Abstract
Rationale: Bronchiolitis during infancy is associated with an increased risk of childhood asthma. Whether winter viral infections cause asthma or are a manifestation of a predisposition to asthma development is unknown.
Objectives: To study the relationship of winter virus infection during infancy and the development of childhood asthma.
Methods: We studied over 95,000 infants born between 1995 and 2000 and followed through 2005 who were enrolled in the Tennessee Medicaid program from birth through early childhood to determine whether infant birth in relationship to the winter virus peak alters the risk of developing early childhood asthma.
Measurements and Main Results: Among 95,310 children studied during five winter virus seasons from birth through early childhood, the risk of developing asthma tracked with the timing of infant birth in relationship to the winter virus peak. Infant birth approximately 4 months before the winter virus peak carried the highest risk, with a 29% increase in odds of developing asthma compared with birth 12 months before the peak (adjusted odds ratio, 1.29; 95% confidence interval, 1.19–1.40). Infant age at the winter virus peak was comparable to or greater than other known risk factors for asthma.
Conclusions: Timing of birth in relationship to winter virus season confers a differential and definable risk of developing early childhood asthma, establishing winter virus seasonality as a causal factor in asthma development. Delay of exposure or prevention of winter viral infection during early infancy could prevent asthma.
Keywords: asthma, respiratory winter virus infection, timing of birth
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Bronchiolitis during infancy is associated with an increased risk of childhood asthma. Whether common respiratory viruses, which infect all infants, cause asthma or whether infection is a marker of those predisposed to developing asthma is unknown.
What This Study Adds to the Field
Timing of birth in relationship to the annual winter virus peak predicts an infant's likelihood of developing childhood asthma. This study provides strong evidence for a causal relationship of winter viruses with early childhood asthma.
Worldwide, the prevalence of asthma rose 100% from 1985 to 2001. About 300 million people have asthma, 255,000 die from it, and deaths could increase by 20% in the next 10 years (1). The problem is severe worldwide, and developing countries are particularly burdened (2). Efforts to find effective and targeted primary and secondary asthma prevention measures are needed.
One potentially modifiable environmental factor associated with asthma development is infant respiratory viral infections. However, whether infant bronchiolitis causes asthma or serves as a marker for those genetically predisposed to develop disease is unknown. This is an important question to address, however, as if it is causal, prevention is possible. Because it is not ethical to randomize infants to early viral exposure or not and because a population-based incidence study would be labor consuming and costly, observational study designs are needed that can answer the question of whether bronchiolitis causes asthma or is a marker of a child with asthma.
The relationship between viral infections and asthma is important from two perspectives. First, it is well established that among infants who require hospitalization for viral bronchiolitis, up to 43% develop asthma by 13 years of age, whereas the estimated prevalence in the U.S. population is approximately 8 to 10% (3–8). Second, viral infections are the most frequent and important cause of asthma exacerbations in children, implicated in up to 85% of disease exacerbations (9–11). Respiratory viral infections during infancy may have an acute and long-term impact on lung and immune system development, resulting in an increased risk for childhood asthma (10, 12–20).
The risk of respiratory syncytial virus (RSV) bronchiolitis is associated with seasonality of birth (21, 22). If similar birth timing determines asthma risk, this would suggest that bronchiolitis or some factor closely associated with bronchiolitis causes asthma. We hypothesized that infant age at the winter virus peak would confer a differential and definable risk of developing early childhood asthma. To test this hypothesis, we conducted an investigation of over 95,000 children born between 1995 and 2000 and followed until early childhood through 2005. Some of the results of these studies have been previously reported in the form of abstracts (23, 24).
MATERIALS AND METHODS
Subjects
We conducted a population-based birth cohort study of 95,310 children who were born in Tennessee from 1995 to 2000 and continuously enrolled in Tennessee Medicaid (TennCare) until 5.5 years of age through 2005, representing 25% of the annual births in Tennessee. Asthma was defined in two ways. First, early childhood asthma was determined between 3.5 and 5.5 years of age using an established algorithm incorporating diagnostic coding (ICD-9 code of 493 in any fields for inpatient, other hospital care, or outpatient physician visit claims) and asthma-specific medication use (25–27). To identify children with significant asthma-related morbidity, children with early childhood asthma who had a history of one or more hospitalizations or visits to an emergency department for asthma or who were given a prescription for a course of corticosteroids as rescue therapy between 4 and 5.5 years of age were classified as having high-risk asthma. Infant birth in relationship to the winter virus peak during the infant's first year of life was the primary predictor. Infant birth in relationship to the winter virus peak was defined for each infant as the infant's age in days from their birth to the first winter virus peak. Because bronchiolitis hospitalizations correlate strongly with the circulation of RSV (28), the annual winter virus peak was defined as the first day of the week with the highest number of bronchiolitis hospitalizations for that winter season. The protocol was approved by the Institutional Review Boards of Vanderbilt University and the Tennessee Department of Health.
Statistical Analysis
Univariate and multivariable logistic regression models were constructed to analyze the relationship of infant age in days at the winter virus peak with the risk of early childhood asthma. Results follow for the group with high-risk asthma determined between ages 4 and 5.5 years; the identical analyses for those with early childhood asthma defined using asthma-specific medications and clinical encounters are presented in the on-line supplement. Restricted cubic splines with four knots were applied to best fit the nonlinear association of infant age at the winter virus peak with childhood asthma and infant bronchiolitis (29). Subgroup analysis of the relationship of infant age in days at the winter virus peak with the risk of childhood asthma was further assessed in subgoups of infants who had experienced clinically significant bronchiolitis or not and subgroups of infants who were born at term (≥37 weeks) or preterm (<37 weeks). Subgroup analyses of children who encountered an early winter viral peak (before December 31) or a late winter viral peak (after January 1) were done, and the relationship of childhood high-risk asthma and infant age at the winter virus peak was assessed. We assessed for interactions between infant age at the winter virus peak and other covariates by including cross products in the model. The 95% confidence intervals (CI) for the infant age at the winter virus peak associated with the highest risk to develop asthma and with the highest bronchiolitis risk were estimated using a bootstrap method (29). A nested subgroup in whom maternal history was available served as the cohort to assess the interaction between infant age at the winter virus peak and the familial risk factor of maternal asthma. Rates of bronchiolitis visits per 1,000 children were calculated for children born in each study season, and the cumulative incidence of current high-risk childhood asthma, offset by 5 years, was graphically represented. The asthma status of children who were born before 1997 and were at least 10 years of age by 2006 was ascertained to determine if similar proportions of children met the definition of asthma at 6 to 10 and 8 to 10 years of age. R-software version 2.6.1 (www.r-project.org) and SAS version 9.1 (SAS Institutes, Cary, NC) were used for data analyses. We used a two-sided 5% significance level for all statistical inferences.
RESULTS
Among the cohort of 95,310 children, the median gestational age was 39.1 weeks, and the median birth weight was 3,203 g (Table 1). Thirteen percent of children had a gestational age less than 37 weeks. The children were from urban (48%), suburban (23%), and rural regions (29%) of the state. Fifty-eight percent of children had at least one living sibling. Twenty percent of children experienced at least one episode of clinically significant bronchiolitis during infancy. Among them, 6% had a hospital visit, 5% had an emergency department visit, and 9% had an outpatient visit (Table 1). Eight percent of study children met the criteria for childhood high-risk asthma. Children with high-risk asthma were more likely to be white and male, to have had a bronchiolitis medical encounter during infancy, and to have mothers who smoked during pregnancy, compared with non-highrisk asthma children. Forty-nine percent (1,058/2,172) of children categorized as high-risk asthma by 5.5 years of age were still enrolled in TennCare by 10 years of age. Among them, 66% met the asthma criteria between 6 and 8 years of age, and 62% met the asthma criteria between 8 and 10 years of age.
TABLE 1.
INFANT AND MATERNAL CHARACTERISTICS OF A COHORT OF CHILDREN BORN IN TENNESSEE BETWEEN 1995 AND 2000 AND ENROLLED IN TENNESSEE MEDICAID THROUGH FIVE-AND-A-HALF YEARS, BY CHILDHOOD ASTHMA (N = 95,310)
| Early Childhood Asthma* (n = 14,074 [14.77%])
|
High-Risk Childhood Asthma† (n = 7,833 [8.22%])
|
No Childhood Asthma (n = 81,236 [85.23%])
|
||||
|---|---|---|---|---|---|---|
| Characteristics | n | % (95% CI)‡ | n | % (95% CI)‡ | n | % (95% CI) |
| Infant race | ||||||
| White | 8,194 | 58.2 (57.4, 59.0) | 4,374 | 55.8 (54.7, 56.9) | 42,757 | 52.6 (52.3, 53.0) |
| Black | 4,857 | 34.5 (33.7, 35.3) | 2,865 | 36.6 (35.5, 37.6) | 32,882 | 40.5 (40.1, 40.8) |
| Other | 1,023 | 7.3 (6.8, 7.7) | 594 | 7.6 (7.0, 8.2) | 5,597 | 6.9 (6.7, 7.1) |
| Infant sex | ||||||
| Male | 8,236 | 58.5 (57.7, 59.3) | 4,722 | 60.3 (59.2, 61.4) | 40,659 | 50.1 (49.7, 50.4) |
| Female | 5,838 | 41.5 (40.7, 42.3) | 3,111 | 39.7 (38.6, 40.8) | 40,577 | 49.9 (49.6, 50.3) |
| Bronchiolitis during infancy | ||||||
| None reported | 9,542 | 67.8 (67.0, 68.6) | 5,196 | 66.3 (65.3, 67.4) | 67,152 | 82.7 (82.4, 82.9) |
| Outpatient visit only | 1,902 | 13.5 (13.0, 14.1) | 1,068 | 13.6 (12.9, 14.4) | 6,720 | 8.3 (8.1, 8.5) |
| Emergency department visit only | 1,035 | 7.4 (6.9, 7.8) | 588 | 7.5 (6.9, 8.1) | 3,232 | 4.0 (3.8, 4.1) |
| Hospitalization visit | 1,595 | 11.3 (10.8, 11.9) | 981 | 12.5 (11.8, 13.3) | 1,595 | 2.0 (1.9, 2.1) |
| Siblings (n = 95,218) | ||||||
| None | 6,068 | 43.2 (42.3, 44.0) | 3,368 | 43.1 (42.0, 44.2) | 33,506 | 41.3 (40.9, 41.6) |
| One or more | 7,990 | 56.8 (56.0, 57.6) | 4,453 | 56.9 (55.8, 58.0) | 47,654 | 58.7 (58.4, 59.1) |
| Region of residence (n = 94,428) | ||||||
| Urban | 5,944 | 42.5 (41.8, 42.6) | 3,334 | 42.9 (41.8, 44.0) | 39,611 | 49.2 (48.9, 49.6) |
| Suburban | 3,494 | 25.0 (24.3, 25.8) | 1,919 | 24.7 (23.7, 25.7) | 18,020 | 22.4 (22.1, 22.7) |
| Rural | 4,521 | 32.4 (31.6, 33.2) | 2,516 | 32.4 (31.3, 33.4) | 22,838 | 28.4 (28.1, 28.7) |
| Maternal marital status (n = 95,308) | ||||||
| Single | 8,728 | 62.0 (61.2, 62.8) | 4,966 | 63.4 (62.3, 64.5) | 52,974 | 65.2 (64.9, 65.5) |
| Married | 5,345 | 38.0 (37.2, 38.8) | 2,867 | 36.6 (35.5, 37.7) | 28,261 | 34.8 (34.5, 35.1) |
| Maternal smoking during pregnancy (n = 95,022) | ||||||
| No | 9,912 | 70.6 (69.9, 71.4) | 5,561 | 71.2 (70.2, 72.2) | 59,690 | 73.7 (73.4, 74.0) |
| Yes | 4,124 | 29.4 (28.6, 30.1) | 2,250 | 28.8 (27.8, 29.8) | 21,296 | 26.3 (26.0, 26.6) |
| Maternal education level (n = 95,133) | ||||||
| <12 yr | 5,919 | 42.1 (41.3, 42.9) | 3,378 | 43.2 (42.1, 44.3) | 36,330 | 44.8 (44.5, 45.2) |
| 12 yr | 6,312 | 44.9 (44.1, 45.7) | 3,419 | 43.7 (42.6, 44.8) | 35,251 | 43.4 (43.1, 43.8) |
| >12 yr | 1,823 | 13.0 (12.4, 13.5) | 1,023 | 13.1 (12.3, 13.8) | 9,498 | 11.7 (11.5, 11.9) |
| Birth weight, g (n = 95,304) | 14,074 | 3,203 (2,835, 3,544)§ | 7,833 | 3,203 (2,835, 3,544)§ | 81,230 | 3,203 (2,863, 3,544)§ |
| Gestational age, wk (n = 95,148) | 14,041 | 39.1 (38.1, 40.1)§ | 7,815 | 39.1 (38.1, 40.1)§ | 81,107 | 39.1 (38.1, 40.1)§ |
| Maternal age, yr (n = 95,125) | 14,042 | 22 (19, 26)§ | 7,806 | 22 (19, 26)§ | 81,093 | 22 (19, 26)§ |
Definition of abbreviations: CI = confidence interval. IQR = interquartile range.
Early childhood asthma is defined by ICD-9 codes for asthma care visits and/or asthma-specific medication use between the ages of 3.5 to 5.5 years.
High-risk childhood asthma is defined among the subset of children with early childhood asthma who experienced a hospitalization, emergency department visit, or course of systematic rescue corticosteroids for asthma between the ages of 4.0 to 5.5 years.
A nonoverlapping 95% CI of percentages for early childhood asthma or high-risk childhood asthma when compared with the no childhood asthma group indicates a statistically significant difference (P < 0.05) between early childhood asthma, high-risk childhood asthma, and no childhood asthma.
Values are expressed as median (IQR).
Among mothers, the median maternal age was 23 years, 65% of women were single, and 44% had less than a high school degree. There were 54,235 (56.9%) children whose mothers also received medical insurance coverage through TennCare at least 180 days before their last menstrual period and throughout their pregnancy and whose medical histories were therefore available. Overall, 3,147 (5.8%) of these women had asthma. Children born to women with asthma were significantly more likely to be classified as having asthma when compared with children whose mothers did not have asthma (14% versus 8%).
To determine the seasonal pattern of bronchiolitis, we studied the 46,613 bronchiolitis hospitalizations among the 426,385 infants who were born during the period July 1994 to June 2001 and were ever enrolled in TennCare during the study period. The weekly frequencies of bronchiolitis hospitalizations were determined for each season (see Figure E1 in the online supplement). The winter virus peak, as determined by bronchiolitis hospitalizations, occurred between December and February of each year (Figure E1). During the study period (1995–2000), the date of the winter virus peak varied by up to 6 weeks. Six analyses follow that define the effect of timing of infant birth in relationship to the winter virus peak, defined as infant age in days from birth to the winter virus peak, on the development of high-risk childhood asthma.
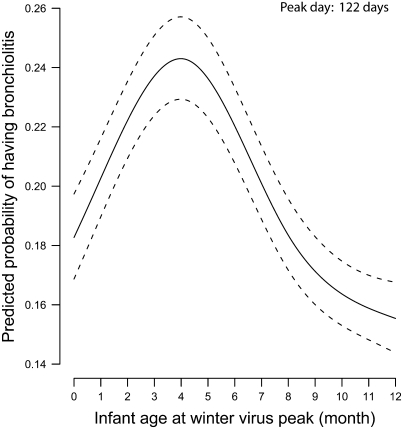
First, timing of infant birth in relationship to the winter virus peak predicted the likelihood of developing clinically significant bronchiolitis, defined as a hospitalization, emergency department visit, or outpatient visit for bronchiolitis (P < 0.001) (Figure 1). Infants who were 122 days of age (95% CI, 118–126) at the winter virus peak had the highest risk of developing clinically significant bronchiolitis after adjusting for gender, race, number of living siblings, birth weight, gestational age, maternal smoking, marital status, maternal education, region of residence, and season.
Figure 1.
Predicted probability and 95% confidence intervals of bronchiolitis requiring a health care visit during infancy (hospitalization, emergency department visit, or outpatient visit) by infant age in months at the winter virus peak (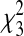 = 345.52; P < 0·001). Results were obtained from a multivariable logistic regression model. Effect was adjusted for gender, infant race, birth weight, gestational age, number of living siblings, region of residence, maternal smoking, marital status, maternal education, and season.
= 345.52; P < 0·001). Results were obtained from a multivariable logistic regression model. Effect was adjusted for gender, infant race, birth weight, gestational age, number of living siblings, region of residence, maternal smoking, marital status, maternal education, and season.
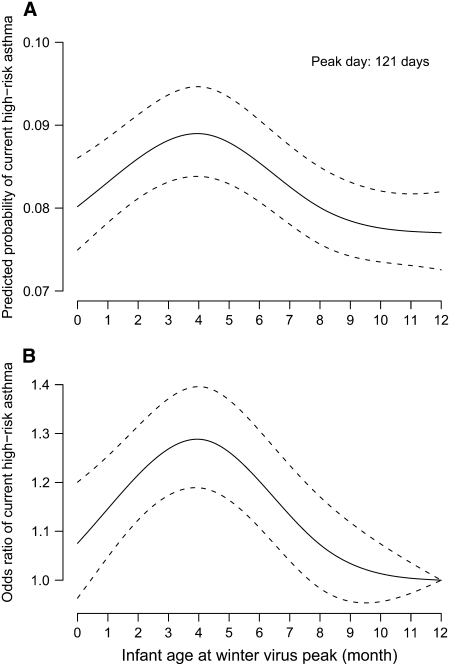
Second, timing of infant birth in relationship to the winter virus peak predicted the likelihood of developing childhood asthma. This observation persisted after adjusting for the above-mentioned covariates (P < 0.001) (Figure 2A). Children who were 121 days of age (95% CI, 108–131) at the winter virus peak had the highest risk of developing high-risk childhood asthma compared with children who were younger or older at the winter virus peak. This relationship was unchanged in a subgroup of children (n = 54,235; 57%) in whom maternal history was available and for whom maternal asthma could be ascertained and adjusted for in the model. Subgroup analysis of children who encountered early winter virus peaks and children who encountered late winter virus peaks showed the same relationship of infant age at the winter virus peak on childhood asthma (P < 0.001 for both analyses).
Figure 2.
Differential risk of developing current high-risk childhood asthma in relationship to infant age at the winter virus peak. Results were obtained from a multivariable logistic regression model adjusted for gender, infant race, birth weight, gestational age, number of living siblings, region of residence, maternal smoking, marital status, maternal education, and season. (A) Predicted probability and 95% confidence intervals (CI) of developing current high-risk childhood asthma by infant age in months at the winter virus peak (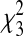 = 49.05; P < 0·001). The area under the curve is equal to the asthma prevalence of the population. (B) Adjusted odds ratio and 95% CI of developing current high-risk childhood asthma relative to children who were 12 months of age at the winter virus peak. Infants who were 1 year of age at the winter virus peak served as the reference group.
= 49.05; P < 0·001). The area under the curve is equal to the asthma prevalence of the population. (B) Adjusted odds ratio and 95% CI of developing current high-risk childhood asthma relative to children who were 12 months of age at the winter virus peak. Infants who were 1 year of age at the winter virus peak served as the reference group.
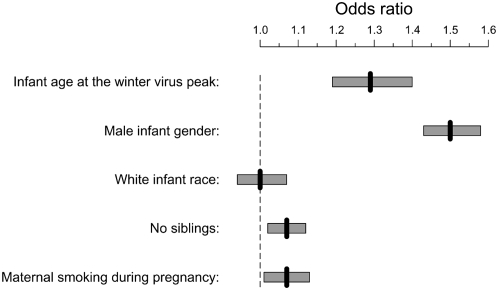
Third, there was a 29% increase in the odds of developing high-risk childhood asthma for children who were 121 days of age at the winter virus peak compared with infants who were 365 days of age at the peak (adjusted odds ratio [AOR], 1.29; 95% CI, 1.19–1.40) (Figures 2B and 3), which is nearly identical to the infant age at the winter virus peak that conferred the highest risk of bronchiolitis. Except for maternal asthma (AOR, 1.82; 95% CI, 1.64–2.03) and infant gender (AOR, 1.50; 95% CI, 1.43–1.58), the maximum relative odds of infant age at the winter virus peak was greater than that of previously established risk factors, such as white race (AOR, 1.00; 95% CI, 0.94–1.07), no siblings (AOR, 1.07; 95% CI, 1.02–1.12), and maternal smoking (AOR, 1.07; 95% CI, 1.01–1.13) (Figure 3).
Figure 3.
Adjusted maximum relative odds ratio and 95% confidence intervals (CI) of developing high-risk childhood asthma by infant age at the winter virus peak compared with other recognized asthma risk factors. Adjusted odds ratios (AOR) were obtained from a multivariable logistic regression model that adjusted for gender, infant race, birth weight, gestational age, number of living siblings, region of residence, maternal smoking, marital status, maternal education, and season. The comparison groups for the variables were infant age 121 days versus 365 days at winter virus peak, male versus female, white versus black, no siblings versus at least one living sibling, and smoking versus no smoking during pregnancy. Among the subgroup of infants in whom maternal history was available (n = 54,235), the AOR of developing high-risk childhood asthma by maternal asthma was 1.82 (95% CI, 1.64–2.03).
Fourth, having clinically significant bronchiolitis at any age during infancy was associated with an increased risk of developing high-risk childhood asthma (P < 0.001). In the 20% of children with a health care visit for bronchiolitis during infancy (n = 18,616), the age at the time of their first bronchiolitis health care visit had no effect on the risk of developing high-risk childhood asthma (P = 0.73). In contrast, the age at the winter virus peak was associated with increased odds of developing high-risk childhood asthma. The overall patterns of infant age at the winter virus peak associated with high-risk childhood asthma were similar among infants who did and those who did not have clinically significant bronchiolitis during infancy. However, the proportion who developed asthma among those with bronchiolitis was substantially higher (Figure E3).
Fifth, neither maternal smoking nor maternal asthma significantly modified the relationship between infant age at the winter virus peak and childhood asthma. However, smoking during pregnancy and maternal asthma increased the odds of developing high-risk asthma by 7% and 82%, respectively, after adjusting for other risk factors (Figure 3).
Additional interaction effects between infant age at the winter virus peak and other covariates (infant race, gender, birth weight, gestational age, other living siblings, region of residence, and maternal education) were assessed by including cross products in the model. There were no interactions between infant age at the winter virus peak and these covariates on the risk of developing asthma (P > 0.05).
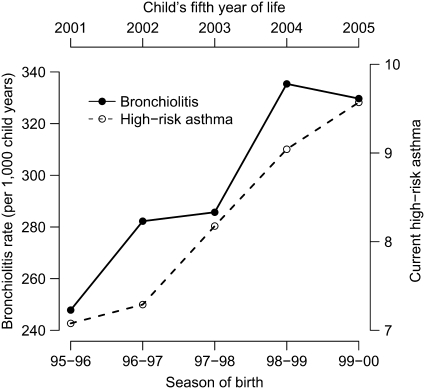
Last, over the five study seasons, the increase in the incidence of bronchiolitis during infancy paralleled the subsequent increase in the cumulative incidence of current high-risk asthma defined between 4 and 5.5 years of age and off-set by 5 years from the birth season in the same cohort of children (Figure 4).
Figure 4.
Bronchiolitis rate (per 1,000 child-years) of each study season (solid line) and current high-risk childhood asthma (dashed line) from 1995 to 2000. The childhood asthma curve is offset by 5 years from that of the bronchiolitis curve to represent the same group of infants who were later 5.5-year-old children.
DISCUSSION
In this large, population-based cohort, we have established that timing of infant birth in relationship to the winter virus peak predicts the likelihood of developing childhood asthma. The following pieces of evidence from our analyses provide compelling support for a causal role of winter viruses in the development of asthma. (1) We confirm the findings of others (21, 22) that infant age at the peak of winter virus activity predicts bronchiolitis, with the highest bronchiolitis risk estimated to be for infants who are approximately 4 months of age at the winter virus peak (122 days; 95% CI, 118–126). (2) We demonstrate for the first time that infant age at the winter virus peak independently predicts asthma development, with the highest risk estimated to be for infants who are 121 days of age (95% CI, 108–131) at the peak, which is nearly identical to the highest-risk age for bronchiolitis, and that this relationship held true across five seasons of study as the winter virus peak shifted by up to 6 weeks in different study years. Similar patterns were observed in both groups of infants who did and did not develop clinically significant bronchiolitis during infancy; however, the magnitude of the effect was much greater for those who developed bronchiolitis. (3) Timing of birth in relationship to the winter virus peak (infant age) conferred a 29% increase in the risk of childhood asthma compared with infants who were 1 year of age at the winter virus peak. (4) We further showed that infant age at the time of a bronchiolitis event did not predict asthma development, suggesting that age at the winter virus peak confers a greater susceptibility to winter viral infection and severe infection when exposed. Thus, children are at higher risk for bronchiolitis around 4 months of age, but bronchiolitis at any age confers a similar and significantly increased risk of developing asthma. (5) We demonstrated that the strength of association of childhood asthma with infant age at the winter virus peak was comparable to or greater than other known risk factors for asthma. Finally, we demonstrated increasing rates of infant bronchiolitis in the last 5 years and the 5-year offset increases in asthma at 5 years of age among the same children (30).
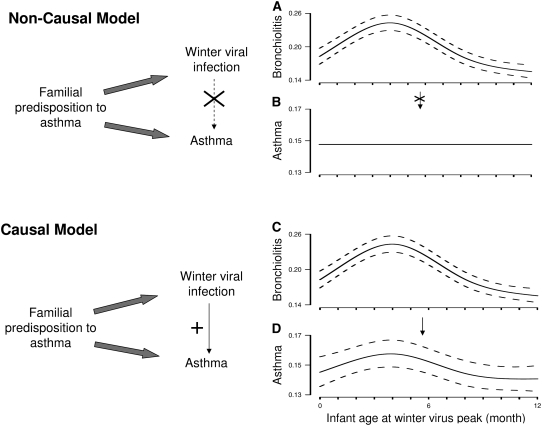
There are two nonmutually exclusive possibilities to explain the relationship between winter virus infection and asthma (Figure 5). The first is that a common genetic predisposition results in severe winter viral infection (bronchiolitis hospitalization) and in asthma. The second is that an environmental exposure such as winter viral infection causes asthma. We have demonstrated that timing of infant birth in relationship to winter virus season relates to the risk of bronchiolitis (Figure 1), early childhood asthma (Figure E2), and high-risk asthma in an identical way (Figure 2A). The risk of progressing from bronchiolitis to asthma is almost certainly influenced by genetic factors; however, if this association were due only to genetic factors, there would be a seasonal effect on infection but not on asthma. In other words, the rate of developing asthma and/or high-risk asthma depending on month of birth should be constant (Figure 5: Non-causal model). Instead, we have shown that there is variation in the risk of developing asthma by timing of birth in relationship to the winter virus peak for every year studied, and this variation is tightly linked to the peak of the first winter virus season after birth even when the timing of the winter virus peak shifts from year to year (Figure 5: Causal model). This supports a causal relationship of childhood asthma with the winter virus peak after birth.
Figure 5.
Noncausal and causal models of the relationship between winter viral infection and early childhood asthma. In the noncausal model, a common familial predisposition to asthma is associated with winter viral infection and asthma and is a confounder of the association between winter viral infection and asthma. Thus, timing of infant birth in relationship to winter virus peak has a seasonal effect on (A) infection but not on (B) asthma. In the causal model, although familial predisposition to asthma relates to winter viral infection and asthma, winter viral infection is in the causal pathway of development of asthma. Timing of infant birth in relationship to winter virus season relates to (C) bronchiolitis and (D) asthma risk in an identical way.
Thus, timing of birth in relationship to winter virus season alters the risk of developing childhood asthma. Depending on the timing of the winter virus peak in a particular study year, birth in August and/or the fall months may confer an increased risk of developing childhood asthma, as shown by Åberg and Wjst and colleagues, because the infant is approximately 4 months of age at the winter virus peak (31, 32). Among our five consecutive seasons, the timing of the winter virus peak varied by up to 6 weeks, and peaks have been demonstrated to vary substantially more in different seasons (33). Because the winter virus peak is known to change by up to 2 months from year to year, it would be expected that the birth date associated with the peak asthma prevalence should also vary from year to year with the peak of winter virus season, which it does for the seasons that we studied. In addition, we used infant age in days at the winter virus peak instead of month or season to capture the continuous relationship of timing of viral exposure and risk of development of childhood asthma. The imprecision in measurements of infant age and the variability in annual winter virus peaks likely explain the different birth months identified to be associated with asthma risk in prior birth month studies (31, 32).
The differential risk of childhood asthma by infant age at the winter virus peak has biologic plausibility. At 4 months of age, infants have lost most maternal antibodies, and infant immunoglobulin G is at its nadir (34–36). Acute lung injury during infancy caused by viral infections is also likely to have chronic airway effects and might affect immune regulation and development (12, 14, 37, 38). Holtzman and colleagues have recently demonstrated that after respiratory paramyxovirus infection (which is similar to RSV) in an experimental mouse model, persistent chronic inflammatory lung disease that resembles asthma develops (39). Furthermore, infants tend to be more sheltered during the first 6 weeks of life and are typically not in daycare. In addition, infants who are 4 months of age at the winter virus peak have increased exposure time throughout the winter virus season before and after the peak. Thus, infants who are 4 months of age at the winter virus peak are more likely to acquire viral infection from an immunologic and an exposure standpoint and are subsequently more likely to develop childhood asthma.
There are several potential limitations of this work. Asthma in early childhood can represent several phenotypes, including transient wheezing, which can resolve generally by 3 or 4 years of age, and IgE-mediated atopic asthma, which persists. Because the diagnosis of asthma in early childhood may represent a prolonged, postviral syndrome and may not represent all children who develop IgE-mediated atopic asthma at 10 years of age or after, we defined asthma in two ways: (1) the use of asthma-specific medication plus asthma-related clinical visits and (2) the presence of the disease during 4 to 5.5 years of age among children who had a hospitalization, emergency department visit, or systemic corticosteroid rescue for asthma. This latter definition uses more stringent criteria in defining asthma associated with increased morbidity. Asthma during childhood, even if transient, is an important problem for children; those who meet the criteria for high-risk asthma have substantial morbidity, and the majority of these children still have asthma at 8 to 10 years of age. However, we believe that it is likely that the relationship between infant respiratory viral infection and asthma inception may be different for children who develop clinical symptoms of asthma later in childhood compared with early childhood, where this environmental exposure is less likely to be an important disease predictor. We used ICD-9 codes and asthma-specific medication use to identify children with asthma; however, this is likely to result in one-way misclassification in missing children with the mildest asthma and classifying those children as nonasthmatics. However, our diagnosis of asthma has been validated and shown to be sensitive and specific in adult populations (25, 26), and the algorithm used is nearly identical to that used by other U.S. Medicaid systems for identifying childhood asthma, having been shown to have 90% sensitivity, 95% specificity, 94% positive predictive value, and 92% negative predictive value in identifying childhood asthma (27). Although our population includes approximately 25% of the annual births in Tennessee (Table E2), there are some demographic differences, such as the study population including a higher proportion of African American subjects, a higher proportion of children with no older siblings, higher urban residency, lower maternal education, and a higher proportion of single mothers. We believe that demographic differences do not impact the relationship between infant viral infection and asthma inception. However, the findings may not be generalizable to other populations, and the results require validation in another population. An additional limitation is that we assumed that infants were most likely exposed to and infected by winter viruses when winter viruses prevail and that infants who were born at the same time of the year in relationship to the winter virus peak have the same risk of being infected. We did this because, although about 70% of infants are infected with RSV during their first year of life (40, 41), only about 20% of infants have clinically significant disease that can be tracked through an outpatient visit or hospitalization for bronchiolitis (42). Next, although recent studies have demonstrated that rhinovirus infection during childhood is a strong predictor of early childhood asthma, we studied the relationship of winter virus infection/exposure because it is the period of greatest respiratory illness morbidity during infancy. The focus on a single viral epidemic period that may not include the peak of each respiratory virus, such as rhinovirus, would attenuate the findings. Additionally, associations observed between infant age at the winter virus peak and childhood asthma may be due to other unknown factors that track with bronchiolitis, such as temperature and light. It seems implausible, however, that those with a genetic predisposition to asthma have differential seasonal fertility or that duration of light affects disease development, particularly because the timing of birth conferring the highest asthma risk predictably shifts in parallel with the winter virus peaks of each year studied. We could not measure other potential confounders, such as daycare attendance and breastfeeding; however, these factors are unlikely to be strongly related to infant age in relationship to the winter virus peak. These results underestimate the impact of winter viruses on early asthma development because not all infants who are approximately 4 months of age at the winter virus peak are infected.
The findings of this study have implications for asthma prevention efforts. Because RSV infection during infancy is nearly ubiquitous, with about 70% of infants infected during their first year of life (40, 41), avoiding winter viral infection during infancy would be difficult. While there are no known interventions to prevent asthma, this study suggests a possible intervention for families whose offspring have a very high risk of asthma because the risk of developing childhood asthma might be altered by delaying infant viral exposure until infants are 9 months of age or older through administration of a vaccine for RSV and/or other winter viruses or RSV immunoprophylaxis. These findings may help to guide immunologic and mechanistic studies to gain insight into differential host response to viral infection during infancy and help define high-risk populations for primary prevention efforts (11, 14).
In summary, we have demonstrated that increasing rates of infant bronchiolitis in the last 10 years parallel the 5-year offset increases in asthma at 5 years of age among these same children. We confirm that infant age at the peak of winter virus activity predicts bronchiolitis, with the highest risk estimated to be age 122 days at the peak, and that bronchiolitis during infancy confers an increased risk of early childhood asthma (3–8), representing a clinical biomarker of developing early childhood asthma. We demonstrate for the first time that timing of birth in relationship to the winter virus peak independently predicts asthma development, with the highest risk estimated to be birth at 121 days before the winter virus peak of any given year. This age confers a 29% increase in risk of developing childhood asthma. These analyses establish seasonality as a causal factor in asthma development, with winter viruses highly likely to be in the causal pathway. Although Simões and colleagues have demonstrated a protective effect of preventing RSV lower respiratory tract infections on subsequent wheezing in a nonrandomized trial (43), a definitive demonstration of a causal relationship between RSV respiratory tract infections and asthma requires a clinical trial of an effective therapy or preventive strategy that proves a reduction in subsequent asthma.
Supplementary Material
Acknowledgments
The authors thank the Tennessee Bureau of TennCare of the Department of Finance and Administration, and the Tennessee Department of Health, Office of Policy, Planning & Assessment, for providing the data.
Supported by research grants from NIH U01 HL 072,471 (T.V.H.), Thrasher Research Fund (P.W., T.V.H. ), and NIH F32 HL 086,048 (P.W., T.V.H.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.200804-579OC on September 5, 2008
Conflict of Interest Statement: P.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. W.D.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.R.G. received an investigator-initiated grant fund from MedImmune. K.N.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. E.F.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. T.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. T.V.H. served on an advisory board for Merck to assist in study design for a RSV-wheezing study and received a total of $750 in advisory board services during 2007.
References
- 1.Asthma Facts from WHO Statistical Information System WHOSIS [accessed June 22, 2008]. Available from: http://www.who.int/respiratory/asthma/en
- 2.Robertson SE, Roca A, Alonso P, Simoes EA, Kartasasmita CB, Olaleye DO, Odaibo GN, Collinson M, Venter M, Zhu Y, et al. Respiratory syncytial virus infection: denominator-based studies in Indonesia, Mozambique, Nigeria and South Africa. Bull World Health Organ 2004;82:914–922. [PMC free article] [PubMed] [Google Scholar]
- 3.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med 2000;161:1501–1507. [DOI] [PubMed] [Google Scholar]
- 4.Sigurs N, Gustafsson PM, Bjarnason R, Lundberg F, Schmidt S, Sigurbergsson F, Kjellman B. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med 2005;171:137–141. [DOI] [PubMed] [Google Scholar]
- 5.Henderson J, Hilliard TN, Sherriff A, Stalker D, Al Shammari N, Thomas HM. Hospitalization for RSV bronchiolitis before 12 months of age and subsequent asthma, atopy and wheeze: a longitudinal birth cohort study. Pediatr Allergy Immunol 2005;16:386–392. [DOI] [PubMed] [Google Scholar]
- 6.Noble V, Murray M, Webb MS, Alexander J, Swarbrick AS, Milner AD. Respiratory status and allergy nine to 10 years after acute bronchiolitis. Arch Dis Child 1997;76:315–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotaniemi-Syrjanen A, Vainionpaa R, Reijonen TM, Waris M, Korhonen K, Korppi M. Rhinovirus-induced wheezing in infancy–the first sign of childhood asthma? J Allergy Clin Immunol 2003;111:66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez FD. Respiratory syncytial virus bronchiolitis and the pathogenesis of childhood asthma. Pediatr Infect Dis J 2003;22:S76–S82. [DOI] [PubMed] [Google Scholar]
- 9.Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, Symington P, O'Toole S, Myint SH, Tyrrell DA. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ 1995;310:1225–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthews SP, Tregoning JS, Coyle AJ, Hussell T, Openshaw PJ. Role of CCL11 in eosinophilic lung disease during respiratory syncytial virus infection. J Virol 2005;79:2050–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Openshaw PJ, Yamaguchi Y, Tregoning JS. Childhood infections, the developing immune system, and the origins of asthma. J Allergy Clin Immunol 2004;114:1275–1277. [DOI] [PubMed] [Google Scholar]
- 12.Gern JE, Rosenthal LA, Sorkness RL, Lemanske RF Jr. Effects of viral respiratory infections on lung development and childhood asthma. J Allergy Clin Immunol 2005;115:668–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh AM, Moore PE, Gern JE, Lemanske RF Jr, Hartert TV. Bronchiolitis to asthma: a review and call for studies of gene-virus interactions in asthma causation. Am J Respir Crit Care Med 2007;175:108–119. [DOI] [PubMed] [Google Scholar]
- 14.Culley FJ, Pollott J, Openshaw PJ. Age at first viral infection determines the pattern of T cell-mediated disease during reinfection in adulthood. J Exp Med 2002;196:1381–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rakes GP, Arruda E, Ingram JM, Hoover GE, Zambrano JC, Hayden FG, Platts-Mills TA, Heymann PW. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care: IgE and eosinophil analyses. Am J Respir Crit Care Med 1999;159:785–790. [DOI] [PubMed] [Google Scholar]
- 16.Saijo M, Takahashi S, Kokubo M, Saino T, In-Yaku F, Ishii T, Takimoto M, Takahashi Y. The role of respiratory syncytial virus in acute bronchiolitis in small children in northern Japan. Acta Paediatr Jpn 1994;36:371–374. [DOI] [PubMed] [Google Scholar]
- 17.Welliver RC, Wong DT, Sun M, Middleton E Jr, Vaughan RS, Ogra PL. The development of respiratory syncytial virus-specific IgE and the release of histamine in nasopharyngeal secretions after infection. N Engl J Med 1981;305:841–846. [DOI] [PubMed] [Google Scholar]
- 18.Welliver RC, Sun M, Rinaldo D, Ogra PL. Predictive value of respiratory syncytial virus-specific IgE responses for recurrent wheezing following bronchiolitis. J Pediatr 1986;109:776–780. [DOI] [PubMed] [Google Scholar]
- 19.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B, Bjorksten B. Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics 1995;95:500–505. [PubMed] [Google Scholar]
- 20.De AA, Walsh EE, Carper HT, La Russa JB, Evans BA, Rakes GP, Platts-Mills TA, Heymann PW. Detection of IgA and IgG but not IgE antibody to respiratory syncytial virus in nasal washes and sera from infants with wheezing. J Pediatr 2001;138:311–317. [DOI] [PubMed] [Google Scholar]
- 21.Boyce TG, Mellen BG, Mitchel EF Jr, Wright PF, Griffin MR. Rates of hospitalization for respiratory syncytial virus infection among children in medicaid. J Pediatr 2000;137:865–870. [DOI] [PubMed] [Google Scholar]
- 22.Holberg CJ, Wright AL, Martinez FD, Ray CG, Taussig LM, Lebowitz MD. Risk factors for respiratory syncytial virus-associated lower respiratory illnesses in the first year of life. Am J Epidemiol 1991;133:1135–1151. [DOI] [PubMed] [Google Scholar]
- 23.Wu P, Gebretsadik T, Dupont WD, Griffin MR, Enriquez R, Carroll KN, Hartert TV. Analysis of respiratory viral exposure and timing of exposure during infancy on the development of childhood asthma [abstract]. Joint Statistical Meeting; August 6–10, 2006; Seattle, WA.
- 24.Wu P, Griffin MR, Carroll KN, Mitchel EF, Enriquez R, Gebretsadik T, Dupont WD, Hartert TV. Timing of infant viral exposure on the risk of childhood asthma [abstract]. Proc Am Thorac Soc 2007;4:A80. [Google Scholar]
- 25.Hartert TV, Togias A, Mellen BG, Mitchel EF, Snowden MS, Griffin MR. Underutilization of controller and rescue medications among older adults with asthma requiring hospital care. J Am Geriatr Soc 2000;48:651–657. [DOI] [PubMed] [Google Scholar]
- 26.Hartert TV, Neuzil KM, Shintani AK, Mitchel EF Jr, Snowden MS, Wood LB, Dittus RS, Griffin MR. Maternal morbidity and perinatal outcomes among pregnant women with respiratory hospitalizations during influenza season. Am J Obstet Gynecol 2003;189:1705–1712. [DOI] [PubMed] [Google Scholar]
- 27.Wakefield DB, Cloutier MM. Modifications to HEDIS and CSTE algorithms improve case recognition of pediatric asthma. Pediatr Pulmonol 2006;41:962–971. [DOI] [PubMed] [Google Scholar]
- 28.Shay DK, Holman RC, Roosevelt GE, Clarke MJ, Anderson LJ. Bronchiolitis-associated mortality and estimates of respiratory syncytial virus-associated deaths among US children, 1979–1997. J Infect Dis 2001;183:16–22. [DOI] [PubMed] [Google Scholar]
- 29.Harrell FE. Regression modeling strategies. New York: Springer; 2001.
- 30.Carroll KN, Gebretsadik T, Griffin MR, Wu P, Dupont WD, Mitchel E, Enriquez R, Hartert TV. Increasing burden and risk factors for bronchiolitis-related medical visits in infants enrolled in a state health care insurance plan. Pediatrics 2008;122:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aberg N. Birth season variation in asthma and allergic rhinitis. Clin Exp Allergy 1989;19:643–648. [DOI] [PubMed] [Google Scholar]
- 32.Wjst M, Dold S, Reitmeir P, Stiepel E, von Mutius E. Month of birth and allergic disease at the age of 10. Clin Exp Allergy 1992;22:1026–1031. [DOI] [PubMed] [Google Scholar]
- 33.Talbot TR, Poehling KA, Hartert TV, Arbogast PG, Halasa NB, Edwards KM, Schaffner W, Craig AS, Griffin MR. Seasonality of invasive pneumococcal disease: temporal relation to documented influenza and respiratory syncytial viral circulation. Am J Med 2005;118:285–291. [DOI] [PubMed] [Google Scholar]
- 34.Hartter HK, Oyedele OI, Dietz K, Kreis S, Hoffman JP, Muller CP. Placental transfer and decay of maternally acquired antimeasles antibodies in Nigerian children. Pediatr Infect Dis J 2000;19:635–641. [DOI] [PubMed] [Google Scholar]
- 35.de Francisco A, Hall AJ, Unicomb L, Chakraborty J, Yunus M, Sack RB. Maternal measles antibody decay in rural Bangladeshi infants: implications for vaccination schedules. Vaccine 1998;16:564–568. [DOI] [PubMed] [Google Scholar]
- 36.Buckley RHT. The T-, B-, and NK-cell systems. In: Behrman RE, Kliegman RM, Arvin AM, Nelson WE, editors. Nelson textbook of pediatrics. Philadelphia, PA: W.B. Saunders Company; 1996. pp. 561–567.
- 37.Sigurs N. Epidemiologic and clinical evidence of a respiratory syncytial virus-reactive airway disease link. Am J Respir Crit Care Med 2001;163:S2–S6. [DOI] [PubMed] [Google Scholar]
- 38.van Rijt LS, Kessel CH, Boogaard I, Lambrecht BN. Respiratory viral infections and asthma pathogenesis: a critical role for dendritic cells? J Clin Virol 2005;34:161–169. [DOI] [PubMed] [Google Scholar]
- 39.Kim EY, Battaile JT, Patel AC, You Y, Agapov E, Grayson MH, Benoit LA, Byers DE, Alevy Y, Tucker J, et al. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat Med 2008;14:633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child 1986;140:543–546. [DOI] [PubMed] [Google Scholar]
- 41.Hall CB. Respiratory syncytial virus. In: Feigin RD, Cherry JD, editors. Textbook of pediatric infectious diseases, 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1998. pp. 2084–2111.
- 42.Carroll KN, Gebretsadik T, Griffin MR, Dupont WD, Mitchel EF, Wu P, Enriquez R, Hartert TV. Maternal asthma and maternal smoking are associated with increased risk of bronchiolitis during infancy. Pediatrics 2007;119:1104–1112. [DOI] [PubMed] [Google Scholar]
- 43.Simões EA, Groothuis JR, Carbonell-Estrany X, Rieger CH, Mitchell I, Fredrick LM, Kimpen JL. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J Pediatr 2007; 151:34–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.