Abstract
Two plasmids which express either nearly intact or truncated filamentous hemagglutinin (FHA) from Bordetella pertussis and which are marked with a tetracycline resistance (Tcr) gene were transformed into Salmonella dublin SL1438, an aroA deletion mutant intended for use as an attenuated oral vaccine against salmonellosis. These S. dublin recombinants, when fed to mice, induced serum immunoglobulin, immunoglobulin M (IgM), and sometimes IgA antibody responses to FHA and S. dublin. In addition, IgA antibodies against FHA were found in gut wash fluids. S. dublin carrying pDB2300, a multicopy plasmid encoding truncated FHA protein, induced a better antibody response than did S. dublin carrying pDB2000, a low-copy-number plasmid encoding full-sized FHA. Administration of tetracycline to mice enhanced the stability of recombinant plasmids, and tetracycline-treated mice developed higher anti-FHA titers. Although neither strain examined is suitable for use in a human oral vaccine, these data demonstrated that an immune response against B. pertussis FHA could be induced by oral administration of live attenuated recombinant strains of S. dublin and suggested that development of a live oral attenuated vaccine against pertussis may be possible.
Full text
PDF

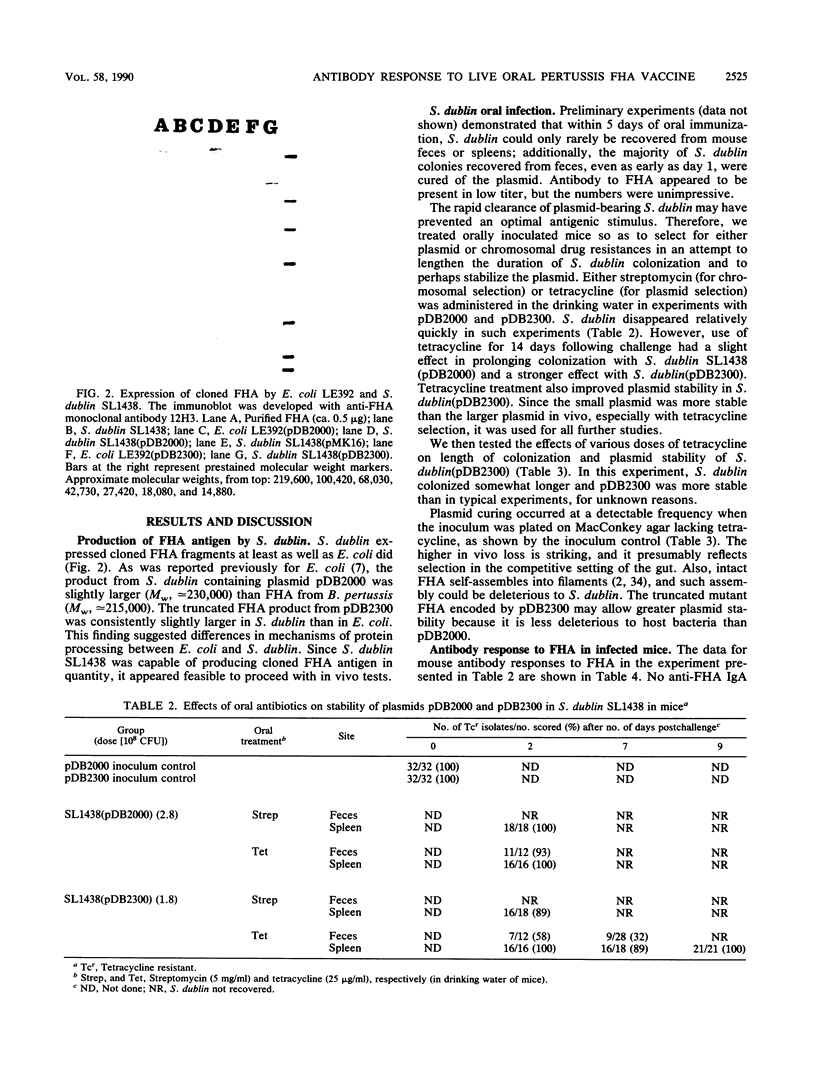

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai H., Sato Y. Separation and characterization of two distinct hemagglutinins contained in purified leukocytosis-promoting factor from Bordetella pertussis. Biochim Biophys Acta. 1976 Oct 22;444(3):765–782. doi: 10.1016/0304-4165(76)90323-8. [DOI] [PubMed] [Google Scholar]
- Armstrong S. K., Parker C. D. Surface proteins of Bordetella pertussis: comparison of virulent and avirulent strains and effects of phenotypic modulation. Infect Immun. 1986 Nov;54(2):308–314. doi: 10.1128/iai.54.2.308-314.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aserkoff B., Bennett J. V. Effect of antibiotic therapy in acute salmonellosis on the fecal excretion of salmonellae. N Engl J Med. 1969 Sep 18;281(12):636–640. doi: 10.1056/NEJM196909182811202. [DOI] [PubMed] [Google Scholar]
- Brown A., Hormaeche C. E., Demarco de Hormaeche R., Winther M., Dougan G., Maskell D. J., Stocker B. A. An attenuated aroA Salmonella typhimurium vaccine elicits humoral and cellular immunity to cloned beta-galactosidase in mice. J Infect Dis. 1987 Jan;155(1):86–92. doi: 10.1093/infdis/155.1.86. [DOI] [PubMed] [Google Scholar]
- Brown D. R., Parker C. D. Cloning of the filamentous hemagglutinin of Bordetella pertussis and its expression in Escherichia coli. Infect Immun. 1987 Jan;55(1):154–161. doi: 10.1128/iai.55.1.154-161.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullas L. R., Ryu J. I. Salmonella typhimurium LT2 strains which are r- m+ for all three chromosomally located systems of DNA restriction and modification. J Bacteriol. 1983 Oct;156(1):471–474. doi: 10.1128/jb.156.1.471-474.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Lyon F. L., Lowe K. L., Farrand A. L., el-Morshidy S. Oral immunization of mice with attenuated Salmonella enteritidis containing a recombinant plasmid which codes for production of the B subunit of heat-labile Escherichia coli enterotoxin. Infect Immun. 1986 Sep;53(3):685–692. doi: 10.1128/iai.53.3.685-692.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Confer D. L., Eaton J. W. Phagocyte impotence caused by an invasive bacterial adenylate cyclase. Science. 1982 Sep 3;217(4563):948–950. doi: 10.1126/science.6287574. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Goldschmidt R. M., Fletchall N. B., Kelly S. M. Avirulent Salmonella typhimurium delta cya delta crp oral vaccine strains expressing a streptococcal colonization and virulence antigen. Vaccine. 1988 Apr;6(2):155–160. doi: 10.1016/s0264-410x(88)80020-3. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Kelly S. M. Salmonella typhimurium deletion mutants lacking adenylate cyclase and cyclic AMP receptor protein are avirulent and immunogenic. Infect Immun. 1987 Dec;55(12):3035–3043. doi: 10.1128/iai.55.12.3035-3043.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Nakayama K., Kelly S. M. Recombinant avirulent Salmonella vaccine strains with stable maintenance and high level expression of cloned genes in vivo. Immunol Invest. 1989 Jan-May;18(1-4):583–596. doi: 10.3109/08820138909112265. [DOI] [PubMed] [Google Scholar]
- Darzins A., Chakrabarty A. M. Cloning of genes controlling alginate biosynthesis from a mucoid cystic fibrosis isolate of Pseudomonas aeruginosa. J Bacteriol. 1984 Jul;159(1):9–18. doi: 10.1128/jb.159.1.9-18.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan G. Molecular characterization of bacterial virulence factors and the consequences for vaccine design. The 1988 Fleming lecture. J Gen Microbiol. 1989 Jun;135(6):1397–1406. doi: 10.1099/00221287-135-6-1397. [DOI] [PubMed] [Google Scholar]
- Frank D. W., Parker C. D. Interaction of monoclonal antibodies with pertussis toxin and its subunits. Infect Immun. 1984 Oct;46(1):195–201. doi: 10.1128/iai.46.1.195-201.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D. W., Parker C. D. Isolation and characterization of monoclonal antibodies to Bordetella pertussis. J Biol Stand. 1984 Oct;12(4):353–365. doi: 10.1016/s0092-1157(84)80060-8. [DOI] [PubMed] [Google Scholar]
- Friedman A. M., Long S. R., Brown S. E., Buikema W. J., Ausubel F. M. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene. 1982 Jun;18(3):289–296. doi: 10.1016/0378-1119(82)90167-6. [DOI] [PubMed] [Google Scholar]
- Goldman W. E., Klapper D. G., Baseman J. B. Detection, isolation, and analysis of a released Bordetella pertussis product toxic to cultured tracheal cells. Infect Immun. 1982 May;36(2):782–794. doi: 10.1128/iai.36.2.782-794.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedenskog S., Granström M., Olin P., Tiru M., Sato Y. A clinical trial of a monocomponent pertussis toxoid vaccine. Am J Dis Child. 1987 Aug;141(8):844–847. doi: 10.1001/archpedi.1987.04460080030021. [DOI] [PubMed] [Google Scholar]
- Hoiseth S. K., Stocker B. A. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981 May 21;291(5812):238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- Kahn M., Kolter R., Thomas C., Figurski D., Meyer R., Remaut E., Helinski D. R. Plasmid cloning vehicles derived from plasmids ColE1, F, R6K, and RK2. Methods Enzymol. 1979;68:268–280. doi: 10.1016/0076-6879(79)68019-9. [DOI] [PubMed] [Google Scholar]
- Kimura A., Mountzouros K. T., Relman D. A., Falkow S., Cowell J. L. Bordetella pertussis filamentous hemagglutinin: evaluation as a protective antigen and colonization factor in a mouse respiratory infection model. Infect Immun. 1990 Jan;58(1):7–16. doi: 10.1128/iai.58.1.7-16.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskell D. J., Sweeney K. J., O'Callaghan D., Hormaeche C. E., Liew F. Y., Dougan G. Salmonella typhimurium aroA mutants as carriers of the Escherichia coli heat-labile enterotoxin B subunit to the murine secretory and systemic immune systems. Microb Pathog. 1987 Mar;2(3):211–221. doi: 10.1016/0882-4010(87)90022-2. [DOI] [PubMed] [Google Scholar]
- Mortimer E. A., Jr Pertussis and its prevention: a family affair. J Infect Dis. 1990 Mar;161(3):473–479. doi: 10.1093/infdis/161.3.473. [DOI] [PubMed] [Google Scholar]
- Nevola J. J., Stocker B. A., Laux D. C., Cohen P. S. Colonization of the mouse intestine by an avirulent Salmonella typhimurium strain and its lipopolysaccharide-defective mutants. Infect Immun. 1985 Oct;50(1):152–159. doi: 10.1128/iai.50.1.152-159.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relman D. A., Domenighini M., Tuomanen E., Rappuoli R., Falkow S. Filamentous hemagglutinin of Bordetella pertussis: nucleotide sequence and crucial role in adherence. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2637–2641. doi: 10.1073/pnas.86.8.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter D. A., Ashworth L. A., Day A., Funnell S., Lovell F., Robinson A. Trial of a new acellular pertussis vaccine in healthy adult volunteers. Vaccine. 1988 Feb;6(1):29–32. doi: 10.1016/0264-410x(88)90010-2. [DOI] [PubMed] [Google Scholar]
- Sato Y., Cowell J. L., Sato H., Burstyn D. G., Manclark C. R. Separation and purification of the hemagglutinins from Bordetella pertussis. Infect Immun. 1983 Jul;41(1):313–320. doi: 10.1128/iai.41.1.313-320.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Kimura M., Fukumi H. Development of a pertussis component vaccine in Japan. Lancet. 1984 Jan 21;1(8369):122–126. doi: 10.1016/s0140-6736(84)90061-8. [DOI] [PubMed] [Google Scholar]
- Schneider D. R., Parker C. D. Effect of pyridines on phenotypic properties of Bordetella pertussis. Infect Immun. 1982 Nov;38(2):548–553. doi: 10.1128/iai.38.2.548-553.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattuck R. L., Oldenburg D. J., Storm D. R. Purification and characterization of a calmodulin-sensitive adenylate cyclase from Bordetella pertussis. Biochemistry. 1985 Nov 5;24(23):6356–6362. doi: 10.1021/bi00344a006. [DOI] [PubMed] [Google Scholar]
- Smith B. P., Reina-Guerra M., Stocker B. A., Hoiseth S. K., Johnson E. Aromatic-dependent Salmonella dublin as a parenteral modified live vaccine for calves. Am J Vet Res. 1984 Nov;45(11):2231–2235. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw A. C., Parton R. Bordetella pertussis toxins. Pharmacol Ther. 1982;19(1):1–53. doi: 10.1016/0163-7258(82)90041-9. [DOI] [PubMed] [Google Scholar]



