Abstract
The naturally competent organism Helicobacter pylori encodes a large number of restriction–modification (R–M) systems that consist of a restriction endonuclease and a DNA methyltransferase. R–M systems are not only believed to limit DNA exchange among bacteria but may also have other cellular functions. We report a previously uncharacterized H. pylori type II R–M system, M.HpyAXII/R.HpyAXII. We show that this system targets GTAC sites, which are rare in the H. pylori chromosome but numerous in ribosomal RNA genes. As predicted, this type II R–M system showed attributes of a selfish element. Deletion of the methyltransferase M.HpyAXII is lethal when associated with an active endonuclease R.HpyAXII unless compensated by adaptive mutation or gene amplification. R.HpyAXII effectively restricted both unmethylated plasmid and chromosomal DNA during natural transformation and was predicted to belong to the novel ‘half pipe’ structural family of endonucleases. Analysis of a panel of clinical isolates revealed that R.HpyAXII was functional in a small number of H. pylori strains (18.9%, n = 37), whereas the activity of M.HpyAXII was highly conserved (92%, n = 50), suggesting that GTAC methylation confers a selective advantage to H. pylori. However, M.HpyAXII activity did not enhance H. pylori fitness during stomach colonization of a mouse infection model.
INTRODUCTION
Helicobacter pylori, a Gram-negative human pathogen, colonizes the stomach and is associated with several gastrointestinal disorders including peptic ulcers and gastric cancers (1). Over half of the world's population is estimated to harbor H. pylori, but only 15–20% of those infected develop severe disease. Helicobacter pylori is known for its remarkably high level of genetic diversity. A comparison of the fully sequenced genomes of three clinical isolates revealed substantial differences in chromosomal structure and a large number of genes that are variably present or absent among strains (up to 25% of the total coding regions) (2,3). These observations have raised many questions, including how genetic diversity is generated in H. pylori and how variable genes contribute to disease outcome.
One class of highly variable genes are restriction–modification (R–M) systems (2), which are typically composed of both a restriction endonuclease (REase) and a cognate DNA methyltransferase (MTase). Generally, the REase cleaves a specific DNA sequence unless it is methylated by the cognate MTase. R–M systems have been divided in type I, II or III, according to their cofactor requirement, molecular structure, mode of action and structure of the recognition sequence site (4). Typical type II R–M systems consist of two independent enzymes that act on the same sequence, usually a 4- to 8-bp palindrome. The recent sequencing of more than 400 bacterial and archaeal genomes has revealed that ∼90% of the genomes contain at least one R–M system and over 80% contain multiple R–M systems (5), but only a small fraction have been comprehensively studied. The H. pylori chromosome encodes a large number of active and inactive R–M systems. Analysis of the sequenced genomes of H. pylori strain 26695 and J99 predicted 26 putative R–M systems including 14 and 16 type II R–M systems, respectively. Comparative genomics of these systems suggests they have a high degree of sequence heterogeneity and that only a small fraction retained full activity (6,7). Interestingly, for some R–M systems, MTase activity may be preserved despite inactivation of the respective REase.
Although still under debate, R–M systems may carry out several biological roles. They have traditionally been implicated in bacterial defense from bacteriophage infection, which have in turn evolved a variety of anti-restriction defense mechanisms (8). R–M systems also regulate genetic exchange among bacteria, particularly relevant for bacteria like H. pylori that are naturally competent for DNA uptake (9). By constituting an effective barrier against DNA transformation in a bacterial population, R–M systems may provide the genetic isolation required for adaptation of the organisms to a special ecological niche (10). Paradoxically, R–M systems were also suggested to promote homologous recombination from the small DNA fragments produced by the incomplete digestion of incoming DNA by REases (11). Kobayashi and colleagues have suggested that R–M systems have attributes of selfish genes, and may act as invasive elements independent of any selective advantage to their host (12,13). This hypothesis emerged from the findings that several R–M systems are horizontally transferred and that a fully active type II R–M system cannot easily be lost from its host because of postsegregational killing. Finally, DNA methylation may have important biological properties independent of the endonuclease function by regulating the interactions between proteins and DNA, in a similar manner to the stand-alone DNA methyltransferase Dam in both α- and γ-Proteobacteria (14,15).
In H. pylori, R–M systems may carry out other biological roles. Several R–M systems have been classified as ‘chronic atrophic gastritis (ChAG) associated genes’ by comparing the genome content of eight ChAG strains to a H. pylori ‘pangenome’ defined previously (3,16). Other R–M genes were acid-regulated (3), undergo phase variation (17,18) and several R–M systems were identified as candidate colonization factors using a mouse model of infection (19). Expression of the REase encoded by gene iceA1 was upregulated upon contact with gastric epithelial cells and its presence was associated with peptidic ulcer disease (20). A mutant in the endonuclease R.HpyC1I, isolated from H. pylori clinical isolate NTUH-C1, exhibited cell elongation and decreased adherence to a gastric cell line (21). In another report, DNA methylation by M.HpyAIV was shown to alter transcription of a selected number of genes including catalase (katA) (22).
Here, we annotate a previously uncharacterized H. pylori R–M system M.HpyAXII/R.HpyAXII encoded by the locus HP0502/0503/0504/0505. We present biochemical and genetic analyses of this R–M system. We additionally explored its conservation among clinical isolates and its role during establishment of infection to query alternative biological functions of M.HpyAXII/R.HpyAXII.
MATERIALS AND METHODS
Bacterial strains and growth conditions
Helicobacter pylori strains (Supplementary Table S1) were grown on solid horse blood agar (HB) plates containing 4% Columbia agar base (Oxoid, Hampshire, UK), 5% defibrinated horse blood (HemoStat Labs, Dixon, CA), 0.2% β-cyclodextrin (Sigma, St Louis, MO), 10 µg/ml vancomycin (Sigma), 5 µg/ml cefsulodin (Sigma), 2.5 U/ml polymyxin B (Sigma), 5 µg/ml trimethoprim (Sigma) and 8 µg/ml amphotericin B (Sigma) at 37°C either under a microaerobic atmosphere generated using a CampyGen sachet (Oxoid) in a gas pack jar or in an incubator equilibrated with 14% CO2 and 86% air. For liquid culture, H. pylori was grown in Brucella broth (Difco, Franklin Lakes, NJ) containing 10% fetal bovine serum (BB10; Invitrogen, Carlsbad, CA) with shaking in a gas pack jar containing a CampyGen sachet. For resistance marker selections, bacterial media were additionally supplemented with 15 µg/ml chloramphenicol (Cm, Sigma), 25 µg/ml kanamycin (Kan, Fisher Scientific, Pittsburgh, PA) or 60 mg/ml sucrose (Fisher Scientific). When culturing bacteria from mouse stomachs, 200 μg/ml bacitracin (Sigma) was added to eliminate normal mouse microbiota contamination.
DNA manipulations
DNA manipulations, such as restriction digestion, PCR and agarose gel electrophoresis, were performed according to standard procedures (23). Helicobacter pylori genomic DNA was prepared by Wizard genomic DNA preparation kits (Promega, Madison, WI). Primers used for PCR and sequencing are in Supplementary Table S2. Plasmid DNA (Supplementary Table S3) was isolated and prepared from Escherichia coli or H. pylori using Qiagen Midiprep kit (Qiagen, Valencia, CA). Sequencing of plasmid DNA and PCR products was performed by the FHCRC Genomics Shared Resource and the resulting sequences analyzed using Sequencher (Gene Codes Corporation, Ann Harbor, MI).
Generation of H. pylori knockout isogenic mutants
Knockout alleles were constructed using a vector-free allelic replacement strategy to generate alleles in which a chloramphenicol acetyl transferase (cat) resistance cassette (24), a nonpolar kanamycin resistance (aphA3) cassette (25), or a cat cassette fused to a sucrose sensitivity marker (sacB) (26) replaced 80–90% of the coding sequence of the gene while preserving the start and stop codons. The primers used for this procedure are designated as 1 through 4 and are given in Supplementary Table S2. The catsacB cassette was generated by fusing cat to sacB with elimination of the terminator sequence using PCR (primers C2SA4 and SB1). After natural transformation (27) with the appropriate PCR product and selection on Cm- or Kan- containing media, four clones were evaluated by PCR to confirm replacement of the wild-type allele with the null allele. Urease activity and flagella-based motility were also confirmed for all the clones generated. Single clones were used for infection experiments and phenotypic characterization.
Generation of H. pylori knockin isogenic mutants
Null alleles generated by replacement with the catsacB cassette were subsequently replaced with alternate alleles by sucrose counter-selection. H. pylori strain NSH57 ΔhpyAXIIM/R::catsacB was naturally transformed with the appropriate plasmid or genomic DNA, and recombinants were selected on sucrose-containing HB plates, screened on Cm-containing media, and evaluated by PCR to confirm they lost the catsacB cassette.
Site-directed mutagenesis
To generate a catalytically inactive DNA MTase in H. pylori strain NSH57, an asparagine in conserved motif IV was replaced by alanine (N87A). A DNA fragment including hpyAXIIM/R was amplified from NSH57 by PCR and cloned into TA vector pCR-TOPO (TOPO cloning, Invitrogen). pCR-TOPO/hpyAXII was digested with EcoRI (NEB, Ipswich, MA) and subcloned into plasmid Bluescript SK+ (Stratagene, La Jolla, CA). Site-directed mutagenesis was performed on hpyAXIIM using the QuikChange kit (Stratagene) following the manufacturer instructions and confirmed by sequencing.
Natural transformation
H. pylori bacteria were freshly grown for 24–32 h on HB plates, transferred as patches onto fresh plates and grown for an additional 6–8 h. Donor DNA (plasmid, genomic or PCR product) was diluted as appropriate in distilled water and 10 μl was added to each patch and incubated overnight. The transformation mixture was harvested from the plate surface and resuspended in 350 μl phosphate buffer saline (PBS). Serial 10-fold dilutions were inoculated onto selective or nonselective HB plates. Transformation frequency was calculated as the number of Kan or Cm resistant colonies per recipient cell per nanogram of donor DNA.
Expression of M.HpyAXII and R.HpyAXII proteins
hpyAXIIM was amplified from DNA of wild-type NSH57 and NSH79 H. pylori by PCR and cloned into a pET-15b vector (Novagen, Gibbstown, NJ) in frame with the N-terminal His-Tag sequence using NdeI and XhoI (New England Biolabs). The resulting pET15b/hpyAXIIM plasmids were transformed into E. coli strain ER2925(DE3), derived from strain ER2925 [NEB and (28)] and engineered to become a λDE3 lysogen using a lysogenization kit (Novagen). Plasmid pET15b/hpyAXIIR was transformed into E. coli strain BL21CodonPlus(DE3)-RIL. Protein expression was induced using 1 mM isopropyl-1-thio-β-d-galactopyranoside (IPTG) induction for 3 h at 37°C for M.HpyAXII and overnight at 16°C for R.HpyAXII to minimize endonuclease activity. Bacterial pellets were resuspended in lysis buffer (50 mM Tris pH 7.9, 100 mM NaCl, 1 mM DTT, 2% glycerol), incubated with 1 mg/ml lysozyme (Sigma) for 30 min on ice, and sonicated. For R.HpyAXII, soluble and insoluble fractions were separated by centrifugation at 18 000 r.p.m. for 20 min at 4°C.
Antibody-based M.HpyAXII methylation activity assays
M. HpyAXII DNA MTase activity was assessed in a dot-blot assay using a rabbit primary antibody raised against DNA with N6-methyladenine (m6A) (29). Escherichia coli ER2925(DE3) transformed with pET15b and pET15b/hpyAXIIM was grown to log phase and induced with 1 mM IPTG for 3 h at 37°C. Genomic DNA of the cultures was isolated and 3 μl cross-linked to a nylon membrane (Hybond-XL, Amersham Biosciences, Piscataway, NJ) by UV (1.2 mJ/cm2 for 30 s). The membrane was blocked in a 3% nonfat dry milk solution and incubated with the m6A polyclonal rabbit antibody (1:10 000 dilution) overnight at 4°C. After a series of washes in PBST (PBS and 0.1% Tween-20), anti-rabbit IgG antibody conjugated to horseradish peroxidase (1:1000 dilution) was added to the membrane and detected using ECL Western Blotting reagent (Amersham Biosciences).
Biochemical R.HpyAXII endonuclease activity assay
One microliter of freshly prepared soluble fraction obtained from E. coli BL21(DE3) expressing R.HpyAXII was incubated for 3 h at 37°C with 500 ng E. coli ER2925 genomic DNA or pET15b plasmid DNA isolated from E. coli DH10B in presence of NEB buffer 1 (New England Biolabs). The product DNA was separated on a 0.8% agarose gel.
Genetic R.HpyAXII endonuclease activity assay
To assess R.HpyAXII activity in a large number of H. pylori strains, we replaced the null allele in H. pylori strain NSH57ΔhpyAXIIM/R::catsacB with that from other strains as described above (Generation of H. pylori knockin isogenic mutants section). R.HpyAXII activity was determined by transforming these H. pylori clones with PCR constructs ΔhpyXIIM::cat and ΔHP0368::cat and enumerating recombinants recovered on Cm- containing HB plates.
Mouse infections
Female C57BL/6 mice 24- to 28-days old were obtained from Charles River Laboratories and certified free of endogenous Helicobacter infection by the vendor. The mice were housed in sterilized microisolator cages with irradiated PMI 5053 rodent chow, autoclaved corn cob bedding, and acidified, reverse-osmosis purified water provided ad libitum. All studies were done under practices and procedures of Animal Biosafety Level 2. The facility is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International, and all activities were approved by the FHCRC Institutional Animal Care and Use Committee. Competition experiments were performed as described previously (24) with 5.0 × 108 bacteria of each strain in the inocula.
In silico genomic analysis
Helicobacter pylori sequences were retrieved from the Comprehensive Microbial Resource (CMR) (30). Homologs of M. and R.HpyAXII and were sought using REBASE Blast, a tool of REBASE (5). Protein structure prediction analysis for M.HpyAXII and R.HpyAXII was carried out using the Protein Homology/Analogy Recognition Engine PHYRE (31). Sequence alignments were carried out with CLUSTALW2 (32) and 3D molecular structures were visualized and modified using PyMOL (33). The genome-wide distribution and frequency of GTAC sites was determined using genome-scale DNA pattern in Regulatory Sequence Analysis (RSA) Tools (http://rsat.ulb.ac.be/rsat/). For H. pylori strain NSH57, the sequence of the derivative strain G27 was used (D.A. Baltrus, M.R. Amieva, A. Covacci, D.S. Merrell, K. M. Ottemann, M. Stein, N.R. Salama, K. Guillemin, personal communication) and genomic GTAC sites were analyzed with the genome tools/restriction digest option of the CMR website.
Sequences are available from the GenBank/EMBL/DDBJ databases with accession numbers FJ233107 to FJ233133.
RESULTS
Structure-based prediction uncovers the existence of a novel H. pylori R–M system
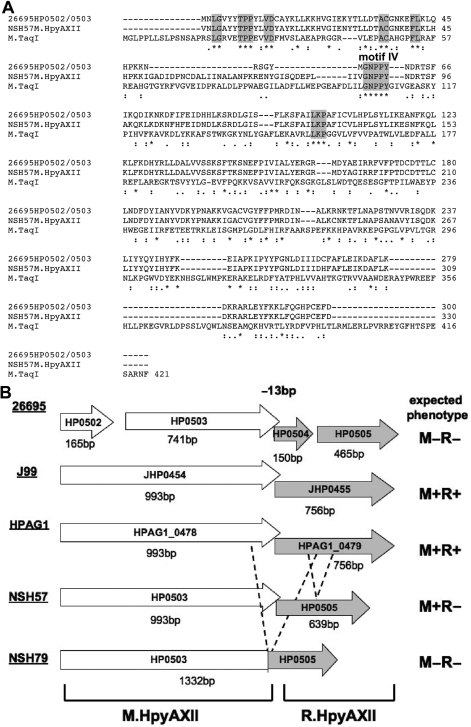
We set out to further characterize the HP0502/0503/0504/0505 locus that we previously identified in our genome-wide screen for candidate colonization factors (19). BLAST search of DNA sequences of these genes from H. pylori strain 26695 failed to identify any homologs. However, several lines of evidence suggested that HP0502/0503 encodes a DNA MTase and that it is organized in an R–M system along with the downstream gene, HP0504/0505. Nobusato et al. (7) previously predicted that this locus encodes a R–M system based on sequence analysis. In agreement with this finding, we used structure prediction algorithms of the program PHYRE (31) and found that the N-terminal domain of the HP0502/0503 polypeptides could be threaded onto a tertiary structure of the M.TaqI DNA MTase, crystallized from Thermus aquaticus (Supplementary Figure S1, E-value 6.1 × 10−15). Despite weak protein sequence homology, several amino acid motifs were shared between HP0502/0503 and M.TaqI (Figure 1A) and mapped to the catalytic domain of M.TaqI (Supplementary Figure S1), further supporting the function of HP0502/0503 as an adenine MTase (34). More recently, the REase R.PabI from Pyrococcus abyssi was characterized and HP0504/0505 was identified as a homolog (28% protein sequence identity) and suggested to recognize the same target sequence, 5′-GTAC-3′ (35). Based on the proposed nomenclature for R–M systems (4), we have named this novel H. pylori R–M system M.HpyAXII/R.HpyAXII, encoded by genes hpyAXIIM and hpyAXIIR, respectively. Since R–M systems are highly polymorphic among H. pylori strains (6), we evaluated the genomic organization of M.HpyAXII/R.HpyAXII in several isolates. As illustrated in Figure 1B, gene hpyAXIIM encodes a polypeptide of 331 amino acids and is predicted to be active in strains J99, HPAG1 and NSH57 but is likely inactive in 26695 and NSH79 where the expected protein products are truncated and fused with R.HpyAXII, respectively. Gene hpyAXIIR encodes a protein of 252 amino acids and is only expected to be functional in strains J99 and HPAG1 based on DNA sequences.
Figure 1.
Genomic organization of the HpyAXII R–M system. (A) Amino acid sequence alignment of 26695 HP0502/0503, NSH57 M.HpyAXII and M.TaqI from Thermus aquaticus reveals conserved motifs (highlighted in gray) in the N-terminal portion of the protein. (*) - identical residues, (:) conserved substitution, (.) semi-conserved substitution. (B) Genomic organization of genes hpyAXIIM and hpyAXIIR in H. pylori strains 26695, J99, HPAG1 and two mouse adapted strains NSH57 and NSH79. The expected phenotype for this R-M system in each strain is given based on DNA sequences. M, DNA MTase; R, REase; +, active; –, inactive. M.HpyAXII is truncated in 26695 and fused to R.HpyAXII in NSH79. R.HpyAXII is truncated in 26695, partially deleted in NSH57 (dashed lines), and fused to M.HpyAXII in NSH79 (in-frame deletion represented with dashed lines).
M.HpyAXII functions as a γ-adenine MTase
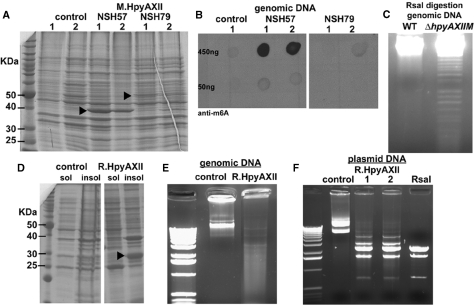
To validate the function of M.HpyAXII as a DNA MTase, we inducibly expressed Histidine-tagged M.HpyAXII from H. pylori strain NSH57 and Histidine-tagged M.HpyAXII fused to R.HpyAXII from strain NSH79 in E. coli (Figure 2A). M.HpyAXII activity was studied using E. coli strain ER2925(DE3) (Supplementary Table S1), defective for all known R–M systems, and presumably harboring chromosomal DNA devoid of adenine or cytosine methylation (Materials and methods section). Genomic DNA from the E. coli cultures expressing M.HpyAXII was isolated and adenine methylation assessed using antibodies against N6-methyladenine. Adenine MTase activity could be detected when M.HpyAXII from NSH57 but not NSH79 was expressed in E. coli (Figure 2B). The lack of MTase activity by NSH79 M.HpyAXII probably reflects the genetic rearrangements observed at the NSH79 locus (Figure 1B). To further confirm that M.HpyAXII acts as an adenine MTase, we used site-directed mutagenesis on the predicted catalytic site of this enzyme. We identified conserved motif IV (D/N/S)PP(Y/F) [Figure 1A and (34)] and the substitution of asparagine with alanine (point mutation designated N87A) in NSH57 M.HpyAXII completely abolished adenine MTase activity without affecting the expression levels of this protein (Supplementary Figure S2). Along with our genomic data, this finding supports the re-annotation of this H. pylori protein as a γ-adenine MTase.
Figure 2.
Biochemical demonstration that HypAXII functions as an R–M system targeting GTAC sites. (A and B) NSH57 M.HpyAXII methylates genomic DNA on adenines when expressed in E. coli. (A) Escherichia coli ER2925(DE3) extracts expressing NSH57 or NSH79 M.HpyAXII (arrowheads) separated by SDS–PAGE gel and stained with Coomassie blue. Control - empty plasmid. Two separate E. coli clones (1 and 2) were analyzed. (B) The indicated amount of genomic DNA from the respective E. coli cultures was cross-linked to a nitrocellulose membrane. DNA N-6 adenine methylation (m6A) was detected using anti-m6A antibodies and showed a signal during NSH57 M.HpyAXII but not NSH79 M.HpyAXII expression. (C–F) The HpyAXII R–M system targets the tetramer 5′-GTAC-3′. (C) Helicobacter pylori strain NSH57 wild-type and ΔhpyAXIIM genomic DNA was isolated and digested with RsaI. Agarose gel separation showed fragmentation for ΔhpyAXIIM genomic DNA but not wild-type. (D) hpyAXIIR from H. pylori strain HPAG1 was cloned and expressed E. coli BL21(DE3) under inducible conditions. Soluble (sol) and insoluble (insol) fractions were separated by SDS–PAGE gel and stained with Coomassie blue. R.HpyAXII was mostly insoluble (arrowhead). Control, empty plasmid. The soluble fractions shown in C were incubated with unmethylated genomic DNA (E) or plasmid DNA (F) and separated by agarose gel. DNA fragmentation was only observed when R.HpyAXII was expressed. Similar DNA restriction patterns (F) indicate that these enzymes cleave the same DNA sequence. Bands of higher sizes seen for with R.HpyAXII suggest incomplete digestion. Two separate E. coli clones are shown (1 and 2).
The HpyAXII R-M system targets the tetramer 5′-GTAC-3′
Two independent approaches defined the target sequence of this novel R–M system. First, using an array of methylation-sensitive endonucleases, we sought to identify an enzyme that could digest H. pylori NSH57 chromosomal DNA isolated from a ΔhpyAXIIM mutant but not from wild-type. Only the enzyme RsaI digested DNA from the ΔhpyAXIIM::cat mutant but not wild-type (Figure 2C), indicating that the DNA MTase M.HpyAXII and the endonuclease RsaI act on the same target sequence, 5′-GTAC-3′, where the adenine residue is methylated by M.HpyAXII. Using this result, we investigated M.HpyAXII activity in the H. pylori strains where the HpyAXII R-M system was sequenced (Figure 1B). Genomic DNA from H. pylori strains 26695 and NSH79 could be digested with RsaI, whereas J99, NSH57 and HPAG1 DNA were protected from RsaI digestion (data not shown), indicating that M.HpyAXII is only active in these three strains and agreeing with our predictions from the genomic sequences. RsaI digestion of DNA isolated from the NSH57hpyAXIIMN87A mutant showed fragmentation when separated on agarose gel (data not shown), confirming that this single amino acid substitution abolishes MTase catalytic activity. In the second approach, we cloned and expressed the putative endonuclease gene hpyAXIIR in E. coli under the control of an inducible promoter (Figure 2D). We chose hpyAXIIR from H. pylori strain HPAG1 because this protein is expected to be functional based on sequence analysis (Figure 1). Incubation of the E. coli soluble fraction expressing HPAG1 R.HpyAXII with genomic DNA unmethylated for GTAC sites showed endonuclease activity as indicated by the resulting smear on agarose gel (Figure 2E). To determine R.HpyAXII cleavage site, the same experiment was carried out with unmethylated plasmid DNA. R.HpyAXII-containing extract cleaved plasmid DNA and gave an identical fragmentation pattern as RsaI (Figure 2F) confirming that R.HpyAXII acts on GTAC sites. In contrast to HPAG1 R.HpyAXII, no endonuclease activity was detected when R.HpyAXII from H. pylori strain J99 was expressed in E. coli (data not shown). While the exact cutting site of R.HpyAXII within the tetramer GTAC remains to be defined, our demonstration that hpyAXIIM and hpyAXIIR encode two independent enzymes suggests that they together constitute a class II R-M system (4).
GTAC sites are scarce in the H. pylori chromosome but are found at high frequency in the rDNA genes
We determined the frequency and distribution of GTAC sites in four sequenced strains of Helicobacter pylori: 26695 (122 sites), J99 (184 sites), HPAG1 (114 sites), G27 (115 sites). This total number of sites is >50-fold lower than the expected number based on frequency of nucleotides and confirms previous observations (35). Interestingly, a significant fraction of the GTAC sites are located within the ribosomal RNA (rRNA) genes. The 23SrRNA contains eight or nine sites depending on the strain analyzed and the 16SrRNA contains two sites (Table 1). Since two copies of each gene exist in the H. pylori chromosome, a total of 20 or 22 GTAC sites are located within the rRNA genes. We were particularly interested in GTAC sites that are strictly conserved among H. pylori strains since they may be biologically important. Of all intragenic sites, ten are conserved in all four H. pylori strains and can be mapped to a total of 10 genes that belong to various functional categories (Table 1). Interestingly, three of these genes belong to the cag pathogenicity island (cagT, cagL and cagH). Some of these sites are located in the promoter region of genes and may influence transcription efficiency.
Table 1.
Distribution of all conserved GTAC sites in four H. pylori strains
| Gene numbera | Gene namea | Gene description | Characteristicsb |
|---|---|---|---|
| 23S rDNA | Ribosomal DNA | 8 sites | |
| 16S rDNA | Ribosomal DNA | 2 sites | |
| HP0200 | rpl32 | Predicted ribosomal protein L32 | 8 bp after start of HP0200 and 200 bp from start of HP0201 |
| HP0486 | hofC | Outer membrane protein | |
| HP0499 | pldA | Phospholipase A1 precursor | |
| HP0532 | cagT | cag pathogenicity island protein T | |
| HP0539 | cagL | cag pathogenicity island protein L | 200 bp from start of cagI |
| HP0541 | cagH | cag pathogenicity island protein H | |
| HP0613 | Predicted ABC transporter | 87 bp after start of HP0613 | |
| HP0630 | mda66 | Modulator of drug activity | 11 bp after start of HP0630 and 170 bp from start of HP0631 |
| HP1197 | rps12 | Predicted ribosomal protein S12 | 190 bp from start of HP1198 |
| HP1228 | invA | Diadenosine polyphosphate hydrolase | 50 bp from start of HP1229 |
aBased on 26695 annotation (61).
bThe number of conserved sites is given when higher than one. The exact position of the site is given only when within 200bp from the start codon of a gene.
The HpyAXII R-M system effectively restricts incoming plasmid DNA
Bacterial R–M systems protect host genomes from invading DNA such as viral, plasmid and other foreign DNA by cleaving exogenous DNA at specific sites when unmethylated. We determined whether the endonuclease R.HpyAXII could restrict incoming plasmid DNA during natural transformation of H. pylori. As shown above, R.HpyAXII is biochemically active in H. pylori HPAG1, a strain difficult to transform (data not shown). We thus replaced the endogenous locus encoding M.HpyAXII/R.HpyAXII in the easily transformable NSH57 strain with that from HPAG1 (Materials and methods section). This engineered strain, NSH57hpyAXIIM/RHPAG1, encodes a fully active HpyAXII R-M system (R + M + phenotype). For comparison, we deleted the endonuclease component R.HpyAXII in the same strain and replaced it with an antibiotic resistance cassette to create a null allele (ΔhpyAXIIR::aphA3, R–M + phenotype). As donor DNA, we used plasmid pTM113-cat (Materials and methods and Supplementary TableS3) that we isolated from H. pylori strain NSH57 wild-type (fully methylated, denoted ‘M+’), from the catalytically inactive mutant NSH57hpyAXIIMN87A (unmethylated for GTAC sites, denoted ‘M−’), or from E. coli (unmethylated at all sites, denoted ‘all M−’). We first verified that the numerous R–M systems encoded by H. pylori strain NSH57 could effectively restrict unmethylated (all M−) plasmid DNA as compared to methylated (M+) plasmid. Transformation frequency of wild-type H. pylori NSH57 decreased over 200-fold when completely unmethylated plasmid DNA prepared from E. coli was used as compared to methylated (M+) plasmid (data not shown), in agreement with previous work (37). We similarly found that transformation frequency involving M− plasmid decreased over 60-fold in the bacteria harboring an active R.HpyAXII endonuclease (R+) (ratio R−/R+, Table 2), demonstrating that this enzyme constitutes an effective barrier against incoming plasmid DNA in vivo.
Table 2.
R.HpyAXII effectively restricts incoming plasmid DNA
| Donor DNAa | Transformation frequency (# transformants/recipient cell/ng × 10−8)b, mean (SEM) |
Ratio | |
|---|---|---|---|
| Recipient strainc |
|||
| R+ | R− | R−/R+ | |
| M− | 12 (8.8) | 760 (120) | 63 |
| M+ | 1900 (400) | 2500 (180) | 1.3 |
aDonor plasmid pTM113-cat contains a total of five GTAC sites and was constructed by substituting cat for aphA3 in pTM113 (Supplementary Table S3). pTM113-cat was isolated from H. pylori strain NSH57 wild-type (M+) or NSH57hpyAXIIMN87A (M−). A total of 3.3 ng of plasmid DNA was used for each transformation.
bTransformation frequency was calculated by dividing the number of transformants recovered by the total number of recipient bacteria and was based on the average of three replicates.
cStrain NSH57hpyAXIIHPAG1 (R+) encodes an active R.HpyAXII and strain NSH57hpyAXIIM/RHPAG1 ΔhpyAXIIR::aphA3 (R−) lacks this enzyme.
The HpyAXII R–M system effectively restricts incoming chromosomal DNA
To investigate the role of the endonuclease R.HpyAXII in restricting incoming chromosomal DNA in vivo, we assessed transformation using donor genomic DNA harboring a kanamycin resistance marker (aphA3) at the 23S rDNA locus. This marker contains a GTAC site and is susceptible to cleavage by R.HpyAXII. As described for plasmid DNA, donor chromosomal DNA was isolated from bacteria encoding an active (M+) or inactive (M−) M.HpyAXII MTase. We determined if the endonuclease R.HpyAXII could restrict incoming chromosomal DNA by comparing transformation efficiency in NSH57 harboring either an active (R+) or inactive (R−) restriction endonuclease R.HpyAXII. When genomic DNA isolated from H. pylori lacking M.HpyAXII MTase activity (M−) was transformed into the bacteria harboring an active R.HpyAXII endonuclease (R+), a 240-fold decrease in transformation frequency was observed (R−/R+, Table 3). We also digested the same donor genomic DNA in vitro with RsaI prior to transformation, which lowered the transformation frequency further to undetectable levels (Table 3). We suggest that cleavage of the GTAC site in gene aphA3 by the endonucleases RsaI and R.HpyAXII inactivates this marker and results in the subsequent decrease in transformation frequency. Overall, we demonstrated that active R.HpyAXII endonuclease effectively limits the incorporation of unmethylated exogenous homologous DNA into the H. pylori chromosome.
Table 3.
R.HpyAXII effectively restricts incoming genomic DNA
| Donor DNAa | Transformation frequency (# transformants/recipient cell/ng × 10−10)b, mean (SEM) |
Ratio | |
|---|---|---|---|
| Recipient strainc |
|||
| R + | R− | R−/R+ | |
| M+ | 18 (0.16) | 44 (17) | 2.4 |
| M+ RsaI | 49 (9.3) | 95 (30) | 1.9 |
| M− | 0.42 (0.26) | 100 (11) | 240 |
| M− RsaI | 0 | 1.4 (0.60) | inf d |
aDonor genomic DNA was constructed by inserting the aphA3 cassette at position 2586bp (from start codon) in one of the two copies of 23S rDNA genes. One GTAC site is present in aphA3 at position #94 bp and three flanking GTAC sites are found in close proximity to the marker at positions #2527, #2660 and #2745. Chromosomal DNA was isolated from H. pylori strain NSH57 wild-type (M+) or NSH57ΔhpyAXIIM::cat (M−), and predigested or not with RsaI. A total of 10 ng genomic DNA per tranformation was used for M− and 7.9 ng for M+.
bTransformation frequency was based on the average of three replicates.
cStrain NSH57hpyAXIIM/RHPAG1 (R+) encodes an active R.HpyAXII and strain NSH57hpyAXIIM/RNSH57 (R−) encodes an inactive enzyme.
dRatio is infinite because no transformants were recovered for R+ recipient strain
The HpyAXII R–M system follows the selfish hypothesis
Kobayashi and colleagues suggested that type II R–M systems act as selfish genetic elements and cannot be easily replaced with a homologous stretch of DNA (36). This phenomenon, more commonly known as postsegregational killing, has been attributed to the gradual dilution of the modification and restriction enzymes in the descendants of the cell that lost the R–M system. Since there is asymmetry between the roles of the MTase and REase, newly replicated chromosomes will be inadequately protected by methylation and rendered susceptible to cleavage by the residual REase, leading to cell death. To determine whether the type II HpyAXII R–M system follows the selfish hypothesis, we deleted this chromosomal locus by allelic replacement from H. pylori strain NSH57 engineered to harbor an active (R+) or inactive (R−) R.HpyAXII endonuclease. We found that R− but not R+ recipient bacteria were easily transformed with donor NSH57 genomic DNA in which hpyAXIIM/R was replaced with a catsacB marker (strain NSH57 ΔhpyAXIIM/R::catsacB) (Table 4, ΔhpyAXIIM/R::catsacB). This 2100-fold decrease in transformation frequency seen for the R + recipient strain is independent of the digestion of GTAC sites in the donor DNA because they are fully protected by DNA methylation. We verified that the recipient strains were not generally defective at transforming DNA by showing allelic replacement at a different chromosomal region (ΔHP0368). In summary, we showed that chromosomal type II HpyAXII R–M system resists its replacement with an empty site in the presence of the active endonuclease R.HpyAXII, as described for other bacterial type II R–M systems (38,39).
Table 4.
The HpyAXII R-M system follows the selfish hypothesis
| Donor DNA | Transformation frequency (# transformants/recipient cell/ng DNA × 10−10)c, mean (SEM) |
Ratio | |
|---|---|---|---|
| Recipient straind |
|||
| R+ | R− | R−/R+ | |
| ΔhpyAXIIM/R::catsacBa | 0.15 (0.08)e | 320 (21) | 2100 |
| ΔhpyAXIIM::catb | 0.17 (0.17)f | 1100 (350) | 6500 |
| ΔHP0368::catb | 310 (9.2) | 180 (38) | 0.58 |
aNSH57 wild-type genomic DNA was constructed by replacing the entire HpyAXII R-M system with the catsacB marker. 500ng genomic DNA per transformation was used.
bA PCR product was engineered to disrupt genes hpyAXIIM or HP0368. The 5′ and 3′ homologous ends of the respective gene was fused to the antibiotic resistance cassette and transformed to generate a null allele by allelic replacement. A 150ng DNA per transformation was used for ΔhpyAXIIM::cat and ΔHP0368::cat.
cTransformation efficiency was based on the average of three replicates.
dAs in Table 2.
eA total of two clones was recovered.
fA total of five clones was recovered.
An active R.HpyAXII REase can only exist in the presence of its active DNA MTase partner
We predicted that bacteria harboring a functional REase must also encode an active cognate DNA MTase to protect the genome from digestion. The R + M− phenotype should therefore be lethal, a condition especially relevant for the HpyAXII R-M system since many of the cognate sites are present within the rRNA genes (Table 1). Thus we deleted hpyAXIIM by allelic replacement using the ΔhpyAXIIM::cat PCR construct in bacteria harboring active (R+) or inactive (R−) endonuclease R.HpyAXII (Supplementary Figure S3). We observed a dramatic reduction in transformation efficiency (6500-fold) for the R + bacteria (Table 4). In comparison, allelic replacement at a different chromosomal region (ΔHP0368) was carried out at a similar efficiency in R + and R- bacteria (Table 4). Importantly, the observed decrease in transformation efficiency is independent of the cleavage of incoming ΔhpyAXIIM::cat donor DNA by the endonuclease R.HpyAXII since this fragment is devoid of GTAC sites. Rather, recombinants for the R+ bacteria probably died from double-stranded breaks in their chromosomal DNA.
We were intrigued by the rare transformants obtained when gene hpyAXIIM was deleted in the R+ bacteria because it suggests that the R+M− phenotype is occasionally viable. We investigated these clones further and found evidence of gene amplification and adaptive mutations at the M.HpyAXII/R.HpyAXII locus. From three independent transformations, we analyzed a total of 16 clones; seven displayed evidence of gene amplification and nine showed adaptive mutations. For gene amplification, hpyAXIIM was present in both a wild-type and a mutated form at the same locus (Supplementary Figure S3), overall resulting in the phenotype R+ M+. This diploidy for hpyAXIIM was unstable and the mutant form of the gene was generally lost after several passages in vitro (data not shown). In other clones, point mutations were found in the promoter region of gene hpyAXIIR, specifically in the ribosome binding sequence and in the start codon of the gene (Figure 3). In all cases, these mutations likely prevent effective translation of the endonuclease R.HpyAXII; the overall phenotype of these clones therefore became R−M−. We conclude that H. pylori bacteria only harbor an active R.HpyAXII endonuclease in the presence of an active M.HpyAXII MTase.
Figure 3.
Deletion of hpyAXIIM from a H. pylori strain harboring an active endonuclease R.HpyAXII yielded rare transformants that display adaptive mutations in the promoter region of hpyAXIIR. Gene hpyAXIIR was sequenced in nine surviving recombinants obtained following hpyAXIIM deletion. When compared to wild-type, single base deletions and substitutions (highlighted in gray) were detected in the transformants in both the ribosomal binding site (RBS) and start codon (START) of hpyAXIIR.
The adenine MTase M.HpyAXII is highly conserved among H. pylori strains
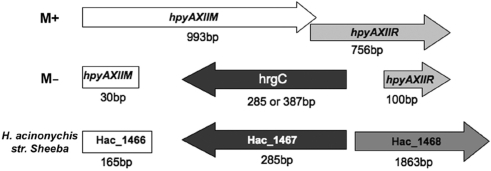
Helicobacter pylori R–M systems are remarkably heterogeneous among strains. Some are present in a fraction of strains, absent in others, or inactivated by deletion or frameshift mutations. It has been estimated that less than one-third of H. pylori R–M systems are fully active (6). We were interested in determining whether M.HpyAXII is present and active in a panel of H. pylori clinical isolates. We found that the HpyAXII R–M system is well conserved as we amplified this locus from all 50 pediatric clinical isolates isolated from 47 patients (S. Talarico, J. Fero, D.T. Thompson, J. Guarner, S. Czinn. B.D. Gold, N.R. Salama, personal communication) though its size was reduced in four (8%) strains (data not shown). To assess M.HpyAXII MTase activity for the clinical isolates, we digested each strain's genomic DNA with the REase RsaI, which only cleaves unmethylated GTAC sites. Of the 50 clinical isolates DNA, only four strains were digested with RsaI, all of which displayed a smaller amplified product for the HpyAXII R–M system (data not shown). M.HpyAXII activity is therefore remarkably conserved among H. pylori strains (92% active as tested). To determine how M.HpyAXII was inactivated in the four H. pylori strains lacking DNA MTase activity, we sequenced the HpyAXII R–M system. In all strains, most of hpyAXIIM and hpyAXIIR coding region was substituted by a gene in reverse orientation (Figure 4). This replacing gene, that we name hrgC (HpyAXII replacing gene C), shows strong homology to Helicobacter acinonychis sheeba Hac_1467 (80% nucleotide identity). Hac_1467 is present at a different chromosomal region in this species (557 kb away from the HpyAXII locus) and encodes a 94 amino acid protein of unknown function. In the four clinical isolates where gene replacement occurred, hrgC encodes a protein of 94- or 128-amino acids. A search for orthologs of Hac_1467 at the H. acinonychis locus using a PCR approach was negative for all 50 clinical isolates and only positive for H. pylori strain NSH57. Surprisingly, in both H. acinonychis sheeba and H. pylori NSH57, Hac_1467 is preceded by a 165 bp DNA fragment (Hac_1466) that shows high homology to the 5′-end of hpyAXIIM (89% sequence identity, Figure 4), suggesting that some recombination event took place at this locus. In summary, we demonstrated that the activity of the DNA MTase M.HpyAXII is highly conserved among H. pylori strains but can be disrupted by insertion of the alternate gene hrgC at the same locus in several unrelated strains.
Figure 4.
M.HpyAXII MTase activity is occasionally disrupted by insertion of the alternate gene hrgC. The HpyAXII R–M system was sequenced in the H. pylori clinical isolates where M.HpyAXII was found inactivated (M−). In all M− strains, most of the HpyAXII locus was replaced with alternate gene hrgC that shows strong homology to Hac_1467 (80% nucleotide identity) from Helicobacter acinonychis sheeba. Depending on the strain analyzed, gene hrgC is either 285- or 387-bp long.
The REase R.HpyAXII is poorly conserved among H. pylori strains
The conservation of M.HpyAXII MTase activity in H. pylori isolates led us to evaluate R.HpyAXII activity in these same isolates. We developed a genetic assay to assess R.HpyAXII endonuclease activity based on the previous finding that the MTase M.HpyAXII cannot be deleted in the presence of an active REase. Since H. pylori strains vary in their ability to be transformed by exogenous DNA, we chose to engineer strain NSH57 to contain the HpyAXII R-M system from the different clinical isolates using allelic replacement and a knockin approach (Materials and methods section). We transformed the resulting clones with the ΔhpyAXIIM::cat donor DNA to delete gene hpyAXIIM. When a large number of transformants were recovered, we deduced that R.HpyAXII from that particular strain lost endonuclease activity. In contrast, when few or no transformants were obtained, we concluded that R.HpyAXII must be active because of the lethality of the R + M− phenotype. The DNA fragment ΔHP0368 was used as transformation control. We first evaluated R.HpyAXII activity in the NSH57 clones engineered to contain the HpyAXII R-M system from H. pylori strains NSH57, J99, NSH79 and HPAG1. As expected from our genomic and biochemical results (Figures 1B and 2), only the clone harboring the HpyAXII R–M system from strain HPAG1 showed a dramatic reduction in transformation efficiency when gene hpyAXIIM was deleted (Supplementary Figure S4). In contrast, clones harboring HpyAXII from strain NSH57, NSH79 and J99 yielded a large number of transformants, confirming that the endonuclease R.HpyAXII is inactive in these strains. This result therefore validates the use of our genetic assay to investigate R.HpyAXII endonuclease activity. We constructed NSH57 clones harboring the HpyAXII R-M system from 37 clinical isolates and found that R.HpyAXII was only active in seven clones (18.9%) based on the number of transformants recovered. All strains could successfully be transformed with the ΔHP0368 PCR product, ruling out a general defect in transformation. We examined the nature of mutations that inactivated R.HpyAXII in the 30 H. pylori isolates by sequencing gene hpyAXIIR: nine strains (30%) contain a large deletion (39AA) similar to the one found in strain NSH57 (Figure 1B), seven strains (23%) harbor nonsense mutations that result in a truncated protein, two strains (7%) have both the deletion and nonsense mutations, and 12 strains (40%) contain single nucleotide polymorphisms (SNPs) in the coding region of gene hpyAXIIR. For four strains, we confirmed that the hpyAXIIR sequence was identical in the engineered strain and in the original isolate, ruling out the possibility that mutations were introduced during the genetic manipulations.
To analyze the inactivating SNPs in more details, we aligned the protein sequences from the 12 inactive and seven active R.HpyAXII enzymes with that of H. pylori strain HPAG1, shown to be active. We identified between two and five amino acid substitutions per strain that may inactivate R.HpyAXII function, resulting in a total of 31 distinct coding SNPs. The nature and frequency of these substitutions are described in Figure 5A and the SNPs for each strain are described in Supplementary Table S3. These coding SNPs were mapped on the primary protein sequence of R.HpyAXII (Figure 5B) as well as on the three-dimensional model based on R.PabI crystal structure (Figure 5C) (40). Out of these 31 SNPs, two (#5 and #18) overlap with residues previously shown to be required for R.PabI activity (40), and seven are found in multiple H. pylori strains (Figure 5A). Several structural motifs (β3, α3, β6, β8, β10 and α4 based on R.PabI) contain a high density of SNPs and may be crucial for R.HpyAXII endonuclease activity.
Figure 5.
Protein sequence analysis of 12 inactive R.HpyAXII enzymes reveal 31 distinct coding SNPs. (A) Comparison of R.HpyAXII protein sequences in 12 H. pylori strains harboring inactive enzyme and in seven strains harboring active enzyme with that from strain HPAG1 identified 31 coding SNPs, enumerated #1 through #31 with the conservation and frequency of each substitution indicated. (C) - conserved, (S) - semiconserved, (-) - unrelated. Changes highlighted in gray were found in multiple strains and changes highlighted in purple were previously shown to be required for R.PabI activity (40). (B) HPAG1 R.HpyAXII and R.PabI protein sequences were aligned and the position of each SNP highlighted. Red, potentially inactivating SNPs numbered #1 through #31; cyan, previously shown to be required for R.PabI activity and target the predicted DNA-binding domain; purple, coding SNPs in R.HpyAXII also required for R.PabI activity. (*) - identical residues, (:) - conserved substitution, and (.) semi-conserved substitution. (C) Coding SNPs were mapped on the three-dimensional model generated for R.HpyAXII based on the R.PabI crystal structure using the same color code used in (B).
H. pylori M.HpyAXII MTase activity is not required for establishing infection in mice
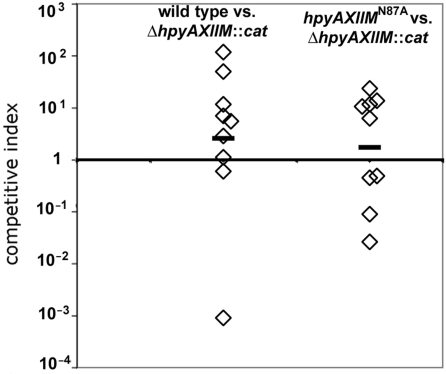
The remarkable preservation of M.HpyAXII activity in H. pylori strains and the intriguing distribution of chromosomal GTAC sites led us to investigate whether this DNA MTase confers a selective advantage to H. pylori during infection. We were also interested in determining whether M.HpyAXII has a role during infection that is independent of its DNA MTase activity. For this reason, we infected mice with H. pylori NSH57 deletion mutant (ΔhpyAXIIM::cat) and with the catalytically inactive mutant (hpyAXIIMN87A), which MTase can presumably still bind DNA but not methylate it. Mouse stomachs were harvested after one week of infection and viable bacteria were recovered and enumerated to calculate the competitive index for each infection [colony forming units (CFU) mutant bacteria/CFU wild-type bacteria] (Materials and methods section). When mice were infected with NSH57ΔhpyAXIIM::cat mutant and wild-type bacteria, equal number of bacteria were recovered (n = 9, Figure 6 left) indicating that they could establish infection at a similar efficiency. M.HpyAXII protein expression is therefore not required for establishing infection in H. pylori strain NSH57. We also infected mice in competition with the catalytically inactive mutant hpyAXIIN87A and the deletion mutant ΔhpyAXIIM::cat. Both strains infected mice similarly (n = 9, Figure 6 right) indicating that M.HpyAXII does not have a role during establishment of infection that is independent of its DNA MTase activity.
Figure 6.
M.HpyAXII MTase activity is not required by H. pylori for establishing infection in a mouse infection model. Mice were infected in 1:1 competition with the indicated H. pylori strains and the competitive index (CI) determined after one week of infection. The CI was calculated by dividing colony forming units (CFU) recovered of null mutant bacteria over CFU wild-type or point mutant bacteria and corrected by dividing the actual input ratio enumerated from plating the inoculums. Left, mice (n = 9) were infected with H. pylori NSH57 wild-type and an isogenic null mutant ΔhpyAXIIM::cat. Both strains infected mice at a similar efficiency (geometric mean CI slightly above one). Right, mice (n = 9) were infected with the NSH57ΔhpyAXIIM::cat mutant and the catalytically inactive mutant (hpyAXIIMN87A) constructed in strain NSH57.
DISCUSSION
Helicobacter pylori naturally takes up exogenous DNA, a condition thought to promote genetic diversification by spreading new alleles in the population. However, genetic exchange is limited by the large number and heterogeneity of H. pylori R–M systems (6). The contribution of individual R–M systems in restricting incoming DNA remains for the most part undetermined since only a few of these systems have individually been tested in H. pylori (37,41). In addition, it is possible that R–M systems carry out other biological roles. In our study, we have biochemically characterized a novel H. pylori type II R–M system, M.HpyAXII/R.HpyAXII, and showed that it targets GTAC sites thus confirming prior predictions (35). The endonuclease R.HpyAXII effectively restricted both unmethylated plasmid (60-fold) and genomic (240-fold) DNA (Tables 2 and 3). Our results agree with previous reports documenting effective restriction of plasmid DNA by R.HpyAIII (encoded by HP0091, GATC) (37), and of chromosomal DNA by R.HpyAII (encoded by HP1366, GAAGA) (41).
Since R–M systems are highly polymorphic in H. pylori, we analyzed the conservation of both M.HpyAXII MTase and R.HpyAXII endonuclease activities in a panel of clinical isolates. We found a striking asymmetry between the highly conserved MTase activity (92% active, n = 50) and that of the REase (18.9% active, n = 37). In fact, our data indicate that M.HpyAXII is the third most highly conserved adenine MTase in H. pylori, preceded only by M.HpyAI (CATG, 100% conserved) and M.HpyAIII (GATC, 97% conserved) (42,43). Other studies have suggested that MTase activities are generally selected for in H. pylori (6,29,44) but how DNA methylation benefits the organism is unknown. To begin to probe how M.HpyAXII affects H. pylori biology, we generated a null allele in gene hpyAXIIM in mouse-adapted strain NSH57 either by deletion or by targeted mutation of the catalytic site of the enzyme (hpyAXIIMN87A). Mouse infection with these strains indicated that M.HpyAXII expression is not required by H. pylori for the establishment of infection (Figure 6) in contrast to previous work (19). As this study did not complement the mutant phenotype, it is possible that second site mutations or polar effects accounted for the defect observed. Further experiments are needed to determine if M.HpyAXII activity is important during persistent infection and to elucidate how GTAC methylation provides a selective advantage in H. pylori. It was proposed that the maintenance of an active MTase allows reactivation of a given R–M system through recombination with the genomic DNA of a strain that encodes an active REase. However, the probability of this event must be relatively low given that R.HpyAXII is active in a small number of strains. It was also postulated that ‘orphan’ MTases are conserved because they act as a ‘molecular vaccine’ against the parasitism of R–M systems. This hypothesis was based on the finding that Dcm in E. coli can alleviate postsegregational killing induced by the EcoRII R–M complex that recognizes the same sequence as Dcm (45). This also seems unlikely because no R–M system that acts on the same site as HpyAXII has been identified in H. pylori. DNA methylation may alternatively be selected for in H. pylori because it confers immunity from the action of the cognate REase and therefore increases the chances that an entire gene is successfully propagated in a population. However, the finding that the average size of recombined fragment is unusually small (417 bp) in H. pylori as compared to other bacteria indicates that incoming DNA is generally susceptible to cleavage by REases (46).
Another important role of DNA methylation is to modulate the interaction between proteins and DNA as demonstrated for the stand-alone adenine MTase Dam in α- and γ-Proteobacteria (14,15,47). DNA MTases involved in R–M systems that are not coupled to an active REase may have evolved similar regulatory functions since they are no longer under the pressure to act on all chromosomal sites and may therefore preferentially methylate sequences required for transcription. The genome-wide analysis of conserved GTAC sites identified genes of various functional classes including several genes of the cag pathogenicity island but it is unknown whether their expression is affected by M.HpyAXII methylation. Although GTAC sites are scarce in the H. pylori chromosome, they are present at high frequency in the 23S and 16S rRNA genes. Our preliminary experiments showed no effect of M.HpyAXII methylation on 23S rRNA transcripts levels or on in vitro growth of the bacteria (data not shown), arguing against a role for GTAC methylation in H. pylori ribosomal biogenesis. It is possible that ribosomal GTAC sites are preserved because they are crucial for the folding of the RNA into functional secondary structures in Helicobacter species and that methylation of these sites does not have any biological role. In agreement with this hypothesis, the analysis of 23S rRNA sequences in 55 Helicobacter strains representing 41 taxa (48) shows conservation of most GTAC sites even though many of these species probably lack a M.HpyAXII enzyme. Alternatively, ribosomal GTAC sites may not have been erased from these chromosomal regions because they evolve at a different rate than the rest of the genome; these elements are duplicated in H. pylori and are subjected to homogenization processes such as gene conversion (49). Overall, the significance of ribosomal GTAC sites in H. pylori remains unsolved but echoes a similar anomaly in site distribution with the tetramer CTAG that is very rare in E. coli and in other proteobacterial genomes and over-represented in rRNA genes (50,51).
The comparison of tetranucleotide combinations in 27 representative microbial genomes revealed that ACGT, GTAC and TCGA are strongly underrepresented in both coding and noncoding DNA in H. pylori (52). A significant correlation between the avoidance of palindromic words and type II R–M systems was suggested (53) presumably because these systems exert significant pressure on an organism when restriction is intact but methylation is incomplete. However, the highly conserved H. pylori R–M systems M.HpyAI/iceA (CATG) or M.HpyAIII/R.HpyAIII (GATC) target sites are not underrepresented in the H. pylori genome. Since cognate site avoidance is probably dependent on the timing of acquisition of a given R–M system during evolution, the HpyAXII R–M system must have been acquired a long time ago, consistent with the finding that this system is present in all H. pylori isolates analyzed. Interestingly, GTAC sites are also scarce in the Helicobacter hepaticus genome even though it does not encode an M.HpyAXII/R.HpyAXII ortholog. This can either be explained by the recent loss of this system or by the existence of a different R–M system that acts on GTAC sites in H. hepaticus.
Our study of the conservation of M.HpyAXII MTase activity uncovered the occasional replacement of the HpyAXII R-M system with an alternate gene that we re-annotate as hrgC (HpyAXII replacing gene C). Other H. pylori R–M systems encoding the two most highly conserved MTases M.HpyAI and M.HpyAIII are similarly replaced with genes of unknown function. In the HpyAIII R–M system, gene hrgA inactivated the REase R.HpyAIII in 33% of the 208 strains analyzed but rarely inactivated M.HpyAIII function (54). hrgA encodes a 370 amino acid protein of unknown function and this gene was associated with gastric cancer in H. pylori isolates from Asian patients but not among Western strains (55). In the HpyAI R–M system, the REase encoded by iceA1 was replaced with iceA2 in 44% of the strains analyzed (56). iceA2 (renamed hrgB) encodes a polymorphic protein of unknown function ranging from 24 to 59 amino acid long depending on the strain studied, and epidemiological studies have correlated the presence of gene iceA1 in H. pylori strains with the incidence of peptidic ulcers disease (57). In our study, we showed that similarly to hrgA and hrgB, hrgC encodes a protein of unknown function but its origin could be traced to a different locus in H. acinonychis sheeba and H. pylori strain NSH57 (G27). Unlike hrgA and hrgB, hrgC replacement always compromised both the MTase and REase activities in our analysis of 37 clinical isolates. Further work will define whether hrgC has any role in H. pylori pathogenicity.
As predicted for a type II R–M system, we showed that deletion of gene hpyAXIIM is lethal in the presence of an active R.HpyAXII endonuclease. The lethal phenotype R+M− can be rescued by adaptive mutations, in the form of gene amplification of hpyAXIIM and null mutations in the promoter region of hpyAXIIR. This result is reminiscent of extensive research describing stress-induced mutations in E. coli with the Lac assay system (58). Based on these findings, we suggest that these adaptive mutations preexisted in the H. pylori population and were selected for as a result of hpyAXIIM deletion. Tandem amplification was previously documented for R–M systems, as when the entire BamHI R–M system was deleted from Bacillus subtilis (38). In another study, large-scale genomic rearrangements were demonstrated when the PaeR71 R–M system was deleted from E. coli (39). These rearrangements involved recombination between copies of the transposon IS3 and were proposed to have resulted from multiple rounds of unequal crossing-over or rolling circle amplification. Our study of the deletion of the MTase gene in the HpyAXII R-M system is the first to demonstrate the occurrence of compensatory mutations in the promoter of the respective REase gene.
Orthologs of M.HpyAXII generally show poor sequence homology except for one gene of unknown function from Campylobacter upsaliensis RM3195 (CUP1644) that shows 43% identity with M.HpyAXII. Other adenine MTase that target GTAC sites such as M. PabI from Pyroccoccus abyssi and M.CviQI from Chlorella virus only show 14.6% and 17.8% protein identity, respectively. M.RsaI (7.9% protein identity) from Rhodopseudomonas sphaeroides and M.MjaV from Methanococcus janaschii (12.7% protein identity) act on the same sequence but methylates cytosines (m4C). GTAC sites are strongly avoided from the genomes of R. sphaeroides and M. jannashi but not from P. abyssi, suggesting that the PabI R–M system invaded this organism recently as discussed previously (35,59). The crystal structure of the endonuclease R.PabI was recently solved and unveiled a novel protein fold termed ‘half-pipe’ (40). Although the two isochizomers R.PabI and R.HpyAXII show relatively weak sequence identity (25.4%), several conserved motifs are shared between the two proteins (Figure 5B). The R.HpyAXII polypeptide could be threaded with high confidence onto the tertiary structure of R.PabI (PHYRE, E-value 3e-26, Figure 5C) suggesting that R.HpyAXII folds into a similar half-pipe structure. Among the thousands of confirmed and hypothetical REase enzymes identified, Orlowski and Bujnicki predicted that only eight present the half-pipe fold (60). R.HpyAXII therefore constitutes an excellent candidate for refined biochemical analyses that will enrich our understanding of this new class of enzymes.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
National Institutes of Health (AI054423 and DK53708 to N.R.S., AI55396 to O.H.). Funding for open access charges: National Institutes of Health (AI054423).
Conflict of interest statement. The project's contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Marion Dorer for critical reading of the article, Laura Sycuro for generating the catsacB cassette, New England Biolabs for providing the m6A antibodies and Barry Stoddard for helpful discussions.
REFERENCES
- 1.Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin. Microbiol. Rev. 2006;19:449–490. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alm RA, Ling LS, Moir DT, King BL, Brown ED, Doig PC, Smith DR, Noonan B, Guild BC, deJonge BL, et al. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;397:176–180. doi: 10.1038/16495. [DOI] [PubMed] [Google Scholar]
- 3.Oh JD, Kling-Backhed H, Giannakis M, Xu J, Fulton RS, Fulton LA, Cordum HS, Wang C, Elliott G, Edwards J, et al. The complete genome sequence of a chronic atrophic gastritis Helicobacter pylori strain: evolution during disease progression. Proc. Natl Acad. Sci. USA. 2006;103:9999–10004. doi: 10.1073/pnas.0603784103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts RJ, Belfort M, Bestor T, Bhagwat AS, Bickle TA, Bitinaite J, Blumenthal RM, Degtyarev S, Dryden DT, Dybvig K, et al. A nomenclature for restriction enzymes, DNA methyltransferases, homing endonucleases and their genes. Nucleic Acids Res. 2003;31:1805–1812. doi: 10.1093/nar/gkg274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts RJ, Vincze T, Posfai J, Macelis D. REBASE—enzymes and genes for DNA restriction and modification. Nucleic Acids Res. 2007;35:D269–D270. doi: 10.1093/nar/gkl891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin LF, Posfai J, Roberts RJ, Kong H. Comparative genomics of the restriction-modification systems in Helicobacter pylori. Proc. Natl Acad. Sci. USA. 2001;98:2740–2745. doi: 10.1073/pnas.051612298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nobusato A, Uchiyama I, Kobayashi I. Diversity of restriction-modification gene homologues in Helicobacter pylori. Gene. 2000;259:89–98. doi: 10.1016/s0378-1119(00)00455-8. [DOI] [PubMed] [Google Scholar]
- 8.Tock MR, Dryden DT. The biology of restriction and anti-restriction. Curr. Opin. Microbiol. 2005;8:466–472. doi: 10.1016/j.mib.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Israel DA, Lou AS, Blaser MJ. Characteristics of Helicobacter pylori natural transformation. FEMS Microbiol. Lett. 2000;186:275–280. doi: 10.1111/j.1574-6968.2000.tb09117.x. [DOI] [PubMed] [Google Scholar]
- 10.Jeltsch A. Maintenance of species identity and controlling speciation of bacteria: a new function for restriction/modification systems? Gene. 2003;317:13–16. doi: 10.1016/s0378-1119(03)00652-8. [DOI] [PubMed] [Google Scholar]
- 11.Price C, Bickle TA. A possible role for DNA restriction in bacterial evolution. Microbiol. Sci. 1986;3:296–299. [PubMed] [Google Scholar]
- 12.Naito T, Kusano K, Kobayashi I. Selfish behavior of restriction-modification systems. Science. 1995;267:897–899. doi: 10.1126/science.7846533. [DOI] [PubMed] [Google Scholar]
- 13.Mochizuki A, Yahara K, Kobayashi I, Iwasa Y. Genetic addiction: selfish gene. Genetics. 2006;172:1309–1323. doi: 10.1534/genetics.105.042895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Low DA, Casadesus J. Clocks and switches: bacterial gene regulation by DNA adenine methylation. Curr. Opin. Microbiol. 2008;11:106–112. doi: 10.1016/j.mib.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Wion D, Casadesus J. N6-methyl-adenine: an epigenetic signal for DNA-protein interactions. Nat. Rev. Microbiol. 2006;4:183–192. doi: 10.1038/nrmicro1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gressmann H, Linz B, Ghai R, Pleissner KP, Schlapbach R, Yamaoka Y, Kraft C, Suerbaum S, Meyer TF, Achtman M. Gain and loss of multiple genes during the evolution of Helicobacter pylori. PLoS Genet. 2005;1:e43. doi: 10.1371/journal.pgen.0010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Woude MW. Re-examining the role and random nature of phase variation. FEMS Microbiol. Lett. 2006;254:190–197. doi: 10.1111/j.1574-6968.2005.00038.x. [DOI] [PubMed] [Google Scholar]
- 18.de Vries N, Duinsbergen D, Kuipers EJ, Pot RG, Wiesenekker P, Penn CW, van Vliet AH, Vandenbroucke-Grauls CM, Kusters JG. Transcriptional phase variation of a type III restriction-modification system in Helicobacter pylori. J. Bacteriol. 2002;184:6615–6623. doi: 10.1128/JB.184.23.6615-6623.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baldwin DN, Shepherd B, Kraemer P, Hall MK, Sycuro LK, Pinto-Santini DM, Salama NR. Identification of Helicobacter pylori genes that contribute to stomach colonization. Infect. Immun. 2007;75:1005–1016. doi: 10.1128/IAI.01176-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peek RM, Jr, Thompson SA, Donahue JP, Tham KT, Atherton JC, Blaser MJ, Miller GG. Adherence to gastric epithelial cells induces expression of a Helicobacter pylori gene, iceA, that is associated with clinical outcome. Proc. Assoc. Am. Physicians. 1998;110:531–544. [PubMed] [Google Scholar]
- 21.Lin TL, Shun CT, Chang KC, Wang JT. Isolation and characterization of a HpyC1I restriction-modification system in Helicobacter pylori. J. Biol. Chem. 2004;279:11156–11162. doi: 10.1074/jbc.M311639200. [DOI] [PubMed] [Google Scholar]
- 22.Skoglund A, Bjorkholm B, Nilsson C, Andersson AF, Jernberg C, Schirwitz K, Enroth C, Krabbe M, Engstrand L. Functional analysis of the M.HpyAIV DNA methyltransferase of Helicobacter pylori. J. Bacteriol. 2007;189:8914–8921. doi: 10.1128/JB.00108-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ausubel F, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Short Protocols in Molecular Biology. New York: John Wiley & Sons; 1997. [Google Scholar]
- 24.Amundsen SK, Fero J, Hansen LM, Cromie GA, Solnick JV, Smith GR, Salama NR. Helicobacter pylori AddAB helicase-nuclease and RecA promote recombination-related DNA repair and survival during stomach colonization. Mol. Microbiol. 2008;69:994–1007. doi: 10.1111/j.1365-2958.2008.06336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menard R, Sansonetti PJ, Parsot C. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 1993;175:5899–5906. doi: 10.1128/jb.175.18.5899-5906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Copass M, Grandi G, Rappuoli R. Introduction of unmarked mutations in the Helicobacter pylori vacA gene with a sucrose sensitivity marker. Infect Immun. 1997;65:1949–1952. doi: 10.1128/iai.65.5.1949-1952.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Roos KP, Taylor DE. Transformation of Helicobacter pylori by chromosomal metronidazole resistance and by a plasmid with a selectable chloramphenicol resistance marker. J. General Microbiol. 1993;139:2485–2493. doi: 10.1099/00221287-139-10-2485. [DOI] [PubMed] [Google Scholar]
- 28.Woodcock DM, Crowther PJ, Doherty J, Jefferson S, DeCruz E, Noyer-Weidner M, Smith SS, Michael MZ, Graham MW. Quantitative evaluation of Escherichia coli host strains for tolerance to cytosine methylation in plasmid and phage recombinants. Nucleic Acids Res. 1989;17:3469–3478. doi: 10.1093/nar/17.9.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kong H, Lin LF, Porter N, Stickel S, Byrd D, Posfai J, Roberts RJ. Functional analysis of putative restriction-modification system genes in the Helicobacter pylori J99 genome. Nucleic Acids Res. 2000;28:3216–3223. doi: 10.1093/nar/28.17.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peterson JD, Umayam LA, Dickinson T, Hickey EK, White O. The comprehensive microbial resource. Nucleic Acids Res. 2001;29:123–125. doi: 10.1093/nar/29.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bennett-Lovsey RM, Herbert AD, Sternberg MJ, Kelley LA. Exploring the extremes of sequence/structure space with ensemble fold recognition in the program Phyre. Proteins. 2008;70:611–625. doi: 10.1002/prot.21688. [DOI] [PubMed] [Google Scholar]
- 32.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 33.DeLano WL. The PyMOL Molecular Graphics System. 2002. Available at http://www.pymol.org.
- 34.Malone T, Blumenthal RM, Cheng X. Structure-guided analysis reveals nine sequence motifs conserved among DNA amino-methyltransferases, and suggests a catalytic mechanism for these enzymes. J. Mol. Biol. 1995;253:618–632. doi: 10.1006/jmbi.1995.0577. [DOI] [PubMed] [Google Scholar]
- 35.Ishikawa K, Watanabe M, Kuroita T, Uchiyama I, Bujnicki JM, Kawakami B, Tanokura M, Kobayashi I. Discovery of a novel restriction endonuclease by genome comparison and application of a wheat-germ-based cell-free translation assay: PabI (5'-GTA/C) from the hyperthermophilic archaeon Pyrococcus abyssi. Nucleic Acids Res. 2005;33:e112. doi: 10.1093/nar/gni113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobayashi I. Behavior of restriction-modification systems as selfish mobile elements and their impact on genome evolution. Nucleic Acids Res. 2001;29:3742–3756. doi: 10.1093/nar/29.18.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ando T, Xu Q, Torres M, Kusugami K, Israel DA, Blaser MJ. Restriction-modification system differences in Helicobacter pylori are a barrier to interstrain plasmid transfer. Mol. Microbiol. 2000;37:1052–1065. doi: 10.1046/j.1365-2958.2000.02049.x. [DOI] [PubMed] [Google Scholar]
- 38.Sadykov M, Asami Y, Niki H, Handa N, Itaya M, Tanokura M, Kobayashi I. Multiplication of a restriction-modification gene complex. Mol. Microbiol. 2003;48:417–427. doi: 10.1046/j.1365-2958.2003.03464.x. [DOI] [PubMed] [Google Scholar]
- 39.Handa N, Nakayama Y, Sadykov M, Kobayashi I. Experimental genome evolution: large-scale genome rearrangements associated with resistance to replacement of a chromosomal restriction-modification gene complex. Mol. Microbiol. 2001;40:932–940. doi: 10.1046/j.1365-2958.2001.02436.x. [DOI] [PubMed] [Google Scholar]
- 40.Miyazono K, Watanabe M, Kosinski J, Ishikawa K, Kamo M, Sawasaki T, Nagata K, Bujnicki JM, Endo Y, Tanokura M, et al. Novel protein fold discovered in the PabI family of restriction enzymes. Nucleic Acids Res. 2007;35:1908–1918. doi: 10.1093/nar/gkm091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aras RA, Small AJ, Ando T, Blaser MJ. Helicobacter pylori interstrain restriction-modification diversity prevents genome subversion by chromosomal DNA from competing strains. Nucleic Acids Res. 2002;30:5391–5397. doi: 10.1093/nar/gkf686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takata T, Aras R, Tavakoli D, Ando T, Olivares AZ, Blaser MJ. Phenotypic and genotypic variation in methylases involved in type II restriction-modification systems in Helicobacter pylori. Nucleic Acids Res. 2002;30:2444–2452. doi: 10.1093/nar/30.11.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vale FF, Vitor JM. Genomic methylation: a tool for typing Helicobacter pylori isolates. Appl. Environ. Microbiol. 2007;73:4243–4249. doi: 10.1128/AEM.00199-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vitkute J, Stankevicius K, Tamulaitiene G, Maneliene Z, Timinskas A, Berg DE, Janulaitis A. Specificities of eleven different DNA methyltransferases of Helicobacter pylori strain 26695. J. Bacteriol. 2001;183:443–450. doi: 10.1128/JB.183.2.443-450.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takahashi N, Naito Y, Handa N, Kobayashi I. A DNA methyltransferase can protect the genome from postdisturbance attack by a restriction-modification gene complex. J. Bacteriol. 2002;184:6100–6108. doi: 10.1128/JB.184.22.6100-6108.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Falush D, Kraft C, Taylor NS, Correa P, Fox JG, Achtman M, Suerbaum S. Recombination and mutation during long-term gastric colonization by Helicobacter pylori: estimates of clock rates, recombination size, and minimal age. Proc. Natl Acad. Sci. USA. 2001;98:15056–15061. doi: 10.1073/pnas.251396098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Casadesus J, Low D. Epigenetic gene regulation in the bacterial world. Microbiol. Mol. Biol. Rev. 2006;70:830–856. doi: 10.1128/MMBR.00016-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dewhirst FE, Shen Z, Scimeca MS, Stokes LN, Boumenna T, Chen T, Paster BJ, Fox JG. Discordant 16S and 23S rRNA gene phylogenies for the genus Helicobacter: implications for phylogenetic inference and systematics. J. Bacteriol. 2005;187:6106–6118. doi: 10.1128/JB.187.17.6106-6118.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liao D. Gene conversion drives within genic sequences: concerted evolution of ribosomal RNA genes in bacteria and archaea. J. Mol. Evol. 2000;51:305–317. doi: 10.1007/s002390010093. [DOI] [PubMed] [Google Scholar]
- 50.Burge C, Campbell AM, Karlin S. Over- and under-representation of short oligonucleotides in DNA sequences. Proc. Natl Acad. Sci. USA. 1992;89:1358–1362. doi: 10.1073/pnas.89.4.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vandenbogaert M, Makeev V. Analysis of bacterial RM-systems through genome-scale analysis and related taxonomy issues. In Silico Biol. 2003;3:127–143. [PubMed] [Google Scholar]
- 52.Pride DT, Meinersmann RJ, Wassenaar TM, Blaser MJ. Evolutionary implications of microbial genome tetranucleotide frequency biases. Genome Res. 2003;13:145–158. doi: 10.1101/gr.335003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gelfand MS, Koonin EV. Avoidance of palindromic words in bacterial and archaeal genomes: a close connection with restriction enzymes. Nucleic Acids Res. 1997;25:2430–2439. doi: 10.1093/nar/25.12.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ando T, Aras RA, Kusugami K, Blaser MJ, Wassenaar TM. Evolutionary history of hrgA, which replaces the restriction gene hpyIIIR in the hpyIII locus of Helicobacter pylori. J. Bacteriol. 2003;185:295–301. doi: 10.1128/JB.185.1.295-301.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ando T, Wassenaar TM, Peek RM, Jr, Aras RA, Tschumi AI, van Doorn LJ, Kusugami K, Blaser MJ. A Helicobacter pylori restriction endonuclease-replacing gene, hrgA, is associated with gastric cancer in Asian strains. Cancer Res. 2002;62:2385–2389. [PubMed] [Google Scholar]
- 56.Figueiredo C, Quint WG, Sanna R, Sablon E, Donahue JP, Xu Q, Miller GG, Peek RM, Jr, Blaser MJ, van Doorn LJ. Genetic organization and heterogeneity of the iceA locus of Helicobacter pylori. Gene. 2000;246:59–68. doi: 10.1016/s0378-1119(00)00054-8. [DOI] [PubMed] [Google Scholar]
- 57.van Doorn LJ, Figueiredo C, Sanna R, Plaisier A, Schneeberger P, de Boer W, Quint W. Clinical relevance of the cagA, vacA, and iceA status of Helicobacter pylori. Gastroenterology. 1998;115:58–66. doi: 10.1016/s0016-5085(98)70365-8. [DOI] [PubMed] [Google Scholar]
- 58.Hastings PJ. Adaptive amplification. Crit. Rev. Biochem. Mol. Biol. 2007;42:271–283. doi: 10.1080/10409230701507757. [DOI] [PubMed] [Google Scholar]
- 59.Chinen A, Uchiyama I, Kobayashi I. Comparison between Pyrococcus horikoshii and Pyrococcus abyssi genome sequences reveals linkage of restriction-modification genes with large genome polymorphisms. Gene. 2000;259:109–121. doi: 10.1016/s0378-1119(00)00459-5. [DOI] [PubMed] [Google Scholar]
- 60.Orlowski J, Bujnicki JM. Structural and evolutionary classification of Type II restriction enzymes based on theoretical and experimental analyses. Nucleic Acids Res. 2008;36:3552–3569. doi: 10.1093/nar/gkn175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tomb JF, White O, Kerlavage AR, Clayton RA, Sutton GG, Fleischmann RD, Ketchum KA, Klenk HP, Gill S, Dougherty BA, et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.