Abstract
Rationale: Linezolid, the first oxazolidinone approved for clinical use, has effective in vitro and promising in vivo activity against Mycobacterium tuberculosis.
Objectives: To evaluate the early and extended early bactericidal activity of linezolid in patients with pulmonary tuberculosis.
Methods: Randomized open label trial. Thirty patients with newly diagnosed smear-positive pulmonary tuberculosis (10 per arm) were assigned to receive isoniazid (300 mg daily) and linezolid (600 mg twice daily or 600 mg once daily) for 7 days. Sputum for quantitative culture was collected for 2 days before and then daily during 7 days of study drug administration. Bactericidal activity was estimated by measuring the decline in bacilli during the first 2 days (early bactericidal activity) and the last 5 days of study drug administration (extended early bactericidal activity).
Measurements and Main Results: The mean early bactericidal activity of isoniazid (0.67 log10 cfu/ml/d) was greater than that of linezolid twice and once daily (0.26 and 0.18 log10 cfu/ml/d, respectively). The extended early bactericidal activity of linezolid between Days 2 and 7 was minimal.
Conclusions: Linezolid has modest early bactericidal activity against rapidly dividing tubercle bacilli in patients with cavitary pulmonary tuberculosis during the first 2 days of administration, but little extended early bactericidal activity.
Clinical trial registered with www.clinicaltrials.gov (NCT00396084).
Keywords: tuberculosis, oxazolidinones, linezolid, pharmacokinetics, antituberculosis agents
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Linezolid has in vitro activity against Mycobacterium tuberculosis (MTB). Because of limited treatment options for multidrug-resistant tuberculosis (TB), it is being used despite scarce information about its bactericidal activity in patients with pulmonary TB.
What This Study Adds to the Field
Linezolid has modest early bactericidal activity (EBA) in patients with pulmonary TB, suggesting that it penetrates into tuberculosis lesions and has bactericidal activity against rapidly growing tubercle bacilli in cavities. However, linezolid has minimal extended EBA after the first 2 days of treatment.
Oxazolidinones are a new class of antimicrobials that inhibit protein synthesis at a site not targeted by other antimicrobials (1). Linezolid, the first of these compounds to be approved by the U.S. Food and Drug Administration, is licensed for the treatment of serious skin and soft tissue infections, bacteremia, and pneumonia due to resistant gram-positive bacteria. It is also active in vitro against many gram-positive actinomycetes, including Nocardia, Actinomadura, and Mycobacterium tuberculosis (2–8). Linezolid blocks bacterial ribosomal protein synthesis by a novel mechanism: binding to the 50S bacterial ribosomal subunit and preventing formation of the initiation complex for protein synthesis (9). It exhibits no cross-resistance with other antituberculosis drugs (10). Linezolid is highly bioavailable and has low protein binding (31%) and good penetration into bronchial mucosa and alveolar lining fluid (11, 12). Its use as an antituberculosis agent was prompted by the limited number of drugs available to treat resistant strains of M. tuberculosis (13–16). Because of the long dividing time of M. tuberculosis, the high cost of linezolid, and myelosuppression and neurotoxicity associated with its long-term use, some clinicians have administered linezolid for the treatment of multidrug-resistant (MDR) tuberculosis (TB) at one-half (600 mg once daily) the dose recommended for treatment of serious gram-positive bacterial infections (14).
Early bactericidal activity (EBA 0–2), the rate at which a drug kills actively metabolizing, rapidly multiplying tubercle bacilli in the sputum of patients with TB during the first 2 days of therapy, has been used to compare the activity of new drugs with that of current drugs and to evaluate the effective dose of new agents (17–19). Measurement of killing rate occurring between Days 2 and 7 (extended early bactericidal activity, EBA 2–7) has been advocated as an early measure of sterilizing activity, the ability of a drug to kill slowly replicating, persistent bacilli in tissues (19, 20). Sterilizing activity is regarded as an important characteristic of drugs capable of shortening TB treatment (21). To date, no controlled study has evaluated the mycobactericidal activity of linezolid in humans with TB. We performed a randomized, open label clinical trial with pharmacokinetic sampling to evaluate the early bactericidal activity and pharmacodynamics of linezolid in adults with smear-positive pulmonary TB. We studied the approved 600-mg twice-daily dose and the 600-mg once-daily dose used by some clinicians for MDR-TB treatment. Some of the results of this study have been previously reported in the form of an abstract (22).
METHODS
Eighteen- to 65-year-old HIV-uninfected Brazilian adults with smear-positive TB who weighed more than 75% of their ideal body weight and who had relatively normal hematologic (hemoglobin ≥ 8 g/dl), renal (serum creatinine < 2 mg/dl), and hepatic (serum aspartate aminotransferase < 1.5 times the upper limit of normal and total bilirubin < 1.3 mg/dl) functions were eligible. Patients with suspected miliary or meningeal TB and patients treated with drugs with known activity against M. tuberculosis during the previous 6 months were excluded. The Brazilian National Council of Ethics on Research and the institutional review boards of the Universidade Federal do Espírito Santo (Vitória, Brazil) and Case Western Reserve University (Cleveland, OH) approved the study. Patients gave written informed consent. See the online supplement for detailed information about study procedures).
Patients were randomly assigned to receive 7 days of oral INH at 300 mg once daily, and linezolid at 600 mg once or twice daily. Staff performing cultures were blinded to treatment assignment. Patients were hospitalized for supervised drug administration and specimen collection. After discharge all received standard TB therapy.
Sputum Collection and Culture
Sputum was collected for 12 hours daily from 8 p.m. to 8 a.m. for 2 days before the study and then for 7 days of study drug administration for all study arms. The morning drug dose was given shortly after completing the previous day's collection. For subjects in the linezolid 600-mg twice-daily arm, the second 600-mg dose was administered at 8 p.m. Sputum culture on selective 7H-10 agar plates were performed as described previously (23). Data were expressed as log10 colony-forming units per milliliter of undiluted sputum. Susceptibility testing was performed on pretreatment, Day 7, and Day 42 isolates from each patient, using standard BACTEC methods for INH, rifampicin, ethambutol, and pyrazinamide (24). Minimal inhibitory concentration (MIC) determinations of linezolid against M. tuberculosis were performed with the BACTEC 460TB system (Becton Dickinson, Franklin Lakes, NJ), using twofold dilutions from 0.125 to 4 μg/ml. The MIC was defined as the lowest concentration for which the change in growth index was less than that of the 1:100 control.
Pharmacokinetic Studies
On Day 5 of drug administration and after overnight fasting, plasma samples were collected 0, 1, 2, 4, 8, and 12 hours after dosing (linezolid arms) and also at 18 and 24 hours for subjects receiving INH. No food was ingested for 2 hours after drug intake. Samples were stored in a −80°C freezer until assay at National Jewish Medical and Research Center (Denver, CO), using a validated high-performance liquid chromatography assay. The plasma standard curve for linezolid ranged from 0.5 to 30 μg/ml. The absolute recovery of linezolid from plasma was 95%.
Safety
A toxicity questionnaire was completed daily during the 9-day inpatient study and on Day 42. Complete blood count, urinalysis, serum aspartate aminotransferase, total bilirubin, creatinine, and glucose were repeated on Days 4 and 7.
Statistical Analysis
The primary study end points, EBA 0–2 and EBA 2–7, were calculated as described by Jindani and coworkers (18, 25). Statistical tests were performed with SAS version 9.1 (SAS Institute, Cary, NC). Comparisons across groups were done by parametric and nonparametric analysis of variance techniques, as appropriate. Significant differences in EBA results (P < 0.05) were followed by post hoc two-way comparisons of INH against each of the other treatments and against the other two treatments combined. Reported significance of P values takes into account multiple comparisons. All tests were two sided. Correlations between EBA and pharmacokinetic or pharmacodynamic parameters were determined by simple linear regression with JMP software (version 7.0.2; SAS Institute). These statistical analyses were performed within the individual treatment groups.
RESULTS
Study Population
Fifty-one adults with suspected pulmonary TB were evaluated for study participation. Twenty-one patients were excluded because of self-reported treatment with drugs with known activity against M. tuberculosis (n = 1), HIV infection (n = 3), hemoptysis (n = 1), sputum smear–negative (n = 1) diabetes mellitus or other comorbidity (n = 4), and low body weight (n = 1), including patients believed by the local investigator to be unlikely to comply with the protocol (n = 10). Thirty-one HIV-uninfected adults (79% males; median age, 35 yr; median body weight, 55.9 kg) with initial episodes of newly diagnosed, smear-positive TB were enrolled and randomly assigned to receive INH 300 mg once per day or linezolid 600 mg once or twice daily. One subject in the INH group was excluded from analysis because of INH resistance on drug susceptibility testing of his initial isolate. This subject was replaced by assigning the next eligible subject to the INH arm, in accordance with the study protocol. One patient in the linezolid twice-daily arm withdrew from the study after randomization before receiving any doses of study medication. Most patients were heavily smear positive and had radiographically far advanced TB (Table 1) (26, 27). Eighty-six percent had cavitary disease.
TABLE 1.
PATIENT CHARACTERISTICS
| Study Drug Arm
|
||||
|---|---|---|---|---|
| Characteristic | Isoniazid (n = 10) | Linezolid, 600 mg twice daily (n = 9)* | Linezolid, 600 mg once daily (n = 10) | Total (n = 29) |
| Age, yr | 26.5 (19.0–36.0) | 45.0 (39.0–48.0) | 33.5 (23.0–42.0) | 35.0 (24.0–45.0) |
| Number of patients, male/female | 8/2 | 7/2 | 8/2 | 23/6 |
| Weight, kg | 55.8 (49.5–62.9) | 53.6 (45.5–58.1) | 56.2 (53.2–57.0) | 55.9 (50.2–58.3) |
| Body mass index, kg/m2 | 19.9 (19.1–21.5) | 19.5 (18.2–20.7) | 19.6 (18.8–21.7) | 19.6 (18.8–21.6) |
| Hemoglobin, g/dl | 13.8 (11.3–14.7) | 12.3 (12.1–14.0) | 12.9 (12.1–13.6) | 12.9 (12.1–14.0) |
| Creatinine, mg/dl | 0.8 (0.7–0.9) | 0.9 (0.8–0.9) | 0.8 (0.6–0.9) | 0.8 (0.7–0.9) |
| Disease extent on chest radiograph† | ||||
| Moderately advanced disease | 1 (10) | 4 (44) | 5 (50) | 11 (38) |
| Far advanced disease | 9 (90) | 5 (56) | 5 (50) | 18 (62) |
| Bilateral disease on chest radiograph | 8 (80) | 5 (56) | 4 (40) | 17 (59) |
| Cavitation on chest radiograph | 9 (90) | 7 (78) | 9 (90) | 25 (86) |
| AFB smear grade‡ | ||||
| 1+ or 2+ | 0 | 1 (11) | 1 (10) | 2 (7) |
| 3+ to 4+ | 10 (100) | 8 (89) | 9 (90) | 27 (93) |
Definition of abbreviations: AFB = acid-fast bacilli; IQR = interquartile range.
Values are median (IQR) or n (%).
One patient in the linezolid twice-daily arm withdrew from the study after randomization before receiving any doses of study medication.
Defined using the standard scheme of the U.S. National Tuberculosis and Respiratory Disease Association (26).
Graded using the standard scheme of the American Thoracic Society and the Centers for Disease Control and Prevention (27).
Bactericidal Activity
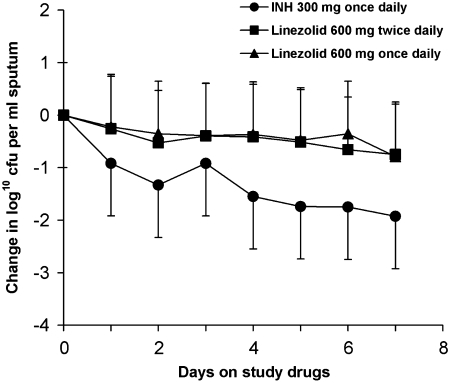
The mean baseline concentration of tubercle bacilli per milliliter of sputum (mean log10 cfu/ml of sputum ± SD) was 6.52 ± 0.63, 6.44 ± 0.87, and 6.34 ± 0.78 for the INH, linezolid twice-daily, and linezolid once-daily arms, and did not differ between groups. The daily change in sputum colony-forming units is shown in Figure 1. Tables 2 and 3 show the mean EBA 0–2 and 2–7 values for the study arms. A significant difference in mean EBA 0–2 was found among the three study arms (P < 0.01). Median EBA 0–2 values were 0.57, 0.13, and 0.17 for the INH, linezolid once-daily, and linezolid twice-daily arms, respectively, and they also were significantly different (P = 0.02). Post hoc comparisons indicated that the mean EBA 0–2 of INH was significantly greater than that of linezolid once daily (P < 0.01) but not significantly different from that of linezolid twice daily (P = 0.07). The mean EBA 0–2 of INH was greater than that of the combined linezolid groups (P < 0.01). No significant differences were found in mean EBA 2–7 (P = 0.25) or mean slope 2–7 (P = 0.42) among the three arms (see Table 3). The difference in EBA 2–7 between the INH arm and the combined linezolid arms was not statistically significant (P = 0.14). Analyses based on median values yielded the same conclusions as those based on mean values and are not presented here.
Figure 1.
Change in colony-forming units (cfu) in sputum before and during 7 days of study drug administration with isoniazid (INH, 300 mg once daily) and linezolid (600 mg, once or twice daily). Sputum was collected for 12 hours for 2 days before and daily during 7 days of drug administration. Data represent the mean change in log10 cfu/ml of sputum ± SD for each of the 7 days of study drug administration. Mean baseline colony-forming unit counts for each treatment group are listed in the text.
TABLE 2.
EARLY BACTERICIDAL ACTIVITY: DAYS 0 TO 2
| Drug | n | Mean EBA (log10 cfu/ml/d) | SD | 95% CI |
|---|---|---|---|---|
| INH, 300 mg once daily | 10 | 0.67 | 0.35 | 0.42 to 0.91 |
| Linezolid, 600 mg twice daily | 9* | 0.26 | 0.42 | −0.06 to 0.59 |
| Linezolid, 600 mg once daily | 10 | 0.18† | 0.27 | −0.01 to 0.37 |
Definition of abbreviations: cfu = colony-forming units; 95% CI = 95% confidence interval; EBA = early bactericidal activity; INH = isoniazid; SD = standard deviation.
One patient in the linezolid 600 mg twice-daily arm withdrew after randomization before receiving any doses of study drug.
P < 0.01 compared with INH.
TABLE 3.
EXTENDED EARLY BACTERICIDAL ACTIVITY: DAYS 2 TO 7
| Drug | n | Mean Extended EBA (log10 cfu/ml/d) | SD (95% CI) | Mean Slope of cfu between Days 2 and 7; b2–7* (log10 cfu/ml/d) | SD (95% CI) |
|---|---|---|---|---|---|
| INH, 300 mg once daily | 8† | 0.16 | 0.11 (0.06 to 0.25) | 0.13 | 0.16 (0.02 to 0.24) |
| Linezolid, 600 mg twice daily | 9‡ | 0.04 | 0.11 (−0.04 to 0.13) | 0.06 | 0.08 (−0.01 to 0.12) |
| Linezolid, 600 mg once daily | 10 | 0.09 | 0.17 (−0.03 to 0.20) | 0.06 | 0.16 (−0.04 to 0.17) |
Definition of abbreviations: cfu = colony-forming units; 95% CI = 95% confidence interval; EBA = early bactericidal activity; INH = isoniazid.
The rate of fall in sputum colony-forming units between Days 2 and 7 (b2–7) was estimated as the slope of the linear regression obtained from fitting the six sputum values from Day 2 to Day 7 (24).
One patient in the INH arm discontinued study drug after 5 days because of minor, self-limited hemoptysis that precluded collection of sputum suitable for the colony-forming unit assay. Quantitative cultures for Days 3 and 7 for another patient in the INH arm were contaminated and colony-forming unit data are not available for this patient for calculation of extended EBA.
One patient in the linezolid twice-daily arm withdrew from the study after randomization, before receiving any doses of study drug.
Pharmacokinetic and Pharmacodynamic Studies
The MIC of linezolid against M. tuberculosis was 0.5 μg/ml for isolates from 11 subjects and 1.0 μg/ml for isolates from 8 subjects. The MIC of linezolid was 1.0 μg/ml for the H37Rv laboratory control strain. The published MIC90 of INH against M. tuberculosis of 0.05 μg/ml was used for pharmacodynamic calculations for the INH treatment group (28).
Median values for plasma maximal drug concentration (Cmax); time to maximal concentration (Tmax); half-life (t1/2); area under the curve during the first 12 or 24 after dosing (AUC0–12 and AUC0–24) for subjects in each group; median values of free (f, unbound) drug for Cmax, AUC, Cmax/MIC, and AUC/MIC; and percent dosing interval above MIC (time above MIC [T > MIC]) are presented in Table 4 (29) and Table 5, respectively. Pharmacokinetic (PK) sampling after 5 days of study drug administration showed that all but one subject in the once-daily linezolid arm had satisfactory drug absorption (defined for linezolid as Cmax > 12 μg/ml). This subject also demonstrated rapid drug elimination (t1/2 = 1.5 h), resulting in an fAUC/MIC of 33 μg·hour/ml. No correlation was found between EBA (0–2 or 2–7) and AUC, Cmax, or fAUC/MIC for either linezolid arm. For twice-daily linezolid only, EBA 0–2 correlated with fCmax/MIC (r2 = 0.59, P < 0.015). In this group, all but one patient had T > MIC values of 100%. No significant correlation was evident between EBA 2–7 and fCmax/MIC for either daily or twice-daily linezolid.
TABLE 4.
MEDIAN PHARMACOKINETIC PARAMETERS (RANGE) AFTER 5 DAYS OF DAILY MONOTHERAPY WITH STUDY DRUGS
| Drug | n | Dose (mg/kg) | Cmax (μg/ml) | Tmax (h) | Half-life (h) | AUC0–12 (μg·h/ml) | AUC0–24 (μg·h/ml) |
|---|---|---|---|---|---|---|---|
| INH, 300 mg once daily | 10 | 5.4 | 3.3 (2.5–5.3) | 1.0 (1.0–2.0) | 3.6* (1.1–4.5) | 17.0 (6.5–26.9) | 19.2 (6.5–29.0) |
| Linezolid, 600 mg twice daily | 9† | 11.2 | 19.4 (11.8–24.9) | 1.0 (1.0–4.0) | 4.56 (2.1–7.0) | 116.4 (50.4–197.2) | 232.9 (100.8–394.4) |
| Linezolid, 600 mg once daily | 10 | 10.7 | 15.0 (11.9–21.3) | 1.5 (1.0–4.0) | 3.20 (1.5–5.0) | 87.0 (47.5–119.3) | 96.9 (47.8–143.7) |
Definition of abbreviations: AUC0–12 and AUC0–24 = area under the curve during the first 12 and 24 hours after dosing, respectively; Cmax = plasma maximal drug concentration; Tmax = time to maximal concentration.
Values are median (IQR).
One patient was a fast acetylator and nine patients were slow acetylators. Patients with t1/2 less than 2 hours were classified as fast acetylators (29).
One patient in the linezolid twice-daily arm withdrew from the study after randomization before receiving any doses of study drug.
TABLE 5.
MEDIAN PHARMACODYNAMIC PARAMETERS (RANGE) ADJUSTED FOR FREE DRUG CONCENTRATIONS AFTER 5 DAYS OF DAILY MONOTHERAPY WITH STUDY DRUGS
| Drug | n | Cmax (μg/ml)* | AUC0–12 (μg·h/ml)* | AUC0–24 (μg·h/ml)* | Cmax/MIC† (IQR) | AUC0–12/MIC† (IQR) | AUC0–24/MIC† (IQR) | Percent Dosing Interval above MIC†‡ (IQR) |
|---|---|---|---|---|---|---|---|---|
| INH, 300 mg once daily | 10 | 3.1 (2.5–4.8) | 15.3 (5.8–24.2) | 17.2 (5.8–26.1) | 62.7 (51.0–77.3) | 306.7 (229.3–405.2) | 344.6 (249.4–449.2) | 95.5 (over 24 h) (76.4–100) |
| Linezolid, 600 mg twice daily | 9§ | 13.4 (8.1–17.2) | 80.3 (34.8–136.1) | 160.7 (134.4–225.8) | 16.2 (14.3–23.0) | 121.6 (79.8–141.6) | 243.2 (159.7–283.2) | 100.0 (over 12 h) (100–100) |
| Linezolid, 600 mg once daily | 10 | 10.3 (8.2–14.7) | 60.1 (32.8–82.3) | 66.8 (33.0–99.2) | 20.0 (10.2–21.9) | 107.8 (63.4–126.3) | 116.2 (71.0–138.4) | 62.8 (over 24 h) (54.6–77.0) |
Definition of abbreviation: AUC0–12 and AUC0–24 = area under the curve during the first 12 and 24 hours after dosing, respectively; Cmax = plasma maximal drug concentration; IQR = interquartile range; INH = isoniazid; MIC = minimal inhibitory concentration.
Cmax and AUC versus time curve for unbound (free) drug in plasma. Linezolid and INH were assumed to be 31 and 10% protein bound, respectively.
Cmax/MIC, AUC0–12/MIC, AUC0–24/MIC, and percent dosing interval above MIC were calculated using the published MIC90 for INH of 0.05 μg/ml (28) and the measured MIC against linezolid for a pretreatment sputum M. tuberculosis isolate from each patient.
Determined by linear extrapolation of concentration-versus-time curve to intersection with MIC.
One patient in the linezolid twice-daily arm withdrew from the study after randomization before receiving any doses of study drug.
Safety
The study drugs were well tolerated and no serious adverse events occurred (leukopenia, thrombocytopenia, worsening anemia, or neurotoxicity). Study drug was discontinued in one subject in the INH arm by the local investigator because of minor, self-limited hemoptysis that precluded collection of suitable sputum specimens for the colony-forming unit assay. All subjects subsequently successfully completed standard anti-TB treatment. No subjects developed acquired resistance to INH, rifampin, ethambutol, pyrazinamide, or linezolid during this trial.
DISCUSSION
Previous studies have shown that linezolid has good in vitro activity against both drug-susceptible and MDR strains of M. tuberculosis, with MIC values of 1 μg/L or lower (2, 30). The clinical use of linezolid in the treatment of TB, however, is limited to five studies of patients with MDR-TB (13–16, 31). In these studies, linezolid was used in combination with other drugs and therefore its bacteriologic activity was not directly demonstrable. We evaluated the mycobactericidal activity of linezolid in a prospective randomized EBA study design with PK sampling to correlate PK and pharmacodynamic parameters with clinical response. Linezolid had a modest EBA 0–2 that was less than that of INH, the first-line TB drug with the highest EBA 0–2 (18), and that of the fluoroquinolones measured in our previous EBA study (23). The mean EBA 0–2 was similar when the linezolid dose was decreased from 600 mg twice to once daily. The modest EBA 0–2 activity of linezolid indicates that it penetrates into tuberculous lesions and is bactericidal against rapidly growing tubercle bacilli present in the sputum of patients with cavitary TB.
Linezolid had little extended EBA (EBA 2–7) after the first 2 days of treatment. The minimal EBA 2–7 of linezolid suggests that it may have limited tissue-sterilizing capacity, which is necessary for long-term nonrelapsing cure, for shortening of TB treatment, and for improving therapy for MDR-TB. However, pyrazinamide, an important tissue-sterilizing drug that allowed shortening of TB treatment by 3 months, has no extended EBA activity. Therefore absence of EBA 2–7 does not exclude sterilizing activity, but points out the limitations of how to interpret negative EBA 2–7 results for TB drugs such as linezolid. Studies examining 2-month culture status or serial sputum culture conversion over the first 2 months of treatment, or phase 3 studies assessing nonrelapsing cure, will be required to determine the tissue-sterilizing capacity of linezolid. The favorable clinical outcomes reported in the five case series of patients with MDR-TB, to whom linezolid combined with other drugs was administered for 6 weeks to 24 months, do not exclude a role for sterilizing activity beyond the mycobactericidal activity we observed.
The bactericidal activity of the INH comparator group (0.67 log10 cfu/ml/d) was within expected values (17), and PK sampling after 5 days of study drug administration showed that all but one subject had satisfactory drug absorption. Linezolid is highly bioavailable and achieves high levels in the lungs. The favorable pharmacodynamics of linezolid (median AUC/MIC over 100 for both doses and excellent percent T > MIC) in our patients with severe pulmonary TB were similar to those associated with excellent activity in treating resistant gram-positive bacterial infections. For other human pathogens, linezolid activity is time and concentration dependent. The correlation of linezolid activity with serum concentration is essentially linear at AUC/MIC values less than 120, but disappears once percent T > MIC reaches 100% (32). In our study, linezolid had modest EBA 0–2 and little EBA 2–7, and these were not correlated with T > MIC or fAUC/MIC.
EBA 0–2 was similar when comparing linezolid 600 mg given once and twice daily. Drug exposure was lower with once-daily administration; however, T > MIC and AUC/MIC with once-daily dosing were similar to those reported effective against resistant gram-positive bacteria, suggesting that once-daily dosing may be useful in TB treatment. In one uncontrolled study, once-daily dosing of linezolid in combination chemotherapy for MDR-TB resulted in sputum culture conversion in less than 3 months in many patients (14). Unfortunately, once-daily dosing still resulted in significant peripheral neuropathy. Alternatively, twice-daily dosing might be considered initially followed by once-daily linezolid therapy.
Our results should be interpreted within the context of the limitations of this study. The small sample size had limited power to detect small differences in EBA 0–2 and EBA 2–7 between study arms, even though we enrolled patients with smear-positive TB and a high sputum bacillary burden to improve chances of detecting differences between treatment arms. Although this study was a randomized open label trial and clinical staff were aware of the drugs each patient received, the primary end point was bacteriologic. Sputum specimens were labeled only with patient identification numbers, and laboratory staff performing quantitative cultures and drug susceptibility testing did not know the patient's treatment arm.
Linezolid was developed primarily for its activity against gram-positive bacteria. Newer oxazolidinones such as DA-7867, DA-7157, RBx 7644, and RBx 8700 have better in vitro activity against M. tuberculosis than linezolid (33, 34) and might be better drugs for TB treatment than linezolid. Our results suggest that further studies are warranted to evaluate the effectiveness of oxazolidinones as anti-TB drugs.
Supplementary Material
Acknowledgments
The authors thank the patients and staff of the Tuberculosis Clinic and Clinical Research Center of the Hospital Universitário Cassiano Antônio de Moraes and the Núcleo de Doenças Infecciosas (NDI) of UFES for their assistance with this study. The authors thank Dr. Solange Alves Vinhas and Tatiana Resende Có for performing the CFU assays. Waleska Ribeiro Meireles, Karina de Souza Fiorotti, Plinio Meira Wetter, and Ana Paula da Silva provided outstanding nursing care during the inpatient phase of the study.
Supported by the Tuberculosis Research Unit (TBRU) at Case Western Reserve University (CWRU), established with funds from the United States National Institutes of Allergy and Infectious Diseases, National Institutes of Health and Human Services, under contract no. NO1-AI95383 and HHSN266200700022C/NO1-AI-70022.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200806-892OC on September 11, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Shinabarger D. Mechanism of action of the oxazolidinone antibacterial agents. Expert Opin Investig Drugs 1999;8:1195–1202. [DOI] [PubMed] [Google Scholar]
- 2.Alcala L, Ruiz-Serrano MJ, Perez-Fernandez Turegano C, Garcia De Viedma D, Diaz-Infantes M, Marin-Arriaza M, Bouza E. In vitro activities of linezolid against clinical isolates of Mycobacterium tuberculosis that are susceptible or resistant to first-line antituberculous drugs. Antimicrob Agents Chemother 2003;47:416–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cynamon MH, Klemens SP, Sharpe CA, Chase S. Activities of several novel oxazolidinones against Mycobacterium tuberculosis in a murine model. Antimicrob Agents Chemother 1999;43:1189–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molicotti P, Ortu S, Bua A, Cannas S, Sechi LA, Zanetti S. In vitro efficacy of linezolid on clinical strains of Mycobacterium tuberculosis and other mycobacteria. New Microbiol 2006;29:275–280. [PubMed] [Google Scholar]
- 5.Rodriguez JC, Ruiz M, Lopez M, Royo G. In vitro activity of moxifloxacin, levofloxacin, gatifloxacin and linezolid against Mycobacterium tuberculosis. Int J Antimicrob Agents 2002;20:464–467. [DOI] [PubMed] [Google Scholar]
- 6.Sood R, Bhadauriya T, Rao M, Gautam R, Malhotra S, Barman TK, Upadhyay DJ, Rattan A. Antimycobacterial activities of oxazolidinones: a review. Infect Disord Drug Targets 2006;6:343–354. [DOI] [PubMed] [Google Scholar]
- 7.Tato M, de la Pedrosa EG, Canton R, Gomez-Garcia I, Fortun J, Martin-Davila P, Baquero F, Gomez-Mampaso E. In vitro activity of linezolid against Mycobacterium tuberculosis complex, including multidrug-resistant Mycobacterium bovis isolates. Int J Antimicrob Agents 2006;28:75–78. [DOI] [PubMed] [Google Scholar]
- 8.Zurenko GE, Yagi BH, Schaadt RD, Allison JW, Kilburn JO, Glickman SE, Hutchinson DK, Barbachyn MR, Brickner SJ. In vitro activities of U-100592 and U-100766, novel oxazolidinone antibacterial agents. Antimicrob Agents Chemother 1996;40:839–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bozdogan B, Appelbaum PC. Oxazolidinones: activity, mode of action, and mechanism of resistance. Int J Antimicrob Agents 2004;23:113–119. [DOI] [PubMed] [Google Scholar]
- 10.Rayner CR, Baddour LM, Birmingham MC, Norden C, Meagher AK, Schentag JJ. Linezolid in the treatment of osteomyelitis: results of compassionate use experience. Infection 2004;32:8–14. [DOI] [PubMed] [Google Scholar]
- 11.Conte JE Jr, Golden JA, Kipps J, Zurlinden E. Intrapulmonary pharmacokinetics of linezolid. Antimicrob Agents Chemother 2002;46:1475–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honeybourne D, Tobin C, Jevons G, Andrews J, Wise R. Intrapulmonary penetration of linezolid. J Antimicrob Chemother 2003;51:1431–1434. [DOI] [PubMed] [Google Scholar]
- 13.Fortun J, Martin-Davila P, Navas E, Perez-Elias MJ, Cobo J, Tato M, De la Pedrosa EG, Gomez-Mampaso E, Moreno S. Linezolid for the treatment of multidrug-resistant tuberculosis. J Antimicrob Chemother 2005;56:180–185. [DOI] [PubMed] [Google Scholar]
- 14.Park IN, Hong SB, Oh YM, Kim MN, Lim CM, Lee SD, Koh Y, Kim WS, Kim DS, Kim WD, et al. Efficacy and tolerability of daily-half dose linezolid in patients with intractable multidrug-resistant tuberculosis. J Antimicrob Chemother 2006;58:701–704. [DOI] [PubMed] [Google Scholar]
- 15.von der Lippe B, Sandven P, Brubakk O. Efficacy and safety of linezolid in multidrug resistant tuberculosis (MDR-TB): a report of ten cases. J Infect 2006;52:92–96. [DOI] [PubMed] [Google Scholar]
- 16.Yew WW, Chau CH, Wen KH. Linezolid in the treatment of “difficult” multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2008;12:345–346. [PubMed] [Google Scholar]
- 17.Donald PR, Sirgel FA, Venter A, Parkin DP, Seifart HI, van de Wal BW, Maritz JS, Fourie PB. Early bactericidal activity of antituberculosis agents. Expert Rev Anti Infect Ther 2003;1:141–155. [DOI] [PubMed] [Google Scholar]
- 18.Jindani A, Aber VR, Edwards EA, Mitchison DA. The early bactericidal activity of drugs in patients with pulmonary tuberculosis. Am Rev Respir Dis 1980;121:939–949. [DOI] [PubMed] [Google Scholar]
- 19.Sirgel FA, Donald PR, Odhiambo J, Githui W, Umapathy KC, Paramasivan CN, Tam CM, Kam KM, Lam CW, Sole KM, et al. A multicentre study of the early bactericidal activity of anti-tuberculosis drugs. J Antimicrob Chemother 2000;45:859–870. [DOI] [PubMed] [Google Scholar]
- 20.Brindle R, Odhiambo J, Mitchison D. Serial counts of Mycobacterium tuberculosis in sputum as surrogate markers of the sterilising activity of rifampicin and pyrazinamide in treating pulmonary tuberculosis. BMC Pulm Med 2001;1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burman WJ. The hunt for the elusive surrogate marker of sterilizing activity in tuberculosis treatment. Am J Respir Crit Care Med 2003;167:1299–1301. [DOI] [PubMed] [Google Scholar]
- 22.Peloquin CA, McGee B, Dietze R, Hadad DJ, Maciel ELN, Johnson DF, Boom WH, Palaci M, Johnson JL. Early bactericidal activity of linezolid in patients with pulmonary tuberculosis [abstract]. First International Workshop on Clinical Pharmacology of Tuberculosis Drugs. Toronto, Canada; May 15, 2008. Abstract no 15.
- 23.Johnson JL, Hadad DJ, Boom WH, Daley CL, Peloquin CA, Eisenach KD, Jankus DD, Debanne SM, Charlebois ED, Maciel E, et al. Early and extended early bactericidal activity of levofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis 2006;10:605–612. [PubMed] [Google Scholar]
- 24.Siddiqi S. Radiometric (BACTEC) tests for slowly growing mycobacteria. In: Isenberg HD, editor. Clinical microbiology procedures handbook. Washington, DC: American Society for Microbiology; 1992. p. 5.14.
- 25.Jindani A, Dore CJ, Mitchison DA. Bactericidal and sterilizing activities of antituberculosis drugs during the first 14 days. Am J Respir Crit Care Med 2003;167:1348–1354. [DOI] [PubMed] [Google Scholar]
- 26.Falk A, O'Connor JB, Pratt PC, Webb WR, Wier JA, Wolinsky E. Classification of pulmonary tuberculosis. In: Diagnostic standards and classification of tuberculosis, 12th ed. New York: National Tuberculosis and Respiratory Disease Association; 1969. pp. 68–76.
- 27.American Thoracic Society, Centers for Disease Control and Prevention. Diagnostic standards and classification of tuberculosis in adults and children [official statement]. Am J Respir Crit Care Med 2000;161:1376–1395. [DOI] [PubMed] [Google Scholar]
- 28.Nuermberger E, Grosset J. Pharmacokinetic and pharmacodynamic issues in the treatment of mycobacterial infections. Eur J Clin Microbiol Infect Dis 2004;23:243–255. [DOI] [PubMed] [Google Scholar]
- 29.Peloquin CA, Jaresko GS, Yong CL, Keung AC, Bulpitt AE, Jelliffe RW. Population pharmacokinetic modeling of isoniazid, rifampin, and pyrazinamide. Antimicrob Agents Chemother 1997;41:2670–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang TS, Liu YC, Sy CL, Chen YS, Tu HZ, Chen BC. In vitro activities of linezolid against clinical isolates of Mycobacterium tuberculosis complex isolated in Taiwan over 10 years. Antimicrob Agents Chemother 2008;52:2226–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Condos R, Hadgiangelis N, Leibert E, Jacquette G, Harkin T, Rom WN. Case series report of a linezolid-containing regimen for extensively drug-resistant tuberculosis. Chest 2008;134:187–192. [DOI] [PubMed] [Google Scholar]
- 32.Rayner CR, Forrest A, Meagher AK, Birmingham MC, Schentag JJ. Clinical pharmacodynamics of linezolid in seriously ill patients treated in a compassionate use programme. Clin Pharmacokinet 2003;42:1411–1423. [DOI] [PubMed] [Google Scholar]
- 33.Sood R, Rao M, Singhal S, Rattan A. Activity of RBx 7644 and RBx 8700, new investigational oxazolidinones, against Mycobacterium tuberculosis infected murine macrophages. Int J Antimicrob Agents 2005;25:464–468. [DOI] [PubMed] [Google Scholar]
- 34.Vera-Cabrera L, Gonzalez E, Rendon A, Ocampo-Candiani J, Welsh O, Velazquez-Moreno VM, Hak Choi S, Molina-Torres C. In vitro activities of DA-7157 and DA-7218 against Mycobacterium tuberculosis and Nocardia brasiliensis. Antimicrob Agents Chemother 2006;50:3170–3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.