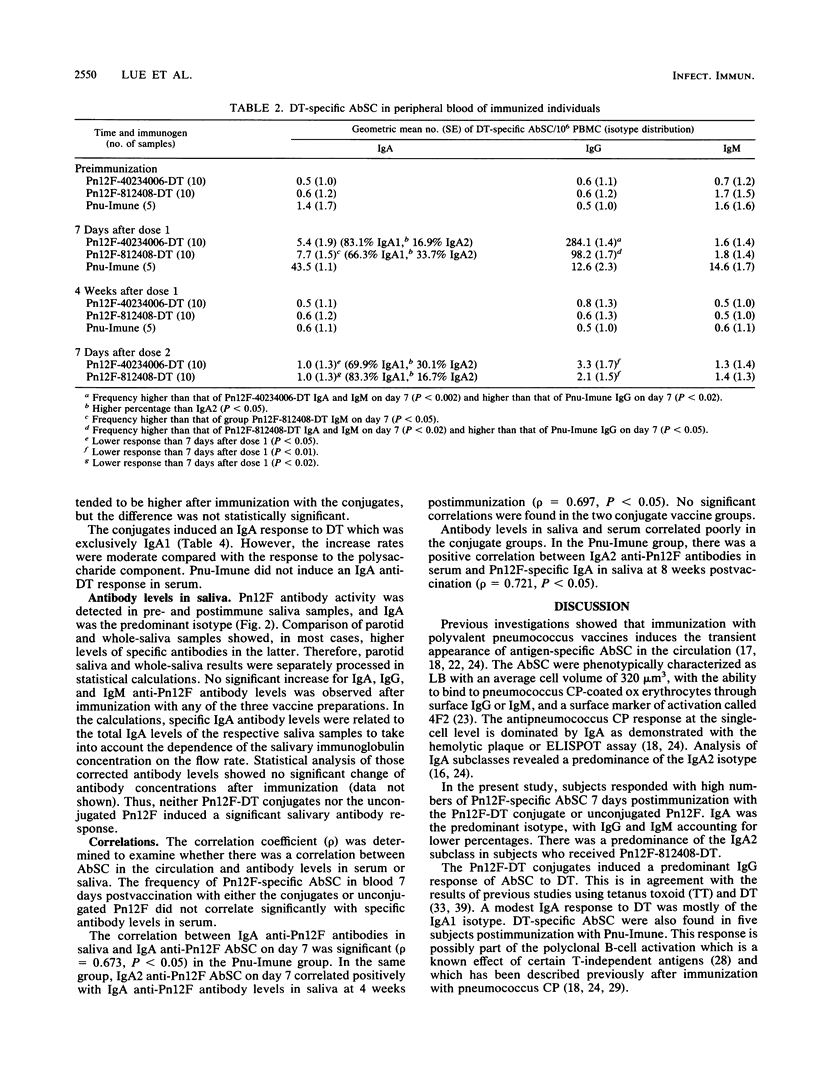
Abstract
Healthy adult volunteers were injected either with one of two conjugates composed of Streptococcus pneumoniae type 12F polysaccharide (Pn12F) covalently coupled to diphtheria toxoid or with Pn12F alone (as a component of Pnu-Imune, a 23-valent pneumococcus vaccine). The conjugates induced Pn12F-specific antibody-secreting cells in peripheral blood with numbers and isotype distribution similar to those induced by Pnu-Imune, with immunoglobulin A (IgA) as the predominant isotype. The conjugates also elicited high numbers of diphtheria toxoid-specific antibody-secreting cells of the IgG class. There was no distinct booster effect, since a second dose of the conjugates induced antibody-secreting cells at significantly lower numbers than after the first dose. In contrast to the cell numbers, the conjugate vaccines induced higher increases of IgA1 Pn12F antibodies in serum than did Pnu-Imune. However, neither the conjugates nor Pnu-Imune induced a secretory antibody response. Antibody levels in serum and saliva correlated poorly with the frequency of antigen-specific antibody-secreting cells. Circulating antibody-secreting cells present 7 days postimmunization were probably not responsible for the high increase of antibodies in serum but rather represented a population of in vivo-activated B cells with the ability to disseminate the humoral response from the antigen recognition site to distant locations of antibody production.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. W., Pichichero M. E., Insel R. A., Betts R., Eby R., Smith D. H. Vaccines consisting of periodate-cleaved oligosaccharides from the capsule of Haemophilus influenzae type b coupled to a protein carrier: structural and temporal requirements for priming in the human infant. J Immunol. 1986 Aug 15;137(4):1181–1186. [PubMed] [Google Scholar]
- Bolan G., Broome C. V., Facklam R. R., Plikaytis B. D., Fraser D. W., Schlech W. F., 3rd Pneumococcal vaccine efficacy in selected populations in the United States. Ann Intern Med. 1986 Jan;104(1):1–6. doi: 10.7326/0003-4819-104-1-1. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Fjellanger I., Gjeruldsen S. T. Human secretory immunoglobulins. I. Salivary secretions from individuals with normal or low levels of serum immunoglobulins. Scand J Haematol Suppl. 1970;12:3–83. [PubMed] [Google Scholar]
- Brieva J. A., Stevens R. H. Human in vivo antigen-induced lymphoblastoid B cells are capable of cyclical antibody production in vitro. J Immunol. 1984 Jul;133(1):147–153. [PubMed] [Google Scholar]
- Brieva J. A., Targan S., Stevens R. H. NK and T cell subsets regulate antibody production by human in vivo antigen-induced lymphoblastoid B cells. J Immunol. 1984 Feb;132(2):611–615. [PubMed] [Google Scholar]
- Claesson B. A., Trollfors B., Lagergard T., Taranger J., Bryla D., Otterman G., Cramton T., Yang Y., Reimer C. B., Robbins J. B. Clinical and immunologic responses to the capsular polysaccharide of Haemophilus influenzae type b alone or conjugated to tetanus toxoid in 18- to 23-month-old children. J Pediatr. 1988 May;112(5):695–702. doi: 10.1016/s0022-3476(88)80684-x. [DOI] [PubMed] [Google Scholar]
- Czerkinsky C., Moldoveanu Z., Mestecky J., Nilsson L. A., Ouchterlony O. A novel two colour ELISPOT assay. I. Simultaneous detection of distinct types of antibody-secreting cells. J Immunol Methods. 1988 Nov 25;115(1):31–37. doi: 10.1016/0022-1759(88)90306-7. [DOI] [PubMed] [Google Scholar]
- Douglas R. M., Hansman D., Miles H. B., Paton J. C. Pneumococcal carriage and type-specific antibody. Failure of a 14-valent vaccine to reduce carriage in healthy children. Am J Dis Child. 1986 Nov;140(11):1183–1185. doi: 10.1001/archpedi.1986.02140250109044. [DOI] [PubMed] [Google Scholar]
- Einhorn M. S., Weinberg G. A., Anderson E. L., Granoff P. D., Granoff D. M. Immunogenicity in infants of Haemophilus influenzae type B polysaccharide in a conjugate vaccine with Neisseria meningitidis outer-membrane protein. Lancet. 1986 Aug 9;2(8502):299–302. doi: 10.1016/s0140-6736(86)90001-2. [DOI] [PubMed] [Google Scholar]
- Fattom A., Lue C., Szu S. C., Mestecky J., Schiffman G., Bryla D., Vann W. F., Watson D., Kimzey L. M., Robbins J. B. Serum antibody response in adult volunteers elicited by injection of Streptococcus pneumoniae type 12F polysaccharide alone or conjugated to diphtheria toxoid. Infect Immun. 1990 Jul;58(7):2309–2312. doi: 10.1128/iai.58.7.2309-2312.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattom A., Vann W. F., Szu S. C., Sutton A., Li X., Bryla D., Schiffman G., Robbins J. B., Schneerson R. Synthesis and physicochemical and immunological characterization of pneumococcus type 12F polysaccharide-diphtheria toxoid conjugates. Infect Immun. 1988 Sep;56(9):2292–2298. doi: 10.1128/iai.56.9.2292-2298.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldbrush T. L., Stewart N. Antigen modulation of the immune response. V. Generation of large memory cells in antigen draining lymph nodes. Cell Immunol. 1978 May;37(2):336–348. doi: 10.1016/0008-8749(78)90202-2. [DOI] [PubMed] [Google Scholar]
- Gray B. M. ELISA methodology for polysaccharide antigens: protein coupling of polysaccharides for adsorption to plastic tubes. J Immunol Methods. 1979;28(1-2):187–192. doi: 10.1016/0022-1759(79)90340-5. [DOI] [PubMed] [Google Scholar]
- Heilmann C., Barington T., Sigsgaard T. Subclass of individual IgA-secreting human lymphocytes. Investigation of in vivo pneumococcal polysaccharide-induced and in vitro mitogen-induced blood B cells by monolayer plaque-forming cell assays. J Immunol. 1988 Mar 1;140(5):1496–1499. [PubMed] [Google Scholar]
- Heilmann C., Henrichsen J., Pedersen F. K. Vaccination-induced circulation of human B cells secreting type-specific antibodies against pneumococcal polysaccharides. Scand J Immunol. 1987 Jan;25(1):61–67. doi: 10.1111/j.1365-3083.1987.tb01047.x. [DOI] [PubMed] [Google Scholar]
- Heilmann C., Pedersen F. K. Quantitation of blood lymphocytes secreting antibodies to pneumococcal polysaccharides after in vivo antigenic stimulation. Scand J Immunol. 1986 Feb;23(2):189–194. doi: 10.1111/j.1365-3083.1986.tb01957.x. [DOI] [PubMed] [Google Scholar]
- Heilmann C. Vaccination-induced activation of human blood T cells suppressing pneumococcal polysaccharide-specific B cells. Acta Pathol Microbiol Immunol Scand C. 1987 Apr;95(2):65–69. doi: 10.1111/j.1699-0463.1987.tb00010.x. [DOI] [PubMed] [Google Scholar]
- Hill S. W. Distribution of plaque-forming cells in the mouse for a protein antigen. Evidence for highly active parathymic lymph nodes following intraperitoneal injection of hen lysozyme. Immunology. 1976 Jun;30(6):895–906. [PMC free article] [PubMed] [Google Scholar]
- Insel R. A., Anderson P. W. Oligosaccharide-protein conjugate vaccines induce and prime for oligoclonal IgG antibody responses to the Haemophilus influenzae b capsular polysaccharide in human infants. J Exp Med. 1986 Feb 1;163(2):262–269. doi: 10.1084/jem.163.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel R. A., Kittelberger A., Anderson P. Isoelectric focusing of human antibody to the Haemophilus influenzae b capsular polysaccharide: restricted and identical spectrotypes in adults. J Immunol. 1985 Oct;135(4):2810–2816. [PubMed] [Google Scholar]
- Kehrl J. H., Fauci A. S. Activation of human B lymphocytes after immunization with pneumococcal polysaccharides. J Clin Invest. 1983 Apr;71(4):1032–1040. doi: 10.1172/JCI110830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrl J. H., Fauci A. S. Identification, purification, and characterization of antigen-activated and antigen-specific human B lymphocytes. J Exp Med. 1983 May 1;157(5):1692–1697. doi: 10.1084/jem.157.5.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue C., Tarkowski A., Mestecky J. Systemic immunization with pneumococcal polysaccharide vaccine induces a predominant IgA2 response of peripheral blood lymphocytes and increases of both serum and secretory anti-pneumococcal antibodies. J Immunol. 1988 Jun 1;140(11):3793–3800. [PubMed] [Google Scholar]
- McCaughan G. W., Adams E., Basten A. Human antigen-specific IgA responses in blood and secondary lymphoid tissue: an analysis of help and suppression. J Immunol. 1984 Mar;132(3):1190–1196. [PubMed] [Google Scholar]
- Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987 Jul;7(4):265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- Möller S. A., Borrebaeck C. A. A filter immuno-plaque assay for the detection of antibody-secreting cells in vitro. J Immunol Methods. 1985 May 23;79(2):195–204. doi: 10.1016/0022-1759(85)90099-7. [DOI] [PubMed] [Google Scholar]
- Nohr C. W., Latter D. A., Meakins J. L., Christou N. V. In vivo and in vitro humoral immunity in surgical patients: antibody response to pneumococcal polysaccharide. Surgery. 1986 Aug;100(2):229–238. [PubMed] [Google Scholar]
- Pichichero M. E., Insel R. A. Mucosal antibody response to parenteral vaccination with Haemophilus influenzae type b capsule. J Allergy Clin Immunol. 1983 Nov;72(5 Pt 1):481–486. doi: 10.1016/0091-6749(83)90585-7. [DOI] [PubMed] [Google Scholar]
- Rijkers G. T., Mosier D. E. Pneumococcal polysaccharides induce antibody formation by human B lymphocytes in vitro. J Immunol. 1985 Jul;135(1):1–4. [PubMed] [Google Scholar]
- Saxon A., Mitsuyasu R., Stevens R., Champlin R. E., Kimata H., Gale R. P. Designed transfer of specific immune responses with bone marrow transplantation. J Clin Invest. 1986 Oct;78(4):959–967. doi: 10.1172/JCI112686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer M. E., Rhodes M., Prince S., Michalek S. M., McGhee J. R. A plastic intraoral device for the collection of human parotid saliva. J Dent Res. 1977 Jul;56(7):728–733. doi: 10.1177/00220345770560070401. [DOI] [PubMed] [Google Scholar]
- Schneerson R., Robbins J. B., Parke J. C., Jr, Bell C., Schlesselman J. J., Sutton A., Wang Z., Schiffman G., Karpas A., Shiloach J. Quantitative and qualitative analyses of serum antibodies elicited in adults by Haemophilus influenzae type b and pneumococcus type 6A capsular polysaccharide-tetanus toxoid conjugates. Infect Immun. 1986 May;52(2):519–528. doi: 10.1128/iai.52.2.519-528.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simberkoff M. S., Cross A. P., Al-Ibrahim M., Baltch A. L., Geiseler P. J., Nadler J., Richmond A. S., Smith R. P., Schiffman G., Shepard D. S. Efficacy of pneumococcal vaccine in high-risk patients. Results of a Veterans Administration Cooperative Study. N Engl J Med. 1986 Nov 20;315(21):1318–1327. doi: 10.1056/NEJM198611203152104. [DOI] [PubMed] [Google Scholar]
- Sorkin E., Landy M. Antibody production by blood leucocytes. Experientia. 1965 Nov 15;21(11):677–680. doi: 10.1007/BF02144077. [DOI] [PubMed] [Google Scholar]
- Stevens R. H., Macy E., Morrow C., Saxon A. Characterization of a circulating subpopulation of spontaneous antitetanus toxoid antibody producing B cells following in vivo booster immunization. J Immunol. 1979 Jun;122(6):2498–2504. [PubMed] [Google Scholar]
- Thomson P. D., Harris N. S. Detection of plaque-forming cells in the peripheral blood of actively immunized humans. J Immunol. 1977 Apr;118(4):1480–1482. [PubMed] [Google Scholar]


