuity is frequently expressed as a Weber ratio, a normalized measure in which the discrimination threshold is expressed as a percentage of the base duration (see Getty, 1975). In auditory duration discrimination studies, the Weber ratio for older control participants falls in the range of 5% to 10%, whereas for patients with cerebellar degeneration, the range is elevated by about 70% (8% to 18%; see Casini & Ivry, 1999; Ivry & Keele, 1989; Mangels et al., 1998; Nichelli et al., 1996). For a 250 ms tone, these differences would lead to the expectation that the discrimination threshold for the patients would be increased by 7.5 to 17.5 ms relative to the controls, a range that is close to the patients’ 20 ms increase in the MMN latency for the duration deviants. The somewhat larger value observed here may be related to the fact that discrimination thresholds are larger with filled intervals (the duration of a tone) than with empty intervals (the time between two tones), as were used in these cited studies (e.g., Grondin, 1993). Importantly, the present findings add to these previous studies by demonstrating that the patients’ impairment in time discrimination is present at an early stage of auditory processing (100–200 ms), even when the task does not require an overt assessment of temporal regularities or even that the stimuli be attended.
A finding at odds with the timing hypothesis was the observed trend towards increased intensity MMN latency in the patients. As a post-hoc account of this unexpected result, we suggest that the delayed detection of both duration and intensity deviants may result from a common deficit in temporal processing. For sounds shorter than 200 ms, perceived stimulus intensity is influenced by stimulus duration, being approximated as the integral of energy over time (Scharf, 1978; Cowan, 1984). Consequently, we chose a standard duration outside this temporal window to ensure that the MMN to the duration deviant – related to sound offset – was not affected by sound intensity. However, the latency of the intensity MMN (around 170 ms from sound onset) suggests that stimulus intensity is determined well before the stimulus ends, and within the temporal window where intensity and duration interact. Perhaps, with an unreliable timer due to cerebellar degeneration, the integration of stimulus energy over time takes longer than usual, resulting in delayed detection of the intensity deviant. This post-hoc conjecture needs to be directly addressed in future research.
Importantly, the pitch and location MMNs were not affected by cerebellar degeneration. This selective sensitivity of the duration (and possibly intensity) MMN to cerebellar pathology is inconsistent with a general role of the cerebellum in sensory prediction as suggested by the sensory prediction hypothesis. Thus, with the possible exception of the intensity MMN, the differential effects of cerebellar degeneration on the MMNs to our four deviant types are largely consistent with the predictions of the timing hypothesis.
It is important to note that the duration deviant was the only “graded” deviant, in the sense that the degree of deviance increased over the extent of stimulus presentation (since we only used deviants longer than the standard). That is, as the long deviant continued past the expected offset time at 250 ms, the listener could detect the difference at various points of time during the subsequent 150 ms. In contrast; the other deviants were “instant” – and rather large. Thus, we cannot exclude the possibility that the use of smaller deviants would have revealed group differences in the MMN response to non-temporal deviants. To overrule this possibility, a parametric study utilizing several deviance magnitudes for each deviant feature would be needed (Pakarinen et al., 2007).
Mismatch negativity - periodicity
Overall, periodicity had limited and somewhat inconsistent effects in our study. Based on previous findings for pitch (Takegata & Morotomi, 1999), periodicity was expected to enhance the MMN response for the controls given that fixed inter-stimulus timing adds a further element of predictability. Based on the timing hypothesis, we predicted that this effect would be reduced or absent in the cerebellar patients, reflecting an impaired ability to utilize the temporal regularity. Consistent with this prediction, the periodicity shortened the latency of the duration MMN in the control group while this effect was not observed in the patients. A lack of sensitivity to the periodicity manipulation was also seen in that the P1 for standards was larger for aperiodic than periodic stimulation in controls, but had equal amplitude across conditions in patients.
However, one aspect of the periodicity results is problematic. The timing hypothesis would predict a global deficit in utilizing the high temporal predictability offered by periodic stimulation. Contrary to this prediction, the patients showed normal amplitude augmentation for pitch changes in the periodic condition. The intensity and location MMNs did not provide a test of the periodicity predictions since they were not affected by periodicity in either controls or patients. Thus, the current results remain inconclusive regarding the effect of periodicity, and consequently provide only partial support to a strong version of the timing hypothesis, as defined in the Introduction.
On a methodological note, the observation of periodicity effects – if somewhat inconsistent – emphasizes that periodicity may be a relevant factor when interpreting cerebellar function in imaging and patient studies. For instance, the two imaging studies that have reported cerebellar activation in an auditory duration MMN task used a fixed SOA (Dittmann-Balçar et al., 2001; Schall et al., 2003). A fixed SOA was also used in a study showing a diminished somatosensory MMN in patients with unilateral cerebellar damage (Restuccia et al., 2007). The present results suggest that these effects might be related to the periodicity of the stimuli. It would be interesting to see if similar results would be obtained with a variable SOA.
Limitations of the present study
The experiment was quite long (approximately two hours). Given that fatigue influences the MMN response (Sallinen & Lyytinen, 1997), this would present a problem if fatigue had a differential effect on the patients and controls. This concern would be especially problematic if we had observed general group differences; that is, if the patients had exhibited abnormal MMNs for all deviant types. The more specific group differences observed in the present study are less likely to be influenced by fatigue effects. Moreover, fatigue seems to primarily affect MMN amplitude (Sallinen & Lyytinen, 1997) and we did not observe any group effects on measures of MMN amplitude. Nonetheless, we reanalyzed the data by computing separate MMNs for stimuli presented in the first and second halves of the experiment. When these two data sets were compared, we did observe a significant reduction in the amplitude of the MMN for the intensity and pitch deviants, suggesting that the participants were experiencing some fatigue. Importantly, these effects did not differ between the patient and control groups, nor were there any changes over time in the latency data for all four deviants. Thus, it is unlikely that fatigue would account for the observed group differences.
A more important limitation with the present experiment is the relative heterogeneity and small sample size of the patient group. While a larger sample size is, of course, desirable, we were limited in the number of participants we could identify who would be comfortable completing a 2-hour EEG study. We note, though, that a small, heterogeneous sample is likely to increase within-group variability, and thus reduce the probability of detecting significant effects. As such, the abnormal MMN latency to the duration deviant would appear to be a robust effect. Sample size and variability issues are more relevant with respect to the null findings for pitch and location, and especially the near-significant effect observed with intensity deviants. Our concerns here are mitigated by the fact that the selective effects on the MMN for the duration deviant were consistent with one of the predictions derived, a priori, for one of the hypotheses under consideration.
Conclusion
The present experiment demonstrates that the auditory mismatch paradigm can reveal important and meaningful data clarifying the functional role of the cerebellum. The results provide support for a cerebellar contribution to the automatic processing and anticipation of auditory stimuli. The absence of impairment in the patients' MMN response to pitch and location deviants further suggests a rather specific cerebellar role in sensory prediction. Although some aspects of the results are at odds with a strong version of the timing hypothesis, they nonetheless indicate that the cerebellar contribution is related to the processing of temporal properties of the stimuli (e.g., stimulus duration). The present findings demonstrate that this impairment is present at an early stage of auditory processing (100–200 ms), even when the task does not require an overt assessment of temporal regularities or even that the stimuli be attended.
Acknowledgements
We wish to thank Shani Shalgi for programming the experiment. This work was supported by the National Institute of Health grants NS21135, PO40813 (to R.T.K.), R01 NS30256 (to R.B.I.). CMK was supported by Ruth L. Kirchstein Predoctoral NRSA F31-MH74342.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackermann H, Graber S, Hertrich I, Daum I. Categorical speech perception in cerebellar disorders. Brain and Language. 1997;60:323–331. doi: 10.1006/brln.1997.1826. [DOI] [PubMed] [Google Scholar]
- Akshoomoff NA, Courchesne E, Townsend J. Attention coordination and anticipatory control. In: Schmahmann JD, editor. The cerebellum and cognition (International Review of Neurobiology, vol. 41) Academic Press; 1997. pp. 575–598. [DOI] [PubMed] [Google Scholar]
- Alain C, Woods DL, Knight RT. A distributed cortical network for auditory sensory memory in humans. Brain Research. 1998;812:23–37. doi: 10.1016/s0006-8993(98)00851-8. [DOI] [PubMed] [Google Scholar]
- Alho K. Cerebral generators of mismatch negativity (MMN) and its magnetic counterpart (MMNm) elicited by sound changes. Ear and Hearing. 1995;16:38–51. doi: 10.1097/00003446-199502000-00004. [DOI] [PubMed] [Google Scholar]
- Alho K, Woods DL, Algazi A, Knight RT, Näätänen R. Lesions of frontal cortex diminish the auditory mismatch negativity. Electroencephalography and Clinical Neurophysiology. 1994;91:353–362. doi: 10.1016/0013-4694(94)00173-1. [DOI] [PubMed] [Google Scholar]
- Allen G, Buxton RB, Wong EC, Courchesne E. Attentional activation of the cerebellum independent of motor involvement. Science. 1997;275:1940–1943. doi: 10.1126/science.275.5308.1940. [DOI] [PubMed] [Google Scholar]
- Amenedo E, Escera C. The accuracy of sound duration representation in the human brain determines the accuracy of behavioural perception. European Journal of Neuroscience. 2000;12:2570–2574. doi: 10.1046/j.1460-9568.2000.00114.x. [DOI] [PubMed] [Google Scholar]
- Anderson CW, Keifer J. The cerebellum and red nucleus are not required for In vitro classical conditioning of the turtle abducens nerve response. Journal of Neuroscience. 1997;17:9736–9745. doi: 10.1523/JNEUROSCI.17-24-09736.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astikainen P, Ruusuvirta T, Korhonen T. Cortical and subcortical visual event-related potentials to oddball stimuli in rabbits. Neuroreport. 2000;11:1515–1517. [PubMed] [Google Scholar]
- Astikainen P, Ruusuvirta T, Korhonen T. Somatosensory event-related potentials in the rabbit cerebral and cerebellar cortices: a correspondence with mismatch responses in humans. Neuroscience Letters. 2001;298:222–224. doi: 10.1016/s0304-3940(00)01747-x. [DOI] [PubMed] [Google Scholar]
- Bell CC. Evolution of cerebellum-like structures. Brain, Behaviour and Evolution. 2002;59:312–326. doi: 10.1159/000063567. [DOI] [PubMed] [Google Scholar]
- Botez-Marquard T, Bard C, Leveille J, Botez MI. A severe frontal-parietal lobe syndrome following cerebellar damage. European Journal of Neurology. 2001;8:347–353. doi: 10.1046/j.1468-1331.2001.00204.x. [DOI] [PubMed] [Google Scholar]
- Bower JM. Control of sensory data acquisition. In: Schmahmann JD, editor. The cerebellum and cognition (International Review of Neurobiology, vol. 41) Academic Press; 1997. pp. 489–513. [DOI] [PubMed] [Google Scholar]
- Bower JM. The organization of cerebellar cortical circuitry revisited: implications for function. Annals of the New York Academy of Sciences. 2002;978:135–155. doi: 10.1111/j.1749-6632.2002.tb07562.x. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Casini L, Ivry RB. Effects of divided attention on temporal processing in patients with lesions of the cerebellum or frontal lobe. Neuropsychology. 1999;13:10–21. doi: 10.1037//0894-4105.13.1.10. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Allen G. Prediction and preparation, fundamental functions of the cerebellum. Learning and Memory. 1997;4:1–35. doi: 10.1101/lm.4.1.1. [DOI] [PubMed] [Google Scholar]
- Cowan N. On short and long auditory stores. Psychological Bulletin. 1984;96:341–370. [PubMed] [Google Scholar]
- Crispino L, Bullock TH. Cerebellum mediates modality-specific modulation of sensory responses of midbrain and forebrain in rat. Proceedings of the National Academy of Sciences. 1984;81:2917–2920. doi: 10.1073/pnas.81.9.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum I, Snitz BE, Ackermann H. Neuropsychological deficits in cerebellar syndromes. International Review of Psychiatry. 2001;13:268–275. [Google Scholar]
- Deouell LY. The frontal generator of the mismatch negativity revisited. Journal of Psychophysiology. (in press) [Google Scholar]
- Deouell LY, Bentin S, Giard MH. Mismatch negativity in dichotic listening: evidence for interhemispheric differences and multiple generators. Psychophysiology. 1998;35:355–365. [PubMed] [Google Scholar]
- Deouell LY, Bentin S, Soroker N. Electrophysiological evidence for an early (pre-attentive) information processing deficit in patients with right hemisphere damage and unilateral neglect. Brain. 2000;123:353–365. doi: 10.1093/brain/123.2.353. [DOI] [PubMed] [Google Scholar]
- Deouell LY, Parnes A, Pickard N, Knight RT. Spatial location is accurately tracked by human auditory sensory memory: evidence from the mismatch negativity. European Journal of Neuroscience. 2006;24:1488–1494. doi: 10.1111/j.1460-9568.2006.05025.x. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Fiez JA. Neuroimaging studies of the cerebellum: language, learning and memory. Trends in Cognitive Science. 1998;2:355–362. doi: 10.1016/s1364-6613(98)01211-x. [DOI] [PubMed] [Google Scholar]
- Devor A. Is the cerebellum like cerebellar-like structures? Brain Research Reviews. 2000;34:149–156. doi: 10.1016/s0165-0173(00)00045-x. [DOI] [PubMed] [Google Scholar]
- Dittmann-Balçar A, Juptner M, Jentzen W, Schall U. Dorsolateral prefrontal cortex activation during automatic auditory duration-mismatch processing in humans: a positron emission tomography study. Neuroscience Letters. 2001;308:119–122. doi: 10.1016/s0304-3940(01)01995-4. [DOI] [PubMed] [Google Scholar]
- Doyon J. Skill learning. In: Schmahmann JD, editor. The cerebellum and cognition (International Review of Neurobiology, vol. 41) Academic Press; 1997. pp. 273–294. [DOI] [PubMed] [Google Scholar]
- Erwin RJ, Buchwald JS. Midlatency auditory evoked responses: Differential recovery cycle characteristics. Electroencephalography and Clinical Neurophysiology. 1986;64:471–423. doi: 10.1016/0013-4694(86)90075-1. [DOI] [PubMed] [Google Scholar]
- Fuerst DR, Gallinat J, Boutros NN. Range of sensory gating values and test-retest reliability in normal subjects. Psychophysiology. 2007;44:620–626. doi: 10.1111/j.1469-8986.2007.00524.x. [DOI] [PubMed] [Google Scholar]
- Getty DJ. Discrimination of short temporal intervals: a comparison of two models. Perception and Psychophysics. 1975;18:1–8. [Google Scholar]
- Ghisolfi ES, Maegawa GH, Becker J, Zanardo AP, Strimitzer IM, Jr, Prokopiuk AS, et al. Impaired P50 sensory gating in Machado-Joseph disease. Clinical Neurophysiology. 2004;115:2231–2235. doi: 10.1016/j.clinph.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Giard MH, Perrin F, Pernier J, Bouchet P. Brain generators implicated in the processing of auditory stimulus deviance: a topographic event-related potential study. Psychophysiology. 1990;27:624–640. doi: 10.1111/j.1469-8986.1990.tb03184.x. [DOI] [PubMed] [Google Scholar]
- Gottwald B, Mihajlovic Z, Wilde B, Mehdorn HM. Does the cerebellum contribute to specific aspects of attention? Neuropsychologia. 2003;41:1452–1460. doi: 10.1016/s0028-3932(03)00090-3. [DOI] [PubMed] [Google Scholar]
- Grill SE, Hallett M, Marcus C, McShane L. Disturbances of kinaesthesia in patients with cerebellar disorders. Brain. 1994;117:1433–1447. doi: 10.1093/brain/117.6.1433. [DOI] [PubMed] [Google Scholar]
- Grondin S. Duration discrimination of empty and filled intervals marked by auditory and visual signals. Perception and Psychophysics. 1993;54:383–394. doi: 10.3758/bf03205274. [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Clarke JM, Heit G, Marinkovic K, Devaux B, et al. Intracerebral potentials to rare target and distractor auditory and visual stimuli. II. Medial, lateral and posterior temporal lobe. Electroencephalography and Clinical Neurophysiology. 1995;94:229–250. doi: 10.1016/0013-4694(95)98475-n. [DOI] [PubMed] [Google Scholar]
- Hallett M, Grafman J. Executive function and motor skill learning. In: Schmahmann JD, editor. The cerebellum and cognition (International Review of Neurobiology, vol. 41) Academic Press; 1997. pp. 297–323. [DOI] [PubMed] [Google Scholar]
- Ito M. Bases and implications of learning in the cerebellum – adaptive control and internal model mechanism. Progress in Brain Research. 2005;148:95–109. doi: 10.1016/S0079-6123(04)48009-1. [DOI] [PubMed] [Google Scholar]
- Ivry R. Cerebellar timing systems. In: Schmahmann JD, editor. The cerebellum and cognition (International Review of Neurobiology, vol. 41) Academic Press; 1997. pp. 555–573. [PubMed] [Google Scholar]
- Ivry R. Exploring the role of the cerebellum in sensory anticipation and timing: commentary on Tesche and Karhuc. Human Brain Mapping. 2000;9:115–118. doi: 10.1002/(SICI)1097-0193(200003)9:3<115::AID-HBM1>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivry RB, Diener HC. Impaired velocity perception in patients with lesion of the cerebellum. Journal of Cognitive Neuroscience. 1991;3:355–366. doi: 10.1162/jocn.1991.3.4.355. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Fiez JA. Cerebellar contributions to cognition and imagery. In: Gazzaniga MS, editor. The new cognitive neurosciences. 2nd ed. Cambridge, MA: The MIT press; 2000. pp. 999–1011. [Google Scholar]
- Ivry RB, Keele SW. Timing functions of the cerebellum. Journal of Cognitive Neuroscience. 1989;1:136–152. doi: 10.1162/jocn.1989.1.2.136. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Keele SW, Diener HC. Dissociation of the lateral and medial cerebellum in movement timing and movement execution. Experimental Brain Research. 1988;73:167–180. doi: 10.1007/BF00279670. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Spencer RM, Zelaznik HN, Diedrichsen J. The cerebellum and event timing. Annals of the New York Academy of Sciences. 2002;978:302–317. doi: 10.1111/j.1749-6632.2002.tb07576.x. [DOI] [PubMed] [Google Scholar]
- Jacobsen T, Schroger E. Measuring duration mismatch negativity. Clinical Neurophysiology. 2003;114:1133–1143. doi: 10.1016/s1388-2457(03)00043-9. [DOI] [PubMed] [Google Scholar]
- Klockgether T, Skalej M, Wedekind D, Luft AR, Welte D, Schulz JB, et al. Autosomal dominant cerebellar ataxia type I. MRI-based volumetry of posterior fossa structures and basal ganglia in spinocerebellar ataxia types 1, 2 and 3. Brain. 1998;121:1687–1693. doi: 10.1093/brain/121.9.1687. [DOI] [PubMed] [Google Scholar]
- Koekkoek SK, Hulscher HC, Dortland BR, Hensbroek RA, Elgersma Y, Ruigrok TJ, et al. Cerebellar LTD and learning-dependent timing of conditioned eyelid responses. Science. 2003;301:1736–1739. doi: 10.1126/science.1088383. [DOI] [PubMed] [Google Scholar]
- Lifshitz S. Two integral laws of sound perception relating loudness and apparent duration to sound impulses. Journal of the Acoustical Society of America. 1933;5:31–33. [Google Scholar]
- Mangels JA, Ivry RB, Shimizu N. Dissociable contributions of the prefrontal and neocerebellar cortex to time perception. Cognitive Brain Research. 1998;7:15–39. doi: 10.1016/s0926-6410(98)00005-6. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Research Reviews. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Molholm S, Martinez A, Ritter W, Javitt DC, Foxe JJ. The neural circuitry of pre-attentive auditory change-detection: an fMRI study of pitch and duration mismatch negativity generators. Cerebral Cortex. 2005;15:545–551. doi: 10.1093/cercor/bhh155. [DOI] [PubMed] [Google Scholar]
- Molinari M, Leggio MG, Silveri MC. Verbal fluency and agrammatism. In: Schmahmann JD, editor. The cerebellum and cognition (International Review of Neurobiology, vol. 41) Academic Press; 1997. pp. 325–339. [DOI] [PubMed] [Google Scholar]
- Näätänen R. Mismatch negativity: clinical research and possible applications. International Journal of Psychophysiology. 2003;48:179–188. doi: 10.1016/s0167-8760(03)00053-9. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Alho K. Mismatch negativity--the measure for central sound representation accuracy. Audiology and Neurootology. 1997;2:341–353. doi: 10.1159/000259255. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Jacobsen T, Winkler I. Memory-based or afferent processes in mismatch negativity (MMN): a review of the evidence. Psychophysiology. 2005;42:25–32. doi: 10.1111/j.1469-8986.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Pakarinen S, Rinne T, Takegata R. The mismatch negativity (MMN): towards the optimal paradigm. Clinical Neurophysiology. 2004;115:140–144. doi: 10.1016/j.clinph.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Paavilainen P, Rinne T, Alho K. The mismatch negativity (MMN) in basic research of central auditory processing: A review. Clinical Neurophysiology. 2007;118:2544–2590. doi: 10.1016/j.clinph.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Tervaniemi M, Sussman E, Paavilainen P, Winkler I. "Primitive intelligence" in the auditory cortex. Trends in Neuroscience. 2001;24:283–288. doi: 10.1016/s0166-2236(00)01790-2. [DOI] [PubMed] [Google Scholar]
- Nawrot M, Rizzo M. Motion perception deficits from midline cerebellar lesions in human. Vision Research. 1995;35:723–731. doi: 10.1016/0042-6989(94)00168-l. [DOI] [PubMed] [Google Scholar]
- Nichelli P, Alway D, Grafman J. Perceptual timing in cerebellar degeneration. Neuropsychologia. 1996;34:863–871. doi: 10.1016/0028-3932(96)00001-2. [DOI] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ, Dean P. Developmental dyslexia: the cerebellar deficit hypothesis. Trends in Neuroscience. 2001;24:508–511. doi: 10.1016/s0166-2236(00)01896-8. [DOI] [PubMed] [Google Scholar]
- Pakarinen S, Takegata R, Rinne T, Huotilainen M, Näätänen R. Measurement of extensive auditory discrimination profiles using the mismatch negativity (MMN) of the auditory event-related potential (ERP) Clinical Neurophysiology. 2007;118:177–185. doi: 10.1016/j.clinph.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Parsons LM, Fox PT. Sensory and cognitive functions. In: Schmahmann JD, editor. The cerebellum and cognition (International Review of Neurobiology, vol. 41) Academic Press; 1997. pp. 255–271. [DOI] [PubMed] [Google Scholar]
- Paulin MG. Neural representations of moving systems. In: Schmahmann JD, editor. The cerebellum and cognition (International Review of Neurobiology, vol. 41) Academic Press; 1997. pp. 515–533. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Simons RF, Graham FK. Prepulse effects as a function of cortical projection system. Biological Psychology. 2001;56:81–111. doi: 10.1016/s0301-0511(01)00075-8. [DOI] [PubMed] [Google Scholar]
- Perrett SP, Ruiz BP, Mauk MD. Cerebellar cortex lesions disrupt learning-dependent timing of conditioned eyelid responses. Journal of Neuroscience. 1993;13:1708–1718. doi: 10.1523/JNEUROSCI.13-04-01708.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nature Reviews Neuroscience. 2007;7:511–522. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Behrens TE, Johansen-Berg H, Richter MC, Pinsk MA, Andersson JL, et al. The evolution of prefrontal inputs to the cortico-pontine system: diffusion imaging evidence from Macaque monkeys and humans. Cerebral Cortex. 2006;16:811–818. doi: 10.1093/cercor/bhj024. [DOI] [PubMed] [Google Scholar]
- Restuccia D, Della Marca G, Valeriani M, Leggio MG, Molinari M. Cerebellar damage impairs detection of somatosensory input changes. A somatosensory mismatch-negativity study. Brain. 2007;130:276–287. doi: 10.1093/brain/awl236. [DOI] [PubMed] [Google Scholar]
- Rosburg T. Left hemispheric dipole locations of the neuromagnetic mismatch negativity to frequency, intensity and duration deviants. Cognitive Brain Research. 2003;16:83–90. doi: 10.1016/s0926-6410(02)00222-7. [DOI] [PubMed] [Google Scholar]
- Ruusuvirta T, Korhonen T, Arikoski J, Kivirikko K. ERPs to pitch changes: a result of reduced responses to standard tones in rabbits. Neuroreport. 1996;7:413–416. [PubMed] [Google Scholar]
- Sallinen M, Lyytinen H. Mismatch negativity during objective and subjective sleepiness. Psychophysiology. 1997;34:694–702. doi: 10.1111/j.1469-8986.1997.tb02144.x. [DOI] [PubMed] [Google Scholar]
- Schall U, Johnston P, Todd J, Ward PB, Michie PT. Functional neuroanatomy of auditory mismatch processing: an event-related fMRI study of duration-deviant oddballs. Neuroimage. 2003;20:729–736. doi: 10.1016/S1053-8119(03)00398-7. [DOI] [PubMed] [Google Scholar]
- Scharf B. Loudness. In: Carterette EC, editor. Handbook of Perception. New York: Academic; 1978. pp. 187–242. [Google Scholar]
- Schmahmann JD, editor. The Cerebellum and Cognition (International Review of Neurobiology, vol. 41) Academic Press; 1997. [Google Scholar]
- Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Spencer RM, Zelaznik HN, Diedrichsen J, Ivry RB. Disrupted timing of discontinuous but not continuous movements by cerebellar lesions. Science. 2003;300:1437–1439. doi: 10.1126/science.1083661. [DOI] [PubMed] [Google Scholar]
- Takegata R, Morotomi T. Integrated neural representation of sound and temporal features in human auditory sensory memory: an event-related potential study. Neuroscience Letters. 1999;274:207–210. doi: 10.1016/s0304-3940(99)00711-9. [DOI] [PubMed] [Google Scholar]
- Tata MS, Ward LM. Early phase of spatial mismatch negativity is localized to a posterior "where" auditory pathway. Experimental Brain Research. 2005;167:481–486. doi: 10.1007/s00221-005-0183-y. [DOI] [PubMed] [Google Scholar]
- Tervaniemi M, Maury S, Näätänen R. Neural representations of abstract stimulus features in the human brain as reflected by the mismatch negativity. Neuroreport. 1994;5:844–846. doi: 10.1097/00001756-199403000-00027. [DOI] [PubMed] [Google Scholar]
- Tesche CD, Karhu JJ. Anticipatory cerebellar responses during somatosensory omission in man. Human Brain Mapping. 2000;9:119–142. doi: 10.1002/(SICI)1097-0193(200003)9:3<119::AID-HBM2>3.0.CO;2-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thach WT. What is the role of the cerebellum in motor learning and cognition? Trends in Cognitive Science. 1998;2:331–337. doi: 10.1016/s1364-6613(98)01223-6. [DOI] [PubMed] [Google Scholar]
- Tiitinen H, May P, Reinikainen K, Näätänen R. Attentive novelty detection in humans is governed by pre-attentive sensory memory. Nature. 1994;372:90–92. doi: 10.1038/372090a0. [DOI] [PubMed] [Google Scholar]
- Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, et al. International Cooperative Ataxia Rating Scale for pharmaco-logical assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. Journal of the Neurological Sciences. 1997;145:205–211. doi: 10.1016/s0022-510x(96)00231-6. [DOI] [PubMed] [Google Scholar]
- Tse CY, Tien KR, Penney TB. Event-related optical imaging reveals the temporal dynamics of right temporal and frontal cortex activation in pre-attentive change detection. Neuroimage. 2006;29:314–320. doi: 10.1016/j.neuroimage.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Winkler I. Interpreting the mismatch negativity (MMN) Journal of Psychophysiology. 2008;21:147–163. [Google Scholar]
- Wolpert DM, Miall RC, Kawato M. Internal models in the cerebellum. Trends in Cognitive Science. 1998;2:338–347. doi: 10.1016/s1364-6613(98)01221-2. [DOI] [PubMed] [Google Scholar]
- Yago E, Corral MJ, Escera C. Activation of brain mechanisms of attention switching as a function of auditory frequency change. Neuroreport. 2001;12:4093–4097. doi: 10.1097/00001756-200112210-00046. [DOI] [PubMed] [Google Scholar]
- Yeo CH, Hesslow G. Cerebellum and conditioned reflexes. Trends in Cognitive Science. 1998;2:338–347. doi: 10.1016/s1364-6613(98)01219-4. [DOI] [PubMed] [Google Scholar]